Abstract
A significant proportion of patients with schizophrenia demonstrate abnormalities in dorsal prefrontal regions including the dorsolateral prefrontal and dorsal anterior cingulate cortices. However, it is less clear to what extent abnormalities are exhibited in ventral prefrontal and limbic regions, despite their involvement in social cognitive dysfunction and aggression, which represent problem domains for patients with schizophrenia. Previously, we found that reduced white matter integrity in right inferior frontal regions was associated with higher levels of aggression. Here, we used resting-state functional magnetic resonance imaging to examine amygdala/ventral prefrontal cortex (vPFC) functional connectivity (FC) and its relation to aggression in schizophrenia. Twenty-one healthy controls and 25 patients with schizophrenia or schizoaffective disorder participated. Aggression was measured using the Buss Perry Aggression Questionnaire. Regions of interest were placed in the amygdala based on previously published work. A voxelwise FC analysis was performed in which the mean time series across voxels for this bilateral amygdala seed was entered as a predictor in a multiple regression model with motion parameters and global, cerebrospinal fluid, and white matter signals as covariates. Patients showed significant reductions in FC between amygdala and vPFC regions. Moreover, in patients, the strength of this connection showed a significant inverse relationship with aggression, such that lower FC was associated with higher levels of self-rated aggression. Similar results were obtained for 2 other measures—Life History of Aggression and total arrests. These results suggest that amygdala/vPFC FC is compromised in schizophrenia and that this compromise is associated with aggression.
Keywords: amygdala, functional connectivity, aggression, schizophrenia
Introduction
Schizophrenia has been characterized as a disorder of brain connectivity.1 This hypothesis has been tested using diffusion tensor imaging (DTI), which has shown that white matter integrity is disrupted in a number of different regions in individuals with schizophrenia,2–4 although the pattern of results differs across studies.5 Abnormalities in white matter integrity in schizophrenia have been associated with reduced visual evoked potential amplitudes,6 hallucinations,7,8 negative symptoms,9 aggression and impulsivity,10,11 and poor cognitive performance.8,12 Moreover, they are present in first-episode8,13 schizophrenia and childhood-onset14 schizophrenia populations. Taken together, these studies provide a strong structural basis for the idea that deficits in connectivity are implicated in the pathophysiology of schizophrenia.
The confirmation of deficiencies in white matter integrity in schizophrenia suggests that dynamic functional interactions between brain regions would also be disrupted in the disorder. These relationships can be examined using resting-state functional connectivity (FC), which refers to the temporal correlation of brain activity across disparate regions. In 1995, Biswal et al15 discovered that spontaneous low-frequency oscillations in the resting-state blood oxygen level–dependent signal were synchronized across motor cortices. Since that time, a number of different cortical and subcortical functionally connected networks have been identified under resting-state conditions, as well as during task performance, during sleep, and under light sedation.16–20 These “intrinsic connectivity networks” (ICNs21) correspond to functionally relevant systems in the brain.15,16,19,22
Several studies of FC have been conducted in schizophrenia. Widespread functional disconnectivity and decreased regional homogeneity (a measure of the strength of correlations between voxels that are members of the same ICN) have been found under resting conditions in schizophrenia.23,24 The FC of the default-mode network (DMN) and its anticorrelated “task-positive” network also seem to be abnormal in schizophrenia, and these abnormalities have been correlated with more severe symptomatology.25,26 Moreover, reduced FC is seen between the dorsolateral prefrontal cortex (DLPFC) and the parietal lobe, posterior cingulate, thalamus, and striatum of first-episode schizophrenia patients.27 In addition, reduced frontotemporal FC during an n-back working memory task was found in medication-naive patients with schizophrenia.28 Finally, patients with schizophrenia have reduced task-based FC between DLPFC and hippocampal cortices.29
Most of these initial studies in schizophrenia have focused on either the DMN broadly or the DLPFC. Little attention has been paid to the role of amygdalar/ventral prefrontal cortex (vPFC) circuitry specifically,30,31 despite its role in social processing and cognition,32 which are particularly associated with functional impairments in schizophrenia. Involvement of these circuits is supported by reduced asymmetry of white matter integrity in the uncinate fasciculus,33 which connects orbitofrontal cortex (OFC) and medial temporal regions, in patients with schizophrenia.
