Abstract
The two widely coexpressed isoforms of β-arrestin (termed βarrestin 1 and 2) are highly similar in amino acid sequence. The β-arrestins bind phosphorylated heptahelical receptors to desensitize and target them to clathrin-coated pits for endocytosis. To better define differences in the roles of β-arrestin 1 and 2, we prepared mouse embryonic fibroblasts from knockout mice that lack one of the β-arrestins (βarr1-KO and βarr2-KO) or both (βarr1/2-KO), as well as their wild-type (WT) littermate controls. These cells were analyzed for their ability to support desensitization and sequestration of the β2-adrenergic receptor (β2-AR) and the angiotensin II type 1A receptor (AT1A-R). Both βarr1-KO and βarr2-KO cells showed similar impairment in agonist-stimulated β2-AR and AT1A-R desensitization, when compared with their WT control cells, and the βarr1/2-KO cells were even further impaired. Sequestration of the β2-AR in the βarr2-KO cells was compromised significantly (87% reduction), whereas in the βarr1-KO cells it was not. Agonist-stimulated internalization of the AT1A-R was only slightly reduced in the βarr1-KO but was unaffected in the βarr2-KO cells. In the βarr1/2-KO cells, the sequestration of both receptors was dramatically reduced. Comparison of the ability of the two β-arrestins to sequester the β2-AR revealed β-arrestin 2 to be 100-fold more potent than β-arrestin 1. Down-regulation of the β2-AR was also prevented in the βarr1/2-KO cells, whereas no change was observed in the single knockout cells. These findings suggest that sequestration of various heptahelical receptors is regulated differently by the two β-arrestins, whereas both isoforms are capable of supporting receptor desensitization and down-regulation.
Signaling via heptahelical receptors is generally terminated by the two-step process of desensitization (1, 2). Initially, the agonist-occupied receptor is phosphorylated by a G protein-coupled receptor kinase that then promotes the high-affinity binding of the β-arrestins. When bound to the receptor, the β-arrestins physically interdict its association with the G protein, thereby attenuating further signaling (1, 2). In addition to associating with the receptor, β-arrestins bind several molecules involved in the machinery for receptor sequestration, including AP-2 (3), clathrin (4), and N-ethylmaleimide-sensitive fusion protein (NSF) (5). Thus, β-arrestins also serve as adaptor molecules that target the heptahelical receptor for internalization. Once internalized the receptor can undergo either dephosphorylation and recycling to the plasma membrane (6) or down-regulation by targeting the receptor for degradation (7).
The family of arrestin molecules comprises the following four members: visual arrestin, cone arrestin, β-arrestin 1, and βarrestin 2 (8–10). Visual and cone arrestin have specialized functions as a result of their limited localization in the visual system. In contrast, β-arrestins 1 and 2 are ubiquitously expressed in all cell types, although in various proportions (8–10). The classical physiological functions of the β-arrestins are heptahelical receptor desensitization and sequestration; however, it is still unknown whether the two β-arrestins play different roles in these processes. Results from several studies have suggested that interactions with the sequestration machinery might differ between β-arrestins 1 and 2. In in vitro assays, clathrin has been found to have a 6-fold greater affinity for β-arrestin 2 than 1 (4). In addition, AP-2 binds preferentially to β-arrestin 2 in yeast two-hybrid assays (3). Moreover, β-arrestin 2 appears to be the more efficient β-arrestin at translocating to the membrane on agonist stimulation of several heptahelical receptors (11). In other studies that used an antisense approach to reduce β-arrestin levels in cells, reduction in either β-arrestin caused some impairment of β2-adrenergic receptor (β2-AR) desensitization and internalization (12). However, because β-arrestin expression was not completely eliminated by this method, it was not possible to define specific values for the contribution of each β-arrestin.
To better define differences in the physiological roles of β-arrestins 1 and 2, we have used the β-arrestin 1 (13) and the β-arrestin 2 (14) knockout mice (βarr1-KO and βarr2-KO, respectively) to generate mouse embryonic fibroblast (MEF) established cell lines. By using MEF lines lacking β-arrestin 1, β-arrestin 2, or both, we have compared the abilities of either β-arrestin to support desensitization, sequestration, and also down-regulation of heptahelical receptors.
Materials and Methods
Materials.
The radiolabeled compounds [125I]iodocyanopindolol, [125I]Tyr4-angiotensin II, [3H]adenine, [14C]cAMP, and myo-[3H]inositol were purchased from NEN Life Science Products. Human AngII was from Peninsula Laboratories. The BCA protein determination kit was obtained from Pierce. All other reagents were purchased from Sigma.
