Abstract
Our previous work has linked verbal learning and memory with cognitive insight, but not clinical insight, in individuals with a first-episode psychosis (FEP). The current study reassessed the neurocognitive basis of cognitive and clinical insight and explored their neural basis in 61 FEP patients. Cognitive insight was measured with the Beck Cognitive Insight Scale (BCIS) and clinical insight with the Scale to assess Unawareness of Mental Disorder (SUMD). Global measures for 7 domains of cognition were examined. Hippocampi were manually segmented in to 3 parts: the body, head, and tail. Verbal learning and memory significantly correlated with the BCIS composite index. Composite index scores were significantly associated with total left hippocampal (HC) volume; partial correlations, however, revealed that this relationship was attributable largely to verbal memory performance. The BCIS self-certainty subscale significantly and inversely correlated with bilateral HC volumes, and these associations were independent of verbal learning and memory performance. The BCIS self-reflectiveness subscale significantly correlated with verbal learning and memory but not with HC volume. No significant correlations emerged between the SUMD and verbal memory or HC volume. These results strengthen our previous assertion that in individuals with an FEP cognitive insight may rely on memory whereby current experiences are appraised based on previous ones. The HC may be a viable location among others for the brain system that underlies aspects of cognitive insight in individuals with an FEP.
Keywords: awareness of illness, cognition, magnetic resonance imaging, neuroimaging, first-episode schizophrenia, overconfidence
Introduction
Lack of insight (ie, poor awareness of illness) is a central clinical characteristic of schizophrenia.1 The clinical concept of insight focuses on the patient's awareness that their symptoms and behavior are abnormal and attributable to a mental disorder, of the social consequences of the disorder and the need for treatment.2,3 This form of insight is determined by observing individuals’ behavior in the context of a clinical examination and is valuable for determining diagnosis, prognosis, and treatment.4,5
Fundamental to an understanding of clinical insight is the study of patients’ capacity to distance themselves from their distorted beliefs and misinterpretations, reflect on them rationally, and recognize erroneous conclusions. These metacognitive processes are studied under the rubric of cognitive insight. To psychometrically assess such cognitions, Beck et al6 developed the brief self-report Beck Cognitive Insight Scale (BCIS). These authors argue that the mental operations underlying cognitive insight can be divided into 2 mechanisms: self-reflectiveness, which includes a willingness to acknowledge fallibility, corrigibility, and recognition of dysfunctional reasoning, and self-certainty, which refers to a tendency to be overconfident. These authors propose that higher levels of certainty may diminish the capacity for self-reflection, and thus a composite index is calculated through subtracting the individual's self-certainty score from her/his self-reflectiveness score. Cognitive insight is of great clinical significance because it directly taps into thinking styles underlying distorted cognitions, which are increasingly acknowledged as target for intervention.7 Cognitive insight has adequate convergent validity with clinical measures of insight.6
The study of cognitive insight has recently been employed to identify aspects of the neurocognitive architecture that may contribute to aberrant thinking styles and cognitive distortions in psychosis.8 In an initial study, in individuals with a first-episode psychosis (FEP), we evaluated associations between cognitive and clinical insight and 7 domains of cognition (verbal learning and memory, visual learning and memory, working memory, speed of processing, reasoning and problem solving, attention, and social cognition).8 Results showed that participants who had higher BCIS composite index scores had better verbal learning and memory than individuals who scored lower on the composite index. We proposed that cognitive insight may rely selectively on verbal memory as it requires reflection and self-searching in memory. Further, the magnitude of verbal learning and memory deficits corresponded with the degree of self-certainty. We suggested that belief inflexibility may cause memories to be held with strong conviction, which may dissuade elaborate searches for previous experiences in memory. Notably, no associations emerged for self-reflectiveness or 2 clinical insight measures, namely, the Scale to assess Unawareness of Mental Disorder (SUMD) item 1 (awareness of mental disorder) and the insight item (G12) from the Positive and Negative Syndrome Scale (PANSS). We concluded that cognitive insight, but not clinical insight, may rely on memory processes whereby current experiences are appraised based on previous ones.
An important question that is yet to be investigated is whether the brain systems underlying cognitive insight in FEP overlap with those underlying verbal memory. Lesion studies have provided evidence for a critical role of the hippocampus (HC) for verbal memory function.9 Moreover, functional neuroimaging studies have demonstrated that the HC is a key neural correlate when normal subjects engage in both memory encoding and memory retrieval.10,11 In comparison, people with schizophrenia show a lack of normal memory-related activity in the HC12–14 as well as volumetric reductions in this region.15 Despite the absence of studies on the neural basis of cognitive insight, the pattern of associations between verbal memory and cognitive insight reported may lead to some tentative hypotheses. First, the finding that increased self-certainty was correlated with poorer verbal memory may imply inefficient HC function in FEP participants with higher certainty judgments. Second, self-reflectiveness and verbal memory were found to be uncorrelated, which may imply that the HC does not play an important role in self-reflectivity.
The aim of this study was to gain an anatomical understanding of the brain systems that underlie cognitive insight in individuals with an FEP. In view of evidence that the verbal memory cognitive system and the HC are functioning suboptimally in schizophrenia and that the HC is critical for verbal memory, an investigation into their role for cognitive insight seemed particularly worthwhile. Against a background of evidence that clinical insight is correlated with neurocognitive ability and with gray matter volumes,16 we also investigated whether correlations would appear with neurocognitive performance and HC volumes. Our prior study detected no substantial influence of verbal memory on clinical insight; thus, we expected no significant impact of verbal memory or HC volume on clinical insight in FEP.