Amygdala/vPFC circuitry is thought to play an important role in the suppression of impulsive/aggressive behaviors.31,34 For example, damage to the vPFC is associated with increased aggression and hostile attitudes.35 Patients with schizophrenia show increased levels of aggression, which is particularly augmented by comorbid substance abuse, although the association is present even in nonusers of illicit substances.36 Aggression in schizophrenia has been associated with abnormal total OFC volumes37,38 and reduced white matter integrity in right inferior frontal regions.10 Similar results for orbitofrontal volumes have been observed in suicidality.39
Accordingly, we sought to examine resting-state FC in amygdala/vPFC circuitry in patients with schizophrenia or schizoaffective disorder compared with healthy volunteers. In order to do so, we selected the amygdala regions of interest (ROIs) defined by Stein et al40 in their analysis of connectivity in a frontolimbic network comprising the cingulate, insula, DLPFC, and parahippocampal gyrus, as well as the amygdala and OFC, which was based on known anatomical connectivity patterns in macaques. Based on the findings of elevated aggression in schizophrenia and our prior work demonstrating that aggression was associated with reduced integrity of ventral prefrontal white matter in patients with schizophrenia,10 we also examined the relationship between amygdala/vPFC FC and aggressive attitudes in these same patients. We predicted that (1) patients would show reduced FC between amygdala and vPFC; (2) patients would show increased levels of aggressive attitudes; and (3) among patients, measures of aggressive attitudes would be associated with FC deficits. To examine the specificity of the findings to regions functionally connected with the amygdala, we also examined correlations between the Buss Perry Aggression Questionnaire (BPAQ)41 and FC to other seeds in the Stein et al40 network (ie, bilateral OFC, subgenual and supragenual anterior cingulate, and bilateral DLPFC). Because we had no a priori hypothesis regarding the laterality of effects, we used bilateral seeds in amygdala, OFC, and DLPFC.
Methods
Participants
Participants were 21 healthy controls and 25 patients who met Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR) criteria for schizophrenia (n = 21) or schizoaffective disorder (n = 4) after a Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient's Version.42 The patient group was comprised of 18 inpatients and 7 outpatients. With regard to schizophrenia subtypes, one patient has schizophrenia—disorganized, 7 have schizophrenia—paranoid, 3 have schizophrenia—residual, and 10 have schizophrenia—undifferentiated.
Participants with a lifetime history of substance dependence or who met criteria for a substance abuse diagnosis within the 6 months prior to assessment were excluded from the study, as were participants with a history of electroconvulsive treatment, head injury with loss of consciousness greater than 10 minutes, neurological disorders, or human immunodeficiency virus seropositivity. Urine toxicology screens for substances of abuse were negative for all subjects. Medication dosages (chlorpromazine equivalents) were computed according to the American Psychiatric Association guidelines.43 The conversion factors for the 2 patients who were taking risperidone Consta were obtained from the Schizophrenia Patients Outcomes Research Team treatment recommendations (http://www.ahrq.gov/clinic/schzrec.htm). Demographic data are given in table 1. All participants signed informed consent as approved by the local institutional review boards.
Table 1.
Demographics of Study Participants
| Variables | Controls (N = 21) | Patients (N = 25) | t | P |
| Age (y) | 40.4 ± 10.8 | 36.7 ± 10.5 | −1.17 | .25 |
| Education (y) | 15.5 ± 3.0 | 12.3 ± 2.1 | −4.22 | .00001 |
| FSIQa,b | 105.5 ± 12.1 | 94.3 ± 13.5 | −2.89 | .003 |
| BPAQa,c | 50.8 ± 12.5 | 61.1 ± 17.4 | 2.25 | .015 |
| LHAa,d | 10.2 ± 5.3 | 14.2 ± 10.3 | 1.60 | .061 |
| PANSS totalc | — | 78.7 ±16.0 | — | — |
| PANSS positivec | — | 18.9 ± 6.2 | — | — |
| PANSS negativec | — | 20.9 ± 6.3 | — | — |
| CPZ equivalents (mg) | — | 1157.8 ± 627.2 | — | — |
| χ2 | ||||
| Gender | 16/5 | 22/3 | 0.44 | .25e |
Note: FSIQ = Full-Scale IQ from Wechsler Abbreviated Scale of Intelligence,44 BPAQ = Buss Perry Aggression Questionnaire total score, LHA = Life History of Aggression total score, PANSS = Positive and Negative Syndrome Scale, CPZ = chlorpromazine.
One tailed.
Missing for 2 subjects.
Missing for 1 subject.
Missing for 3 subjects.
By Fisher exact test.
Assessments
Psychopathology was assessed using the Positive and Negative Syndrome Scale.45 Aggressive attitudes were measured using the BPAQ,41 which is a 29-item self-report scale. The total score served as the dependent measure in this study. Data were missing for one patient on these measures.