Preparation of MEFs.
βarr1-KO and βarr2-KO MEFs were prepared from day 10.5 to day 13.5 embryos derived from crosses between βarr1(+/−) (13) or βarr2(+/−) (14) mice set up to produce littermate wild-type (WT) and knockout embryos. Double knockout MEFs, βarr1/2-KO, were generated from the crosses of βarr1(−/−) βarr2(+/−) and βarr1(+/−) βarr2(−/−) mice to increase the chance of acquiring double knockout embryos. MEF established cultures were prepared according to the 3T3 protocol of Todaro and Green (15). None of the cell cultures appeared to differ in their ability to spontaneously transform or to become established cell lines.
Infection and Transfection of MEFs.
Overexpression of β2-AR was achieved by infecting cells with a recombinant β2-AR adenovirus (Ad; ref. 16) at a multiplicity of infection sufficient for expression of 100–300 fmol of receptor per mg of protein. For the overexpression of AT1A-R, an Ad component system was used (17). Briefly, a complex of empty Ad, poly-l-lysine (Mr, 34,000–48,000), and pcDNA3-Flag-AT1A-R (a gift from Marc G. Caron, Duke University Medical Center, Durham, NC) was formed and then incubated with the cells for 2 h at 37°C. An expression level of 200–350 fmol/mg of AT1A-R was achieved. Replacement of either β-arrestin was accomplished with recombinant βarr1 or βarr2 Ad. βarr1-Ad and βarr2-Ad were generated by inserting the 1.3-kb βarr1 or βarr2 cDNA into pAdTrack-CMV (18) at HindIII/XbaI or KpnI/XbaI sites, respectively. The Ad βarr1 and βarr2 expression plasmids were generated by homologous recombination with pAdEasy-1 in Escherichia coli. These recombinant Ad vectors for βarr1 and βarr2 were used to transfect HEK293 cells to produce βarr1-Ad and βarr2-Ad, as described (18).
Immunoblotting and Quantification of β-Arrestins 1 and 2 Expression Levels in MEFs.
Total cell lysates were prepared from MEFs. Equal amounts of protein were separated by SDS/PAGE and immunoblotted with rabbit polyclonal anti-β-arrestin antibody (A1CT) (10). Protein standards for β-arrestin 1 and 2, respectively, were prepared from HEK293 cells transfected with pcDNA3βarr1-flag or pcDNA3βarr2-flag (19). Cells were lysed, lysates were centrifuged, and the β-arrestins were immunoprecipitated with anti-Flag M2 agarose conjugate beads. To quantitate protein concentrations of purified β-arrestins, various amounts of sample were separated by PAGE with known quantities of BSA standards. Gels were stained and protein bands were quantitated by densitometry with a Bio-Rad Fluor-S imager.
Receptor Sequestration Assays.
Agonist-induced β2-AR and AT1A-R sequestration were measured in intact cells by radioligand binding, as described (20, 21).
Second Messenger Accumulation Assays.
To determine agonist-stimulated cAMP accumulation in cells, the conversion of [3H]adenine to [3H]cAMP was measured as described (22). AngII-induced phosphatidylinositol hydrolysis was determined as described (23).
Data Analysis.
Data are expressed as mean ± SEM. Time course and dose-response data were analyzed with GRAPHPAD PRISM software. Statistical significance was determined by an unpaired, two-tailed t test.
Results
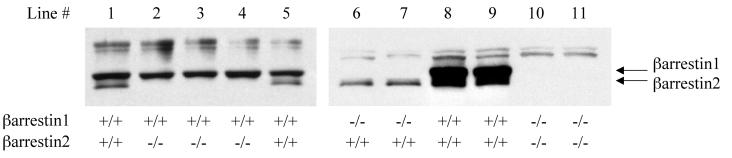
To date, differences in the functions of the ubiquitously expressed arrestins, β-arrestins 1 and 2, have not been clearly demonstrated, due in large part to the lack of appropriate systems in which each β-arrestin can be studied individually. Accordingly, we generated MEF lines from β-arrestin knockout animals with the 3T3 protocol (15). The β-arrestin expression profile of each of the 11 MEF lines generated was analyzed by Western blotting cell lysates with a rabbit polyclonal anti-β-arrestin antiserum (A1CT; Fig. 1). This antiserum, which recognizes β-arrestins 1 and 2, detects the 47-kDa β-arrestin 1 and 46.3-kDa β-arrestin 2 proteins (10) in a pattern that matches exactly that predicted from the genotyping of the primary cell cultures.
Figure 1.