Methods
Participants
All participants (N = 61) were part of a longitudinal naturalistic outcome study of FEP treated in a specialized early intervention service, the Prevention and Early Intervention Program for Psychoses (PEPP-Montreal), Douglas Hospital in Montreal, Canada. FEP participants were diagnosed based on a Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition),17 conducted by a trained interviewer and confirmed through a consensus meeting attended by at least 2 senior research psychiatrists (R.J. and A.M.). Duration of untreated psychosis (DUP) was calculated as part of the initial assessment as the time between onset of psychotic symptoms to the time of adequate treatment with antipsychotics.18 The research assistants and graduate students who perform symptom ratings using the PANSS have established an intraclass correlation coefficient (ICC) = .75. The SUMD is administered by the same research assistants and graduate students who administered the PANSS. Although interrater reliability data for the SUMD is not available, our raters receive extensive training and supervision with reliability measured at least once a year for the PANSS. Interrater reliability for the PANSS insight item (G12) was found to be high (ICC = .79). Interrater reliability for estimating DUP independently by 2 raters was conducted on 12 randomly selected cases and was found to be high (ICC = .81–.98).
The type and dosage of antipsychotic taken at each clinical assessment were recorded and converted to a standard chlorpromazine equivalent (see table 1). At the time of testing, patients were receiving mostly (n = 54) novel antipsychotic medications. One patient was receiving typical antipsychotic medication and 6 were unmedicated. Medication adherence was based on a 5-point scale ranging from 0 (never) to 4 (fully compliant).18 Patients were asked how often they missed a dose over the past month, and adherence was calculated as a percent of prescribed doses taken. Similar methodology was employed by clinical staff and adherence recorded as a percentage in clinical notes. Correlational analyses among scores based on information obtained from patients and clinical notes, and a more objective measure of pill counting, available for a subset of the sample were found to be high.19 In addition to pharmacotherapy, case managers provide individualized supportive psychotherapy and education to individuals with an aim to increase awareness (ie, insight) of the nature of their symptoms. Demographic and clinical descriptions of the sample are reported in table 1.
Table 1.
Demographic and Clinical Characteristics of the Sample
| First-Episode Psychosis (N = 61) |
|||
| Mean | SD | Range | |
| Age at hospitalization (y) | 23.4 | 3.7 | 17.5–30.8 |
| Education | 11.8 | 2.6 | 7–18 |
| PANSS positive total | 12.1 | 5.2 | 7–28 |
| PANSS negative total | 13.5 | 4.8 | 7–30 |
| DUP (d) | 53.2 | 94.2 | 2–591 |
| Calgary Depression Scale (total) | 2.0 | 2.9 | 0–11 |
| Hamilton Anxiety Scale (total) | 3.6 | 3.6 | 0–14 |
| BCIS | |||
| Self-reflectiveness | 13.3 | 4.0 | 3–22 |
| Self-certainty | 8.3 | 3.2 | 2–18 |
| Composite index | 5.0 | 5.7 | −11–19 |
| SUMD | |||
| Awareness of mental disorder | 2.4 | 1.4 | 1–5 |
| Awareness of response to medication | 2.1 | 1.4 | 1–5 |
| Awareness of need for treatment | 2.5 | 1.5 | 1–5 |
| Medication adherencea | 3.1 | 1.4 | 0–4 |
| Antipsychotic (dose [mg])b | 235.9 | 277.7 | 0–1250 |
| N | % | ||
| Gender (M:F) | 43:18 | 70:30 | |
| Diagnostic category | |||
| Schizophrenia | 37 | 60.7 | |
| Schizoaffective disorder | 9 | 14.8 | |
| Schizophreniform disorder | 1 | 1.6 | |
| Psychosis not otherwise specified | 7 | 11.5 | |
| Delusional disorder | 1 | 1.6 | |
| Bipolar disorder | 5 | 8.2 | |
| Undetermined | 1 | 1.6 | |
Note: PANSS, Positive and Negative Syndrome Scale; DUP, duration of untreated psychosis; BCIS, Beck Cognitive Insight Scale; SUMD, Scale to assess Unawareness of Mental Disorder.
Medication adherence: 0 (never taken) to 4 (always taken).
Antipsychotic medication reported as chlorpromazine equivalent dosage in milligrams. Six patients were unmedicated.
Magnetic resonance scans were acquired for 61 people with an FEP. All participants completed the BCIS, 54 completed the SUMD, and 59 completed neurocognitive assessments. In the current investigation, we report neurocognitive data for the same 50 participants as in our first study8 plus an additional 9 participants who were tested since the previous publication.
Cognitive insight was assessed with the BCIS,6 a 15-item self-report inventory. Self-reflectiveness (ability to reflect on previous experiences), self-certainty (overconfidence), and composite index (self-reflectiveness − self-certainty) scores were computed. Each question is rated on a 4-point scale from 0 (do not agree at all) to 3 (agree completely). Clinical insight was quantified with an abbreviated version of the SUMD.20 We limited our exploration of the SUMD to item 1, awareness of mental disorder, but for completeness, we report descriptive data for the first 3 items (see table 1). Items are rated on a 5-point scale from 1 (aware) to 5 (unaware). Psychotic symptom severity was assessed with the PANSS,21 depression with the Calgary Depression Scale,22 and anxiety with the Hamilton Anxiety Scale.23
Neurocognitive Assessment
Neurocognitive assessments were incomplete for some of the participants as they either refused or were unable to complete some of the tasks. FEP participants were assessed within an average of 11.3 weeks (SD = 15.2) after treatment initiation and only when in a stable though not necessarily asymptomatic condition.