Aggressive histories were measured using the total score on the Life History of Aggression (LHA46), which is an 11-item self-report interview. Data were missing for 3 subjects on this measure.
In a prior study on men with schizophrenia that included patients with comorbid substance abuse/dependence,10 we found that scores on the Buss Durkee Hostility Inventory (BDHI)47 (the predecessor of the BPAQ) were significantly correlated with arrests for violent charges as gleaned from New York State criminal history records, which are available for inpatients at the study site, r = .65, n = 14, P = .02. This suggests that the BDHI could be used as a valid measure of aggression in patients. The BPAQ, used in the current study, is a more refined set of items derived from the BDHI. The range of arrests for violent offenses was restricted in the current sample (range = 0–2), possibly because none of the patients in the current study had significant substance abuse/dependence histories. However, BPAQ total scores were correlated with total arrests (range = 0–5) in the current sample (r = .78, n = 17, P = .0002, arrest data unavailable for 9 patients). These results support the validity of the BPAQ as a measure for aggression in our population.
Magnetic Resonance Imaging
Scanning took place on the 1.5T Siemens Vision Scanner (Erlangen, Germany) at the Nathan Kline Institute Center for Advanced Brain Imaging. Participants received a magnetization prepared rapidly acquired gradient echo (MPRAGE) T1-weighted scan (repetition time [TR] = 11.6 ms, echo time [TE] = 4.9 ms, inversion time [TI] = 1122 ms, matrix = 256 × 256, field of view (FOV) = 256 mm, number of excitations (NEX) = 1, slice thickness = 1 mm, 190 slices, no gap) and a resting-state functional magnetic resonance imaging (fMRI) scan (TR = 2000 ms, TE = 50 ms, matrix = 64 × 64, field of view (FOV) = 224 mm, number of excitations (NEX) = 180, 5-mm slice thickness, 22 slices, no gap). For the resting-state scan, participants were instructed to close their eyes and remain awake.
Postprocessing
Resting-state data were processed as described elsewhere in detail.19,48,49 Briefly, the first 10 volumes were discarded to eliminate T1 relaxation effects. Thereafter, images were motion corrected, time shifted, and despiked using AFNI.50 Next, time series were smoothed using a 6-mm full width-half maximum (FWHM) Gaussian kernel, temporally filtered, and normalized to Montreal Neurological Institute (MNI) space (1 × 1 × 1 mm3 resolution) using FSL (www.fmrib.ox.ac.uk/fsl).
The MPRAGE also was normalized to MNI space and was segmented using FSL's FAST software. The gray matter, cerebrospinal fluid (CSF), and total brain signal time series were then extracted using masks derived from the MPRAGE segmentation. These time series as well as the time series for the 6 motion parameters were used as covariates in a general linear model (GLM).
FC Analyses
To obtain time series for each seed in each participant, we (1) transformed the subject's time series into MNI space using a 12 degrees of freedom linear affine registration implemented in FMRIB's Linear Image Registration Tool (FLIRT) (voxel size = 1 × 1 × 1 mm3) and (2) calculated the spatial mean time series (across all voxels) for 2 mask spheres centered around the 2 spherical ROI (radius = 4 mm) based on the coordinates reported by Stein et al40 (amygdala coordinates: x = ±26, y = 0, z = −20). Additional identically sized spheres were placed in other parts of the effective connectivity network identified by Stein et al40 and included subgenual cingulate (0, 15, −14), supragenual cingulate (0, 34, 30), and bilateral DLPFC (±56, 26, 25) and OFC (±46, 31, −9).
For each ROI, individual participant analyses were carried out with the GLM implemented in FSL's FEAT toolbox using the seed-based regression approach employed in our prior work.19,48 The time series for the ROI as well as for the nuisance covariates (time series regressors for global signal intensity, white matter, CSF, and 6 motion parameters) were entered as predictors. We produced individual subject-level maps of all positively correlated voxels for each ROI seed, correcting for multiple comparisons at the cluster level using Gaussian random field theory (minimum z > 2.3; cluster significance: P < .05, corrected).
Group-level analyses were conducted using FMRIB's Local Analysis of Mixed Effects (FLAME), which produced thresholded z-score maps of activity associated with each ROI. These maps revealed networks for patients and controls, as well as difference maps (ie, group difference maps). Talairach coordinates were derived from the MNI coordinates using the MNI to Talairach Coordinate Converter (http://www.bioimagesuite.org/Mni2Tal/index.html), and brain localizations were derived from the Talairach Daemon (http://www.talairach.org/daemon.html). Surface maps of images in Talairach space were generated using SUMA, a part of AFNI.50
Correlations between aggression and resting-state FC were performed using a 2-tailed α level of .05.