Analysis of β-arrestin expression in MEF cell lines. Whole cell lysates were prepared from 11 MEF cell lines and resolved (50–70 μg of protein per lane) by SDS/PAGE. Proteins were transferred to a nitrocellulose sheet and immunoblotted with the polyclonal anti-β-arrestin antibody A1CT. The genotype of each MEF line is described beneath the immunoblot. Lines 1–5 are littermates of a βarr2(+/−) × βarr2(+/−) cross, lines 6–9 are littermates from a βarr1(+/−) × βarr1(+/−) cross, and lines 10 and 11 are littermates from a βarr1(+/−) βarr2(−/−) × βarr1(−/−) βarr2(+/−) cross.
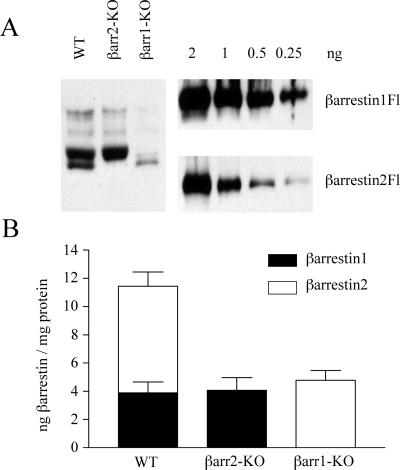
The amount of each β-arrestin expressed was then calculated for each line to determine whether direct comparison of βarrestin function between lines was possible. Shown in Fig. 2A is a representative immunoblot of equivalent amounts of the MEF cell lysates WT (line 1), βarr2-KO (line 2), and βarr1-KO (line 6) blotted with the A1CT antiserum. Known amounts of purified β-arrestin1-Flag and β-arrestin2-Flag were electrophoresed beside the lysates to measure levels of the β-arrestins (Fig. 2A). Of note is that, although the A1CT antibody recognizes both β-arrestins 1 and 2, it has a 5-fold higher affinity for β-arrestin 1. Analysis of the expression of the endogenous β-arrestins (Fig. 2B) determined that in the WT line 1 there is 2-fold more β-arrestin 2 (7.56 ± 1.01 ng of βarr2 per mg of protein, n = 7) than β-arrestin 1 (3.88 ± 0.78 ng of βarr1 per mg of protein, n = 7). When compared with WT line 1, βarr1-KO line 6 shows a reduced level of expression of the remaining β-arrestin 2 (4.78 ± 0.69 ng of βarr2 per mg of protein, n = 4), whereas βarr2-KO line 2 maintains similar amounts of β-arrestin 1 (4.07 ± 0.89 ng of βarr1 per mg of protein, n = 5) to that found in WT line 1. As depicted in Fig. 2B, the MEFs βarr1-KO line 6 and βarr2-KO line 2 have similar concentrations of the remaining β-arrestin and, therefore, offer an ideal system in which to compare the functions of the β-arrestins. In fact, from Fig. 1, it is evident that the level of β-arrestin 1 in all three βarr2-KO lines is approximately equivalent, as is β-arrestin 2 in both the βarr1-KO lines, making comparisons between all these lines possible.
Figure 2.
Quantitation of β-arrestin levels in MEF cell lines. (A) Whole cell lysates (50 μg) from WT (line 1), βarr1-KO (line 6), and βarr2-KO (line 2) (Left) and known quantities of β-arrestin1-Flag and β-arrestin2-Flag proteins (Right) were separated by SDS/PAGE, transferred to nitrocellulose, and immunoblotted with the A1CT antibody. (B) Concentrations of β-arrestin 1 and β-arrestin 2 in the above MEF lines were quantitated by densitometric analysis of the immunoblots. The resulting β-arrestin levels are plotted as ng of β-arrestin per mg of cell protein. Data are expressed as the mean ± SEM of four to seven experiments.