Cognitive ability was examined by separating various neuropsychological tests into 7 cognitive domains as suggested by the National Institute of Mental Health-Measurement and Treatment Research to Improve Cognition in Schizophrenia group.24,25 Verbal learning and memory was derived from the logical memory26 subtests of the Wechsler Memory Scale—Third Edition.27 Other domains were visual learning and memory, working memory, speed of processing, reasoning/problem solving, attention, and social cognition. The neuropsychological tests that comprise these domains are listed in table 2 and described in detail elsewhere.28
Table 2.
Neurocognitive Performance of the Sample
| Mean | SD | Range | |
| Verbal learning and memory | |||
| Logical memory I immediate recall | 34.8 | 10.9 | 10–60 |
| Logical memory II delay recall | 20.5 | 8.2 | 4–39 |
| Logical memory II recognition | 25.0 | 2.9 | 17–30 |
| Visual learning and memory | |||
| Visual reproduction I immediate recall | 89.2 | 13.3 | 37–104 |
| Visual reproduction II delay recall | 72.2 | 20.5 | 14–104 |
| Visual reproduction II recognition | 44.9 | 2.8 | 36–48 |
| Working memory | |||
| Digit span forward | 9.5 | 2.2 | 4–14 |
| Digit span backward | 6.2 | 2.4 | 2–12 |
| Spatial span forward | 8.5 | 2.2 | 4–14 |
| Spatial span backward | 7.6 | 2.1 | 2–13 |
| Speed of processing | |||
| Trail A | 35.6 | 10.4 | 19–61 |
| Digit symbol | 62.7 | 15.0 | 38–107 |
| Reasoning and problem solving | |||
| Trail B | 77.4 | 32.2 | 34–228 |
| Block design | 44.2 | 11.6 | 21–64 |
| Tower of London: number of movementsa | 8.8 | 2.9 | 5–17 |
| Attention | |||
| D2 Test of Attention | 147.6 | 43.8 | 61–279 |
| Social cognition | |||
| 4-Factor tests of social intelligence | 31.1 | 7.6 | 12–46 |
| Hinting task | 15.5 | 2.9 | 8–20 |
The mean and SD exclude the results of the 3-movement subtest.
Magnetic Resonance Imaging Acquisition
Structural magnetic resonance data were acquired at the Montreal Neurological Institute (MNI) on a 1.5-T Siemens Sonata whole-body system magnetic resonance imaging (MRI) scanner outfitted with a standard head coil (Siemens, Erlangen, Germany). Participants’ heads were stabilized by a vacuum cushion. High-resolution T1-weighted anatomical MR images were acquired from each participant using the standard MNI structural acquisition protocol. Contiguous sagittal slices perpendicular to the anterior-posterior commissure line, with 1-mm slice thickness, were acquired using a gradient echo pulse sequence (repetition time = 22 ms, echo time = 9.2 ms, flip angle = 30°, voxel size = 1 × 1 × 1 mm3). MR images were transferred to an external workstation for image processing.
Manual Segmentation of the HC
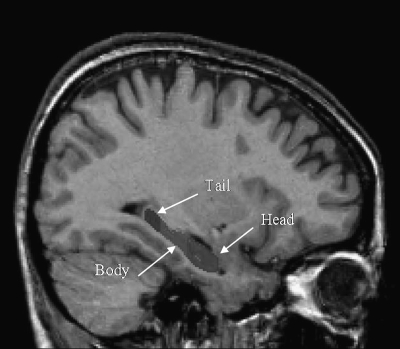
Prior to manual HC segmentation, the following preprocessing steps were performed: nonuniformity correction, registration into standard stereotaxic space, and signal intensity normalization. Volumetric analyses were performed with the interactive software package DISPLAY developed at the Brain Imaging Center of the MNI. HC volumes were calculated automatically by the software. Manual tracing of the HC was performed using a validated protocol,29 and manual segmentation into HC body, head, and tail regions was performed according to an updated protocol by the same author (J. C. Pruessner, PhD, unpublished data, 2006) (figure 1). Measurements were performed by a trained rater (Y.C.) who was blind to clinical information. To assess intrarater reliability of the obtained volumes, volumetric measurements of the HC were performed twice for 10 participants and was found to be high (left HC, ICC = .98; right HC, ICC = .99). Interrater reliability for estimating HC volumes independently by one rater (C.C.) was conducted on 5 randomly selected cases and was found to be high (left HC, ICC = .98; right HC, ICC = .98).
Fig. 1.
Sagittal Section of the Brain and Delineation of the Hippocampus Into Body, Head, and Tail Subregions.
Procedure
Clinical interviews and the neuropsychological assessment were administered by a trained professional who was not involved with the patient's treatment. The BCIS and SUMD/PANSS were performed within 3 weeks of each other (mean = 20.6 d, SD = 51.4). The MRI scan was performed within 2 weeks of the insight assessments (BCIS: mean = 11.3 d, SD = 25.2; SUMD/PANSS; mean = 7.3 d, SD = 61.4). The neurocognitive assessment was administered within 57.0 days (SD = 120.6) of the SUMD/PANSS interviews and within 78.4 days (SD = 125.1) of the BCIS interviews. The difference between these latter assessment dates was significant, t52 = 2.60, P = .01. Owing to the stability of neurocognitive functions in FEP,30–32 it is unlikely that this difference was of direct significance for the primary purpose of this study. Moreover, virtually all individuals were tested at least 3 months after admission to PEPP and were clinically relatively stable at the time of insight evaluation.
After a comprehensive description of the study, written informed consent was obtained from all participants. Research protocols were approved by the Douglas Hospital Human Ethics Review Board.