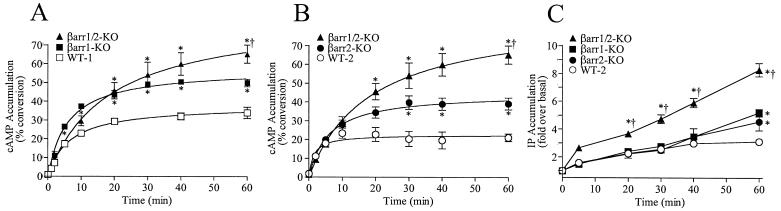
β-Arrestins 1 and 2 have been ascribed roles in both heptahelical receptor desensitization and sequestration (2). We first examined the ability of the β-arrestin knockout MEFs to undergo heptahelical receptor-mediated desensitization of second messenger generation. For this purpose we analyzed cAMP accumulation stimulated by the Gs protein-coupled β2-AR, and phosphatidylinositol hydrolysis stimulated by the Gq protein-coupled AT1A-R. MEF lines were infected with sufficient β2-AR Ad to express 100 fmol/mg of receptor. The total cAMP accumulated in response to isoproterenol treatment in the βarr1-KO and βarr2-KO MEFs is significantly greater than that in the WT cells (Fig. 3 A and B), indicating that a reduction in the levels of β-arrestin (to approximately one-third of that in WT cells) impairs the ability of these cells to desensitize the β2-AR. No significant differences in isoproterenol-induced β2-AR desensitization were noted between the βarr1-KO and βarr2-KO cells. However, the double knockout βarr1/2-KO cells were even more impaired in their desensitization profile than the single knockout cells. Thus, when the single β-arrestin knockout lines have completely desensitized, the βarr1/2-KO still generate cAMP. Taken together, these findings suggest that the remaining β-arrestin 1 or 2 in the single knockout cells can equivalently mediate some β2-AR desensitization, although at a slower rate than WT cells containing a normal complement of β-arrestins. However, the double knockout cells appear to be greatly impaired in their ability to desensitize.
Figure 3.
Effect of reduced β-arrestin levels on second messenger generation. (A and B) Littermate WT (line 8) and βarr1-KO (line 6) cell lines (A) or littermate WT (line 1) and βarr2-KO (line 2) cell lines (B), as well as the βarr1/2-KO cell line 10, all expressing approximately100 fmol of β2-AR per mg of protein, were stimulated with 10 μM isoproterenol as indicated. Isoproterenol-induced cAMP accumulation in the MEF lines was determined as the percent conversion of [3H]adenine into [3H]cAMP and then normalized to total forskolin (50 μM)-stimulated cAMP accumulation for each cell line. Data are the mean ± SEM of three to six experiments and were analyzed with GRAPHPAD PRISM software. (C) WT (line 1), βarr1-KO (line 6), βarr2-KO (line 2), and βarr1/2-KO (line 10) MEF cell lines, all expressing AT1A-R at 200–350 fmol/mg of protein, were stimulated with 100 nM AngII for the indicated times. Accumulation of inositol phosphates was measured as the fold difference over basal accumulation. Data are the mean ± SEM of 10 experiments. Unpaired, two-tailed t tests were performed for total cAMP and total inositol phosphate accumulations between WT and βarr1-KO, βarr2-KO, or βarr1/2-KO lines (*, P < 0.005) and between βarr1/2-KO and βarr1-KO or βarr2-KO lines (†, P < 0.03).
A similar approach was used to examine the ability of βarrestins 1 and 2 to mediate agonist-induced desensitization of the AT1A-R. Fig. 3C shows AngII-stimulated phosphatidylinositol hydrolysis in the knockout and WT lines overexpressing the AT1A-R (200–350 fmol/mg of protein). A comparison of the total phosphatidylinositol hydrolysis after 60 min of AngII stimulation showed significant increases in inositol phosphate accumulation in the single knockout MEFs and double knockout MEFs in comparison to the WT cells but no appreciable difference when the two single knockout lines were compared with each other. This pattern was the same as that observed for the β2-AR, where desensitization was clearly mediated by βarrestins but no significant difference was observed between the ability of β-arrestin 1 and 2 to uncouple the receptor from its cognate G protein.
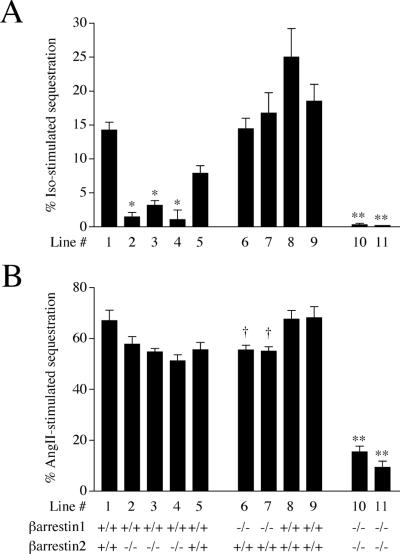
Differences between β-arrestin 1- and 2-mediated heptahelical receptor sequestration were next assessed (Fig. 4). Cells were infected with the β2-AR-recombinant Ad or transfected with the AT1A-R expression plasmid and stimulated with the appropriate ligand, and then the number of internalized receptors was measured by ligand binding assays. In all βarr2-KO lines, isoproterenol-induced β2-AR sequestration was significantly impaired compared with WT control lines, and it was abolished in the βarr1/2-KO cell lines (Fig. 4A). In contrast, β2-AR sequestration was not significantly different between the WT and the βarr1-KO cell lines. Thus, these data suggest that β-arrestin 2 is the β-arrestin mainly responsible for regulating β2-AR sequestration.