Statistical Analyses
Pearson correlations were used to evaluate associations between HC volumes and cognitive and clinical insight scores. The critical P value was set at .05 for analyses of total left and right HC volumes. When considering HC head, body, and tail volumes, the critical P value was set at .02 following Bonferroni correction. Calculations of the 7 cognitive domains are described elsewhere.8 All statistical tests were 2 tailed and performed using SPSS version 16.0.33
Results
Relations Between Cognitive and Clinical Insight and Verbal Memory
Table 2 presents the mean performance of the tests or subtests used in each of the different neurocognitive domains. The global measure of verbal learning and memory was significantly correlated with the BCIS composite index, r57 = .33, P = .01, and with the BCIS self-reflectiveness subscale, r58 = .31, P = .02. No significant correlations emerged for self-certainty or awareness of mental disorder scores, r57 = −.22, P = .10, or r52 = −.11, P = .45, respectively. The only other modestly significant correlation between any of the insight variables and the cognitive domains was between the global measure of social cognition and the BCIS composite index, r50 = .28, P = .05.
Relations Between Cognitive and Clinical Insight and Hippocampal Volumes
Mean and SDs and minimum and maximum HC volumes are shown in table 3. HC volumes for our FEP participants appear to be bigger than those reported in studies of HC volumes in FEP.15 However, these studies have employed a variety of different methods for calculating HC volumes than the current manual tracing technique. Differences in anatomical boundaries across protocols need to be taken in to account, and this hinders the comparison of results. Notably, the current protocol has been shown to allow a more precise definition of the HC.29
Table 3.
Mean and SD and Minimum and Maximum Volumes for the Right and Left Hippocampus in 61 Individuals With First-Episode Psychosis
| Mean | SD | Minimum | Maximum | |
| Right hippocampus | ||||
| Total | 4208.6 | 417.3 | 3303 | 5370 |
| Head | 2207.6 | 338.6 | 1396 | 2881 |
| Body | 1257.1 | 238.7 | 623 | 1998 |
| Tail | 743.8 | 163.2 | 392 | 1245 |
| Left hippocampus | ||||
| Total | 4106.5 | 368.3 | 3296 | 5017 |
| Head | 2029.0 | 346.6 | 1292 | 2841 |
| Body | 1337.0 | 220.8 | 797 | 1929 |
| Tail | 740.4 | 197.9 | 295 | 1194 |
Note: All values are in cubic millimeters.
Self-reflectiveness
The analyses revealed no significant correlations between self-reflectivity scores and HC volume in either hemisphere (left HC: r59 = .11, P = .40; right HC: r59 = .07, P = .61). An examination of the subdivisions of the HC revealed no significant effects (left HC—head: r59 = .18, P = .16; body: r59 = −.11, P = .42; tail: r59 = .01, P = .97; and right HC—head: r59 = .12, P = .37; body: r59 = −.18, P = .17; tail: r59 = .18, P = .16).
Self-certainty
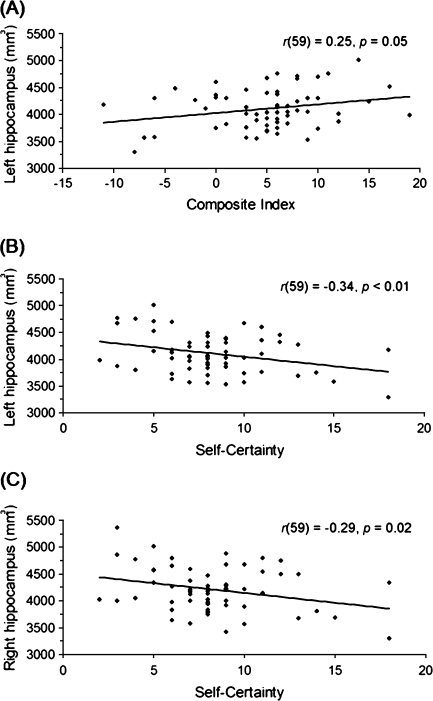
Negative correlations revealed that FEP participants with smaller HC volumes had higher self-certainty scores (left HC: r59 = −.34, P < .01; right HC: r59 = −.29, P = .02) (see figure 2). When the subdivisions of the HC were examined, no regional specificity of this effect was observed (left HC—head: r59 = −.16, P = .22; body: r59 = −.14, P = .30; tail: r59 = −.19, P = .14; and right HC—head: r59 = −.17, P = .19; body: r59 = −.12, P = .38; tail: r59 = −.24, P = .07).
Fig. 2.
Significant Correlations Between Beck Cognitive Insight Scale Scores and Hippocampal Volume in First-Episode Psychosis Patients. (A) Correlations of composite index scores and hippocampal volume in the left hemisphere; N = 61. (B and C) Correlations of self-certainty scores and hippocampal volume in the left and right hemispheres; N = 61.
Composite Index
The analysis revealed a significant correlation such that FEP participants with higher composite index scores showed greater HC volume in the left hemisphere, r59 = .25, P = .05 (see figure 2). No significant effect emerged for the right hemisphere, r59 = .21, P = .11. Examination of the subdivisions of the HC revealed a positive correlation between composite index scores and right HC tail volume, r59 = .29, P = .03; however, this became nonsignificant following a Bonferroni correction. No other significant effects emerged (left HC—head: r59 = .21, P = .11; body: r59 = −.02, P = .91; tail: r59 = .12, P = .35; and right HC—head: r59 = .18, P = .17; body: r59 = −.09, P = .51).
Awareness of Mental Disorder
The analyses revealed no significant correlations between awareness of mental disorder scores and HC volume in either hemisphere (left HC: r52 = −.08, P = .58; right HC: r52 = −.11, P = .43) or with subdivisions of the HC (left HC—head: r52 = .00, P = .98; body: r52 = −.15, P = .30; tail: r52 = .02, P = .92; and right HC—head: r52 = −.05, P = .71; body: r52 = −.19, P = .14; tail: r52 = .09, P = .50).
Complementary Analyses
Against the background of severe problems with verbal memory34,35 and reduced HC volumes in individuals with schizophrenia,15 and in view of the association between verbal memory and cognitive insight in FEP8, we directly tested the specificity of the HC to cognitive insight when controlling statistically for verbal memory performance.
First, we followed up on the significant correlation between the BCIS composite index and left HC volume. Partial correlations were computed between composite index scores and left HC volume, with verbal memory performance as a covariate. The result became a nonsignificant trend, r56 = .25, P = .06. Second, we followed up on the significant correlations between BCIS self-certainty and HC volumes. Partial correlations performed between self-certainty scores and both left and right HC volumes, with verbal memory as a covariate, did not change interpretation of results (left HC: r55 = −.32, P = .01; right HC: r55 = −.27, P = .04).
The impact of positive symptomatology as a possible mediating variable was also explored. Entering PANSS positive scale scores as a second covariate in all abovementioned analyses did not affect interpretation of results, with the exception of the following: left HC volume reemerged as a significant correlate of composite index scores, r56 = .28, P = .04.
When correlating the verbal memory scores with HC volumes, no significant correlations were observed in either hemisphere (left HC: r57 = .02, P = .86; right HC: r57 = .09, P = .50) nor with the subdivisions of the HC (left HC—head: r57 = .00, P = .99; body: r57 = −.01, P = .94; tail: r57 = .06, P = .67; and right HC—head: r57 = .15, P = .27; body: r57 = −.15, P = .26; tail: r57 = .15, P = .27). PANSS positive scale score did not correlate with verbal memory, r56 = −.15, P = .27, or any index of HC volume, r59 values = −.02 to .23, P values > .49.
Discussion
To our knowledge, this is the first demonstration that in individuals with an FEP cognitive insight is associated with HC volume. Most importantly, our volumetric analysis revealed that greater self-certainty correlated with diminished bilateral HC volumes, and this relationship was independent of verbal memory performance. Self-reflectiveness correlated with verbal memory but not with HC volume. Further, higher BCIS composite index scores were associated with greater volume of the left HC. A modest proportion of this relationship was attributable to verbal memory performance. When considering clinical insight, no correlations were observed between awareness of mental disorder scores and verbal memory or HC volumes. The results strengthen the claim that the neurocognitive system involved in verbal memory is important for cognitive insight, but perhaps not for clinical insight, in people with an FEP. The HC may be a viable location among others for the brain system that underlies aspects of cognitive insight in people with an FEP.
The current analysis revealed that higher self-reflectiveness was associated with better verbal memory performance. This result was not seen in our previous work (r = .22 for self-reflectiveness and verbal memory in our initial published study),8 and presumably, the larger sample size yielded increased statistical power to detect this effect. A possible explanation of this finding is that in people with an FEP poor verbal memory ability precludes accurate reflection on the mental episodes of previous experiences,8 possibly through impaired discrimination of valid from invalid information in memory.36 Consequentially, aberrant memory information is used to guide current beliefs and judgments. A related possibility is that self-reflectiveness is achieved through recognition that memories are internally generated.37 It has been recognized that for 4 of 9 items on the self-reflectiveness subscale reflection on past life experiences is required, eg, “Some of my experiences that have seemed very real may have been due to my imagination, and I have jumped to conclusions too fast.”38 At a conceptual level, it may be that in people with an FEP distorted memory for specific experiences may influence everyday judgments. This may occur by compromising the capacity to consider alternative explanations or acknowledge others’ superior objectivity, or by promoting belief inflexibility (the remaining 5 self-reflectiveness items address these biases). Replication is needed to strengthen this hypothesis.
The neural substrates underlying self-reflectiveness do not appear to include the HC in FEP. As mentioned above, we propose that self-reflectiveness relies on discrimination of accurate from inaccurate information in memory and that information in memory, irrespective of its validity, is used to guide current beliefs and judgments. These cognitive functions clearly draw on episodic memory, for which the HC is thought to play a major role.9,39 This interpretation would suggest an association between HC volume and self-reflectiveness; however, this finding did not emerge in the current FEP sample. One explanation of the negative results may be the nonsignificant correlation between verbal memory and HC volumes, suggesting our verbal memory task was not sensitive to hippocampal integrity. Alternatively, other neural regions beyond the HC may mediate the association between verbal memory performance and self-reflection. Regions in the prefrontal cortex (PFC) are thought to play a role in episodic memory processes; for example, the ventrolateral PFC supporting memory of items40 and the dorsolateral PFC facilitating relational processing between items.41 This may place the involvement of PFC as a candidate mechanism for self-reflectiveness. Another factor is that, relative to healthy participants, patients with schizophrenia often show decreased hippocampal recruitment during episodic memory performance.14,42 The absence of an expected triangulated relationship between self-reflectiveness, HC volume, and verbal memory may have been due in part to this factor. The precise role of the HC for self-reflectiveness in normal cognition (ie, in healthy participants) compared with participants with an FEP merits investigation.