Figure 4.
Effect of reduced levels of β-arrestins on heptahelical receptor sequestration. (A) Littermate MEF βarr2-KO lines 1–5, βarr1-KO lines 6–9, and βarr1/2-KO lines 10 and 11 expressing 200–300 fmol of β2-AR per mg of protein were stimulated with 10 μM isoproterenol (iso) for 20 min at 37°C. Receptor sequestration was subsequently measured with a ligand binding assay. Percent isoproterenol-stimulated sequestration was determined as the difference between the agonist-stimulated internalized β2-ARs and the nonstimulated basally internalized β2-ARs. (B) Littermate MEF βarr2-KO lines 1–5, βarr1-KO lines 6–9, and βarr1/2-KO lines 10 and 11 expressing 200–350 fmol of AT1A-R per mg of protein were stimulated with 0.2 nM 125I-labeled AngII for 20 min at 37°C. Percent AngII-stimulated sequestration was determined as acid-resistant cpm divided by the total cpm bound. Data are the mean ± SEM of 5–10 experiments. An unpaired two-tailed t test was used to test statistical significance. *, P < 0.0001 between βarr2-KO (lines 2–4) cell lines and their WT controls (lines 1 and 5); †, P < 0.01 between βarr1-KO (lines 6 and 7) cell lines and their WT control (lines 8 and 9); **, P < 0.0001 between βarr1/2-KO (lines 10 and 11) cell lines and all WT lines (lines 1, 5, 8, and 9).
The AngII-stimulated sequestration of the AT1A-R, however, showed a very different pattern (Fig. 4B). There was no difference in the ability of the βarr2-KO lines to sequester the AT1A-R compared with their WT controls. In contrast, the βarr1-KO lines were slightly impaired by 18% in the sequestration of the AT1A-R. The βarr1/2-KO lines, however, exhibited a dramatic (82%) reduction in agonist-induced AT1A-R internalization. These results suggest that in these cells the AT1A-R is primarily internalized in a β-arrestin-dependent manner and that either β-arrestin 1 or 2 can be used.
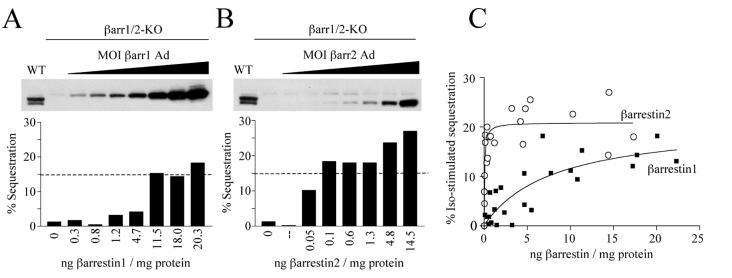
To further quantify the difference between β-arrestin 1 and 2 in promoting β2-AR internalization, we used the βarr1/2-KO cells as a background to reintroduce various concentrations of either β-arrestin 1 or 2 (Fig. 5). The actual concentrations of the β-arrestins achieved were determined by Western blotting followed by comparison to standard curves of known amounts of β-arrestin protein. In this manner data from multiple experiments were pooled to produce dose–response curves for the ability of both β-arrestins to support β2-AR internalization (Fig. 5C). Analysis of the curves revealed that, maximally, both β-arrestins could promote the same level of internalization (21%). However, the apparent affinity (EC50) for sequestration by β-arrestin 1 was 8.22 ± 4.5 ng/mg of cellular protein and for β-arrestin 2 was 0.063 ± 0.023 ng/mg of protein. Thus, these data indicate that both β-arrestins can mediate the same maximal level of sequestration but that β-arrestin 2 attains this level at 1/100th the concentration of that required for β-arrestin 1.
Figure 5.
Reconstitution of agonist-induced β2-AR sequestration by β-arrestin 1 or 2 in βarr1/2-KO MEFs. (A and B) βarr1/2-KO cells (line 10) were infected with various multiplicities of infection of βarr1-Ad (A) or βarr2-Ad (B) and sufficient β2-AR Ad to express β2-AR at approximately 200 fmol/mg. The level of β-arrestin expression in each infection was determined by Western blotting of cell lysates (Upper) followed by comparison to a standard curve of β-arrestin1-Flag and β-arrestin2-Flag proteins. Isoproterenol-induced β2-AR sequestration for each infection was then determined (Lower). A and B show a representative experiment (n = 5). For comparison, the same protein concentration from WT cell lysates was immunoblotted and its isoproterenol-induced sequestration is represented as a dashed line in the bar graph. (C) Pooled data from all experiments showing effect of β-arrestin expression on the ability of βarr1/2-KO cells to sequester the β2-AR.