Although in our initial study poorer verbal memory was associated with self-certainty,8 in the current work this relationship was nonsignificant. The significant result in our previous study may be attributable to the sensitivity of the verbal memory effect to a smaller sample size. Moreover, all 6 items on the self-certainty subscale appraise current judgments,38 supporting conceptually the negative result in the current study. One plausible explanation is related to the fact that 3 of 6 self-certainty items require comparison of one's own beliefs and judgments to those of another person (“I know better than anyone else what my problems are; When people disagree with me, they are generally wrong; and I cannot trust other people's opinion about my experiences”). In this regard, it may be that the cognitive system involving source memory,43,44 and in particular the ability to distinguish self-generated from externally perceived information (reality monitoring) or imagined vs actual events (internal source monitoring) in memory, may be important for understanding self-certainty. This hypothesis is an extension of Moritz et al's45 observation that people with schizophrenia hold false source attributions in high conviction, relative to healthy individuals. It can also be noticed that the self-certainty subscale taps overconfidence in the context of everyday social interactions. This leads to a proposal that source misattributions for an incident involving a personal problem or a disagreement (the scale includes items that directly address both of these) may form the basis for increased confidence in one's current judgments. To illustrate, consider a disagreement in which a person attributes self-generated information (eg, I think that she is at fault) as external (She admitted that she was at fault),43,46 thus demonstrating fallible source memory. In subsequent disagreements, this person will likely show higher confidence that others are wrong than a person who has reliable source memory. Exploration of the relationship of source monitoring to self-certainty may be of interest for future studies.
Bilateral HC volume reductions were associated with increased self-certainty—independent of verbal memory performance—in our FEP sample. The observation that cognitive insight is associated with HC volumes in individuals experiencing an FEP suggests that this association is not an artifact of illness chronicity or lengthy psychotropic medication use. Reduced volume of the HC could reflect disturbances in neuronal cell count, distribution or size, or failed adult hippocampal neurogenesis.47 One interpretation is that larger HC volumes may be a protective factor for appropriate confidence levels. A related suggestion is that the HC may form part of a neural circuit whose dysfunction manifests as overconfidence in people with psychosis. Our postulate that self-certainty engages source memory processes is congruent with previous findings that the HC, along with distributed neural networks, contributes to (1) successful avoidance of source misattributions,48 (2) source memory for spatial contextual information,48 (3) source recollection for an item's contextual details,49,50 (4) later ability to distinguish the task performed on an item during encoding,51 and (5) hearing another person's voice when expecting to hear one's own voice.52 We propose that the decreased HC volumes seen in this study in relation to overconfidence is related to inadequate monitoring of self- vs outer-generated information (reality monitoring) and/or imagined vs actual events (internal source monitoring) in memory. That is, engaging false memory information to direct current thinking may bias individuals to hold their own beliefs and judgments in high confidence via the HC. This hypothesis deserves further investigation.
It should be noted that overconfidence may relate to other hippocampal mediated cognitive functions such as working and/or spatial memory. This hypothesis, however, was not supported, given the absence of a correlation between self-certainty and our working memory domain (which incorporated a spatial memory task). Moreover, it is possible that overconfidence relates to symptoms of psychosis via the HC. In line with the suggestion of Warman et al38 that self-certainty applies preferentially to delusions, structural abnormalities in the HC53 and pathology in its connectivity,54,55 as well as global medial temporal lobe dysfunction,56 have been associated with delusions.
In agreement with our forerunner investigation,8 the BCIS composite index correlated with verbal memory, suggesting that, as we previously hypothesized, in individuals with an FEP enhanced cognitive insight may depend on an ability to retrieve past memories. At a conceptual level, it is possible that people scoring at antipodal extremes on the composite index may display different cognitive profiles. For example, the presence of low composite index scores, reflecting low self-reflectiveness (poor memory accuracy) and concurrent high self-certainty (inappropriate confidence), may relate to knowledge corruption typically observed in people with schizophrenia,36,57 whereby false judgments are held with strong conviction.
Composite index scores and left HC volume were positively correlated in our FEP sample. This association remained significant when variance due to verbal memory and positive symptom severity was subtracted out. When proposing a neural system for global cognitive insight as indexed by the composite index, it is important to emphasize that composite index scores reflect the synthesis of self-reflectiveness and self-certainty scores. An apposite explanation would be that the brain system that underlies global cognitive insight reflects those that underpin its individual components (self-reflectiveness and self-certainty). The present neurological view of cognitive insight traces hyperconfidence at least in part to HC pathology and posits an empirically testable role for the PFC in self-reflectiveness. It should be highlighted that the composite index and self-certainty, but not self-reflectiveness, correlated with HC volumes, raising the possibility that overconfidence is driving the left HC result for the global cognitive insight measure. A related point is that self-certainty may capture more of a deficit-like “trait” process that is detectable as a structural brain change. On the other hand, the neural system of self-reflectiveness may be better captured in a more fluid process such as positron emission tomography or other physiologically based imaging procedures.58 The functional basis of cognitive insight remains to be explored.
The lack of significant correlation between awareness of mental disorder scores and HC volume is in agreement with the only other study that has examined this relationship in schizophrenia.59 The negative finding is strengthened by the nonsignificant correlation between awareness of mental disorder scores and verbal memory, a hippocampal mediated cognitive measure.9 This set of results may support the view that clinical insight impairments relate to dysfunction of PFC regions.59–63 In line with this argument, there is consistent evidence linking clinical insight to prefrontally mediated cognitive tasks such as the Wisconsin Card Sorting Task.16,35 Other recent data suggest a role for the parietal and temporal cortices in clinical insight in schizophrenia.64 Alternatively, the patient's unwillingness to admit their mental disorder may be contingent on psychological characteristics. Evidence for this suggestion comes from the observations that poor insight in schizophrenia is associated with a tendency to give self-reports that are honest but positively biased,65 present oneself in a socially desirable manner,66 and attribute negative events to an external source.67
One issue when interpreting the behavioral results of this study is that we did not apply a correction for multiple comparisons to our statistical threshold. However, we did calculate global measures for each of the 7 neurocognitive domains that afforded a better construct validity. Secondly, we did not include a healthy comparison group. Future research needs to explore the role of verbal memory and HC volumes for cognitive insight in normal cognition to understand how this system becomes dysfunctional in psychosis. Third, it should be noted that the neurocognitive evaluation and the BCIS interviews were conducted on different test dates (average = 78.4 d). Further study is necessary to evaluate other areas of the neural network that supports cognitive insight, such as prefrontal, temporal, and parietal cortical regions, to shed light on some of the hypotheses made above and to strengthen the current model.
The results strengthen our account holding that the cognitive processes underlying verbal memory are compatible with those involved in cognitive insight. Our data suggest that hippocampal pathology may adversely affect certainty judgments. These data place cognitive insight among other metacognitive problems observed in individuals with psychosis, including knowledge corruption.45 It remains to be explored whether poorer cognitive insight occurs in conjunction with this metacognitive bias in FEP individuals. Examination of the cognitive insight–metacognition link at both the behavioral and neural levels will be important for cognitive insight modeling.
Funding
Canadian Institute of Health Research (CIHR) (operating grant 68961; studentship to L.B.); Sackler Foundation (to M.L., A.M.); Fonds de la Recherche en Santé du Québec (FRSQ) (salary award to M.L.; studentship to L.B.); Canada Research Chairs Program (to A.M.).
Acknowledgments
Declaration of interest: None. We thank Michael Bodnar and Clifford Cassidy for their comments on the Methods. We thank PEPP-Montreal research staff for their help with recruitment and for conducting the clinical assessments and Douglas Brain Imaging Group staff for conducting the neuropsychological assessments and MRI scans. Finally, we are grateful to all the people who participated in the study.
References
- 1.Carpenter WT, Jr, Strauss JS, Bartko JJ. Flexible system for the diagnosis of schizophrenia: report from the WHO International Pilot Study of Schizophrenia. Science. 1973;182:1275–1278. doi: 10.1126/science.182.4118.1275. [DOI] [PubMed] [Google Scholar]
- 2.Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM. Assessment of insight in psychosis. Am J Psychiatry. 1993;150:873–879. doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- 3.David AS. Insight and psychosis. Br J Psychiatry. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- 4.Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61:75–88. doi: 10.1016/s0920-9964(02)00316-x. [DOI] [PubMed] [Google Scholar]
- 5.Amador XF, David AS, editors. Insight and Psychosis. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 6.Beck AT, Baruch E, Balter JM, Steer RA, Warman DM. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68:319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 7.Turkington D, Kingdon D, Turner T. Effectiveness of a brief cognitive-behavioural therapy intervention in the treatment of schizophrenia. Br J Psychiatry. 2002;180:523–527. doi: 10.1192/bjp.180.6.523. [DOI] [PubMed] [Google Scholar]
- 8.Lepage M, Buchy L, Bodnar M, Bertrand MC, Joober R, Malla A. Cognitive insight and verbal memory in first episode of psychosis. Eur Psychiatry. 2008;23:368–374. doi: 10.1016/j.eurpsy.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 12.Weiss AP, Heckers S. Neuroimaging of declarative memory in schizophrenia. Scand J Psychol. Jul 2001;42:239–250. doi: 10.1111/1467-9450.00234. [DOI] [PubMed] [Google Scholar]
- 13.Boyer P, Phillips JL, Rousseau FL, Ilivitsky S. Hippocampal abnormalities and memory deficits: new evidence of a strong pathophysiological link in schizophrenia. Brain Res Rev. 2007;54:92–112. doi: 10.1016/j.brainresrev.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Achim AM, Lepage M. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 15.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 16.Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86:54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Patient Edition (SCID-I/P V and SCID-I/NP Version 2.0) New York, NY: Biometric Research Department; 1998. [Google Scholar]
- 18.Malla A, Norman R, Schmitz N, et al. Predictors of rate and time to remission in first-episode psychosis: a two-year outcome study. Psychol Med. 2006;36:649–658. doi: 10.1017/S0033291706007379. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy CM, Rabinovitch M, Joober R, Malla A. Presented at: Winter Workshop on Schizophrenia and Bipolar Disorders. Is any one method of measuring medication adherence in first-episode psychosis better than others? February 5, 2007; Montreux, Switzerland. [Google Scholar]
- 20.Amador XF, Flaum M, Andreasen NC, et al. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry. 1994;51:826–836. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 23.Riskind J, Beck A, Brown G, Steer R. Taking the measure of anxiety and depression: validity of the reconstructed Hamilton Scales. J Nerv Ment Dis. 1987;175:474–479. doi: 10.1097/00005053-198708000-00005. [DOI] [PubMed] [Google Scholar]
- 24.MATRICS. Results of the MATRICS RAND Panel Meeting: Average Medians for the Categories of Each Candidate Test. MATRICS 2003;Measurement and Treatment Research to Improve Cognition in Schizophrenia. http://www.matrics.ucla.edu/meetings/september-2003/RAND-Panel-Medians.pdf. Accessed October 6, 2008. [Google Scholar]
- 25.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Brinkenkamp R, Zillmer E. The d2 Test of Attention. Seattle, Wash: Hogrefe & Huber Publishers; 1998. [Google Scholar]
- 27.Wechsler D. Weschler Memory Scale—3rd Edition. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- 28.Bodnar M, Malla A, Joober R, Lepage M. Cognitive markers of short-term clinical outcome in first-episode psychosis. Br J Psychiatry. 2008;193:297–304. doi: 10.1192/bjp.bp.107.040410. [DOI] [PubMed] [Google Scholar]
- 29.Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 30.Albus M, Hubmann W, Scherer J, et al. A prospective 2-year follow-up study of neurocognitive functioning in patients with first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2002;252:262–267. doi: 10.1007/s00406-002-0391-4. [DOI] [PubMed] [Google Scholar]