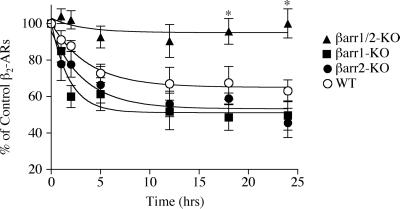
The sustained activation of most hepathelical receptors results in their down-regulation, leading to a reduction in the total number of receptors in the cell. There are two alternative hypotheses for the mechanism underlying receptor down-regulation; one requires prior internalization of the receptor (7) and one does not (24). We sought to evaluate this phenomenon in the β-arrestin knockout MEFs impaired in β2-AR sequestration. Cells were treated with agonist (isoproterenol, 10 μM) for up to 24 h, and the total density of cellular receptors was measured by ligand binding (Fig. 6). βarr1/2-KO MEFs, which did not sequester β2-AR on agonist stimulation, did not down-regulate the β2-AR. In contrast, the WT and both single knockout cell types displayed similar patterns of receptor down-regulation. Thus, although the βarr2-KO cells were greatly impaired in their ability to sequester the receptor, they nonetheless were able to down-regulate the β2-AR, apparently to the same extent as the WT and βarr1-KO cells.
Figure 6.
Effect of reduced β-arrestin expression on down-regulation of the β2-AR. WT (line 1), βarr1-KO (line 6), βarr2-KO (line 2), and βarr1/2-KO (line 10) cell lines expressing β2-AR at approximately 150 fmol/mg of protein or endogenous β2-AR (approximately 25–50 fmol/mg of protein) were stimulated with 10 μM isoproterenol as indicated. Experiments with overexpressed β2-AR (n = 3) and with endogenous β2-AR (n = 4) showed similar results, and thus data were pooled. Receptor number was determined by ligand binding. An unpaired two-tailed t test was used to determine statistical significance as follows. *, P < 0.01 between WT and βarr1/2-KO cells.
Discussion
The ubiquitously expressed β-arrestins 1 and 2 are structurally highly homologous, sharing 78% amino acid identity (8, 10). There has been extensive investigation of these two proteins to determine whether they serve different roles in heptahelical receptor signaling or are functionally redundant. Studies have used the overexpression of the wild-type or dominant negative proteins, as well as antisense mRNA to elucidate these differences (12, 25, 26). However, to date there is little compelling evidence bearing on these issues. To further this investigation, we have generated βarr1-KO, βarr2-KO, and βarr1/2-KO MEFs to study the functions of the individual β-arrestins.
Desensitization of heptahelical receptors occurs when the phosphorylated receptor binds β-arrestin and its coupling to G proteins is disrupted. The function of β-arrestin in this process has been extensively examined in reconstituted systems of purified proteins (10), by overexpression of proteins (25, 26), and by antisense reduction of β-arrestin levels in cells (12). When β-arrestin is overexpressed in cells, there is enhanced desensitization of heptahelical receptors in response to their respective agonists (25). Using an antisense approach to reduce the endogenous levels of β-arrestins 1 and 2, Mundell et al. (12) have shown that in cells lacking 50% of β-arrestin 1 and 75% of β-arrestin 2, compared with control cells, β2-AR desensitization was significantly attenuated. However, the individual contributions of β-arrestins 1 and 2 still could not be discerned with the above experimental strategies. In our study, when compared with WT cells, βarr1-KO and βarr2-KO cells showed similar magnitudes of impairment of agonist-stimulated β2-AR and AT1A-R desensitization, as demonstrated by increased maximal accumulation of second messengers in knockout cells. Furthermore, cells lacking both β-arrestins have an even slower rate of desensitization (higher total cAMP and inositol phosphate accumulations) than either of the single knockouts. Thus, we show that β-arrestins are necessary for maximal desensitization of heptahelical receptors and that β-arrestins 1 and 2 are equally efficacious in this process for both types of receptors. Albeit diminished, the βarr1/2-KO cells still eventually demonstrate desensitization of the β2-AR despite the complete absence of β-arrestins. This is presumably due to other pathways of desensitization such as protein kinase A- or C-mediated heterologous desensitization (27) and/or postreceptor alterations. Alternatively, desensitization could result from protein kinase A-mediated switching of the β2-AR from Gs protein coupling to Gi protein coupling, thus leading to decreased activation of adenylyl cyclase (28).