- 31.Gold S, Arndt S, Nopoulos P, O'Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- 32.Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am J Psychiatry. 1999;156:1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- 33.SPSS for Windows [computer program]. Version Release 16.0.1. Chicago: SPSS; 2007. [Google Scholar]
- 34.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 35.Aleman A, Agrawal N, Morgan KD, David AS. Insight in psychosis and neuropsychological function: meta-analysis. Br J Psychiatry. 2006;189:204–212. doi: 10.1192/bjp.189.3.204. [DOI] [PubMed] [Google Scholar]
- 36.Moritz S, Woodward TS. Memory confidence and false memories in schizophrenia. J Nerv Ment Dis. 2002;190:641–643. doi: 10.1097/00005053-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs AA, David AS. Delusion formation and insight in the context of affective disturbance. Epidemiol Psichiatr Soc. 2003;12:167–174. doi: 10.1017/s1121189x00002943. [DOI] [PubMed] [Google Scholar]
- 38.Warman DM, Lysaker PH, Martin JM. Cognitive insight and psychotic disorder: the impact of active delusions. Schizophr Res. 2007;90:325–333. doi: 10.1016/j.schres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 40.Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- 42.Achim AM, Bertrand MC, Sutton H, et al. Selective abnormal modulation of hippocampal activity during memory formation in first-episode psychosis. Arch Gen Psychiatry. 2007;64:999–1014. doi: 10.1001/archpsyc.64.9.999. [DOI] [PubMed] [Google Scholar]
- 43.Bentall RP, Baker GA, Havers S. Reality monitoring and psychotic hallucinations. Br J Clin Psychol. 1991;30(pt 3):213–222. doi: 10.1111/j.2044-8260.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 44.Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- 45.Moritz S, Woodward TS, Ruff CC. Source monitoring and memory confidence in schizophrenia. Psychol Med. 2003;33:131–139. doi: 10.1017/s0033291702006852. [DOI] [PubMed] [Google Scholar]
- 46.Woodward TS, Menon M, Hu X, Keefe RS. Optimization of a multinomial model for investigating hallucinations and delusions with source monitoring. Schizophr Res. 2006;85:106–112. doi: 10.1016/j.schres.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- 48.Ross RS, Slotnick SD. The hippocampus is preferentially associated with memory for spatial context. J Cogn Neurosci. 2008;20:432–446. doi: 10.1162/jocn.2008.20035. [DOI] [PubMed] [Google Scholar]
- 49.Dougal S, Phelps EA, Davachi L. The role of medial temporal lobe in item recognition and source recollection of emotional stimuli. Cogn Affect Behav Neurosci. 2007;7:233–242. doi: 10.3758/cabn.7.3.233. [DOI] [PubMed] [Google Scholar]
- 50.Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. J Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu CH, Vythelingum GN, Brammer MJ, et al. An fMRI study of verbal self-monitoring: neural correlates of auditory verbal feedback. Cereb Cortex. 2006;16:969–977. doi: 10.1093/cercor/bhj039. [DOI] [PubMed] [Google Scholar]
- 53.Degreef G, Ashtari M, Bogerts B, et al. Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry. 1992;49:531–537. doi: 10.1001/archpsyc.1992.01820070025004. [DOI] [PubMed] [Google Scholar]
- 54.Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 55.Prasad KM, Rohm BR, Keshavan MS. Parahippocampal gyrus in first episode psychotic disorders: a structural magnetic resonance imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:651–658. doi: 10.1016/j.pnpbp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 56.Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- 57.Moritz S, Woodward TS. The contribution of metamemory deficits to schizophrenia. J Abnorm Psychol. 2006;115:15–25. doi: 10.1037/0021-843X.15.1.15. [DOI] [PubMed] [Google Scholar]
- 58.Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 59.Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and prefrontal cortex in first-episode schizophrenia. NeuroImage. 2004;22:1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 60.Lee KH, Brown WH, Egleston PN, et al. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–1933. doi: 10.1176/ajp.2006.163.11.1926. [DOI] [PubMed] [Google Scholar]
- 61.Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci. 2001;13:255–257. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- 62.Shad MU, Muddasani S, Keshavan MS. Prefrontal subregions and dimensions of insight in first-episode schizophrenia–a pilot study. Psychiatry Res. 2006;146:35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Sapara A, Cooke M, Fannon D, et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res. 2007;89:22–34. doi: 10.1016/j.schres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 64.Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res. 2008;103:40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore O, Cassidy E, Carr A, O'Callaghan E. Unawareness of illness and its relationship with depression and self-deception in schizophrenia. Eur Psychiatry. 1999;14:264–269. doi: 10.1016/s0924-9338(99)00172-8. [DOI] [PubMed] [Google Scholar]
- 66.Subotnik KL, Nuechterlein KH, Irzhevsky V, Kitchen CM, Woo SM, Mintz J. Is unawareness of psychotic disorder a neurocognitive or psychological defensiveness problem? Schizophr Res. 2005;75:147–157. doi: 10.1016/j.schres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Langdon R, Corner T, McLaren J, Ward PB, Coltheart M. Externalizing and personalizing biases in persecutory delusions: the relationship with poor insight and theory-of-mind. Behav Res Ther. 2006;44:699–713. doi: 10.1016/j.brat.2005.03.012. [DOI] [PubMed] [Google Scholar]