It has been proposed that heptahelical receptors can be grouped into two classes based on the differences in kinetics of their agonist-induced recruitment of the two fluorescently tagged β-arrestins (11). Class A receptors, which include the β2-AR, recruit β-arrestin 2 at a faster rate than β-arrestin 1, and class B receptors, which include the AT1A-R, have similar recruitment profiles for both β-arrestins. In this study we have shown that the βarr2-KO cells are severely impaired in their ability to sequester the β2-AR, whereas the βarr1-KO cells are not and the βarr1/2-KO cells are completely impaired. The notion that β-arrestin 2 is required for proper sequestration of the β2-AR is further substantiated by experiments where βarrestins 1 and 2 are expressed in increasing amounts in βarr1/2-KO cells. These experiments demonstrated that, although both β-arrestins mediate β2-AR sequestration, 100-fold more βarrestin 1 than β-arrestin 2 is necessary to reconstitute WT sequestration. Sequestration of heptahelical receptors is a multistep process that includes translocation and binding of the β-arrestin to the receptor and its subsequent linkage to the sequestration machinery through AP-2 and clathrin. The 100-fold enhancement in sequestration of the β2-AR by β-arrestin 2 over β-arrestin 1 could represent a composite of higher affinities of β-arrestin 2 for several components of the internalization machinery. Oakley et al. (11) have reported that β-arrestin 2 can be recruited to the β2-AR with 10-fold greater efficiency than can β-arrestin 1. Furthermore, β-arrestin 2 binds clathrin with 6-fold greater affinity than β-arrestin 1 (4). Thus, it is reasonable to propose that the greater ability of β-arrestin 2 than β-arrestin 1 to mediate each of several steps of the internalization process ultimately results in the observed 100-fold greater efficacy of sequestration of the β2-AR.
Recently, Mundell et al. (12) reported that, in HEK293 cells, a 50% reduction in β-arrestin 1 protein by the expression of antisense mRNA results in reduced β2-AR internalization. The reason for the difference between these results and results of the present study is unknown. However, a possible explanation for this discrepancy is that β-arrestin 1 antisense mRNA expression might have additional nonspecific effects on other components of the internalization machinery. Any such nonspecific effect, however, was overcome by the overexpression of β-arrestin 2, which can perhaps be explained by the much greater capacity for β-arrestin 2 than β-arrestin 1 to direct receptor internalization, even in cells in which other sequestration components are compromised to some extent.
The agonist-induced sequestration of the AT1A-R has a very different profile than that of the β2-AR. From studies with dominant negative β-arrestin mutants, it has been suggested that AT1A-R internalization is β-arrestin-independent (29). However, recently it has been demonstrated that green fluorescent protein-tagged β-arrestins 1 and 2 are recruited equally well to the AT1A-R and are internalized with the receptor (11). In the present study, although the βarr1-KO cells show a slight statistically significant impairment in the sequestration of the AT1A-R, neither βarr1-KO or βarr2-KO cells are greatly impaired in AT1A-R internalization. However, internalization in the βarr1/2-KO cells is impaired by 82% compared with the WT cells. Thus, it appears that the AT1A-R uses primarily a βarrestin-dependent pathway for internalization and, to a lesser extent, a β-arrestin-independent pathway. Furthermore, in agreement with the observations of Oakley et al. (11), β-arrestins 1 and 2 can substitute for each other in the sequestration of the AT1A-R.
The relationship between endocytosis and down-regulation of receptors has only recently begun to be explored. One model proposed for heptahelical receptor down-regulation postulates that endocytosis of the receptors is a required initial step in the down-regulation pathway (7). Our results appear consistent with this hypothesis because in the βarr1/2-KO MEFs, where sequestration of the β2-AR is completely abolished, there is no agonist-stimulated down-regulation over a 24-h period. However, this result may also be interpreted as there being a β-arrestin requirement for down-regulation unrelated to sequestration. Furthermore, normal down-regulation is observed in the βarr2-KO cells that are 87% impaired in β2-AR sequestration, suggesting that the remaining reduced capacity of these cells to internalize the receptor is nonetheless sufficient to subserve the function of down-regulation or that an alternative pathway that is independent of internalization also exists (24).
On the basis of these results we have been able to clearly define differences in the function of β-arrestins 1 and 2. One of the specialized functions of β-arrestin 2 appears to lie in the sequestration of the β2-AR and possibly of other class A receptors (11) that remain to be tested. Furthermore, we have shown that the AT1A-R sequesters mainly in a β-arrestin-dependent manner and that no differences are observed in the efficiency of β-arrestins 1 and 2 for mediating this process, consistent with its classification as a class B receptor (11). Finally, the β-arrestin knockout cells we have generated not only allow the detailed study of differences in functions of β-arrestins 1 and 2 but also provide a system in which β-arrestin-dependent signaling pathways can be more clearly delineated.
Acknowledgments
We thank Donna Addison, Mary Holben, and Julie Turnbough for excellent secretarial assistance and Sabrina Exum for excellent technical assistance. We also thank Drs. Audrey Claing and Stéphane Laporte for critical reading of this manuscript. This work was supported in part by National Institutes of Health Grant HL16037 (to R.J.L.). R.J.L. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- MEF
mouse embryonic fibroblast
- WT
wild type
- β2-AR
β2-adrenergic receptor
- AT1A-R
angiotensin II type 1A receptor
- AngII
angiotensin II
- Ad
adenovirus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041608198.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041608198
References
- 1.Pitcher J A, Freedman N J, Lefkowitz R J. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz R J. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 3.Laporte S A, Oakley R H, Zhang J, Holt J A, Ferguson S S, Caron M G, Barak L S. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 5.McDonald P H, Cote N L, Lin F T, Premont R T, Pitcher J A, Lefkowitz R J. J Biol Chem. 1999;274:10677–10680. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 6.Oakley R H, Laporte S A, Holt J A, Barak L S, Caron M G. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 7.Gagnon A W, Kallal L, Benovic J L. J Biol Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- 8.Krupnick J G, Benovic J L. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 9.Lohse M J, Benovic J L, Codina J, Caron M G, Lefkowitz R J. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 10.Attramadal H, Arriza J L, Aoki C, Dawson T M, Codina J, Kwatra M M, Snyder S H, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- 11.Oakley R H, Laporte S A, Holt J A, Caron M G, Barak L S. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 12.Mundell S J, Loudon R P, Benovic J L. Biochemistry. 1999;38:8723–8732. doi: 10.1021/bi990361v. [DOI] [PubMed] [Google Scholar]
- 13.Conner D A, Mathier M A, Mortensen R M, Christe M, Vatner S F, Seidman C E, Seidman J G. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 14.Bohn L M, Lefkowitz R J, Gainetdinov R R, Peppel K, Caron M G, Lin F T. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 15.Todaro G J, Green H. J Cell Biol. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drazner M H, Peppel K C, Dyer S, Grant A O, Koch W J, Lefkowitz R J. J Clin Invest. 1997;99:288–296. doi: 10.1172/JCI119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohout T A, O'Brian J J, Gaa S T, Lederer W J, Rogers T B. Circ Res. 1996;78:971–977. doi: 10.1161/01.res.78.6.971. [DOI] [PubMed] [Google Scholar]
- 18.He T C, Zhou S, da Costa L T, Yu J, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald P H, Chow C W, Miller W E, Laporte S A, Field M E, Lin F T, Davis R J, Lefkowitz R J. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 20.Lohse M J, Benovic J L, Caron M G, Lefkowitz R J. J Biol Chem. 1990;265:3202–3211. [PubMed] [Google Scholar]
- 21.Laporte S A, Servant G, Richard D E, Escher E, Guillemette G, Leduc R. Mol Pharmacol. 1996;49:89–95. [PubMed] [Google Scholar]
- 22.Freedman N J, Liggett S B, Drachman D E, Pei G, Caron M G, Lefkowitz R J. J Biol Chem. 1995;270:17953–17961. doi: 10.1074/jbc.270.30.17953. [DOI] [PubMed] [Google Scholar]
- 23.Cotecchia S, Ostrowski J, Kjelsberg M A, Caron M G, Lefkowitz R J. J Biol Chem. 1992;267:1633–1639. [PubMed] [Google Scholar]
- 24.Jockers R, Angers S, Da Silva A, Benaroch P, Strosberg A D, Bouvier M, Marullo S. J Biol Chem. 1999;274:28900–28908. doi: 10.1074/jbc.274.41.28900. [DOI] [PubMed] [Google Scholar]
- 25.Pippig S, Andexinger S, Daniel K, Puzicha M, Caron M G, Lefkowitz R J, Lohse M J. J Biol Chem. 1993;268:3201–3208. [PubMed] [Google Scholar]
- 26.Ferguson S S, Downey W E, 3rd, Colapietro A M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 27.Yuan N, Friedman J, Whaley B S, Clark R B. J Biol Chem. 1994;269:23032–23038. [PubMed] [Google Scholar]
- 28.Daaka Y, Luttrell L M, Lefkowitz R J. Nature (London) 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Ferguson S S G, Barak L S, Menard L, Caron M G. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]