Results
Functional Connectivity
Talairach coordinates and cluster information are given in table 2. Across groups, FC analyses for the amygdala seed revealed significant correlations within a very large cluster that included bilateral lentiform nuclei and parahippocampal gyri, right inferior frontal gyrus (Brodmann area [BA] 47), and right superior temporal gyrus (STG; BA 38).
Table 2.
Amgydala/Ventral Prefrontal Cortex Resting-State Activity in Controls and Patients With Schizophrenia or Schizoaffective Disorder
| Regions | Cluster Information |
||||||
| BA | xa | y | z | Sizeb | z score | P | |
| All subjects | |||||||
| PHG | — | 27 | −1 | −11 | 23810 | 8.07 | <10−45 |
| PHG | — | −24 | −3 | −10 | — | 7.8 | — |
| Lentiform nucl | — | 27 | 6 | −3 | — | 6.17 | — |
| Lentiform nucl | — | −21 | 6 | 0 | — | 5.82 | — |
| STG | 38 | 38 | 9 | −15 | — | 5.34 | — |
| IFG | 47 | 38 | 14 | −17 | — | 5.3 | — |
| Patients < controls | |||||||
| ACC | 32 | 3 | 40 | 3 | 2244 | 4.41 | 5.22 × 10−10 |
| ACC | 24 | 3 | 25 | 3 | — | 3.82 | — |
| ACC | 32 | −10 | 38 | 3 | — | 3.79 | — |
| ACC | 24 | −6 | 38 | 3 | — | 3.75 | — |
| MedFG | 10 | 0 | 55 | 3 | — | 3.67 | — |
| ACC | 10 | 13 | 35 | −5 | — | 3.64 | — |
| IFG | 47 | −50 | 16 | 1 | 733 | 3.82 | .00036 |
| MFG | 47 | −47 | 44 | −7 | — | 3.53 | — |
| IFG | 47 | −39 | 25 | 2 | — | 3.47 | — |
| IFG | 47 | −43 | 30 | −6 | — | 3.32 | — |
| Lentiform nucl | − | −27 | 13 | 2 | — | 3.29 | — |
| IFG | 47 | −33 | 31 | −7 | — | 3.19 | — |
Note: BA = Brodmann area, PHG = parahippocampal gyrus, Nucl = nucleus, STG = superior temporal gyrus, IFG = inferior frontal gyrus, ACC = anterior cingulate cortex, MedFG = medial frontal gyrus.
Maximum.
Number of voxels.
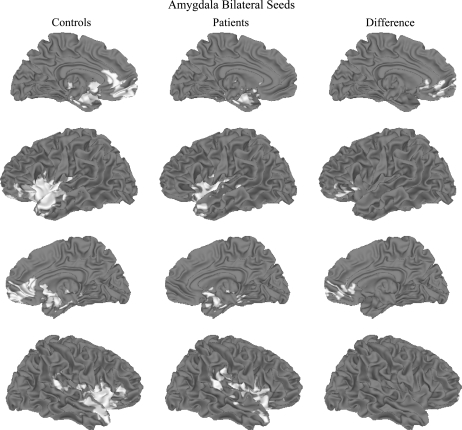
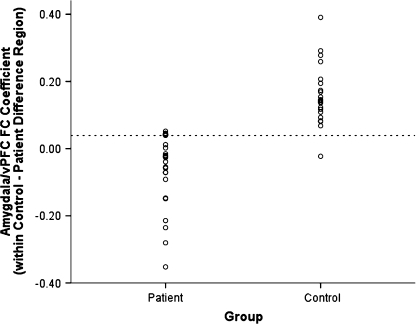
Group analyses revealed marked reductions in FC between the amygdala seed and portions of vPFC (see figure 1). More specifically, compared with controls, patients exhibited reduced amygdala-based FC bilaterally with rostral anterior cingulate cortex (BAs 24 and 32) and medial prefrontal gyrus (BA 10). They also showed reduced amygdala-based FC with left inferior frontal gyrus (BA 47) and middle frontal gyrus, as well as left lentiform nucleus. Overall, the FC regions appear to be more diffuse in patients (figure 1, second and fourth rows). A scatterplot of group differences in FC is presented in figure 2.
Fig. 1.
Functional Connectivity (FC) Associated With an 8-mm Diameter Spherical Seed Placed in Amygdala Bilaterally for Healthy Controls (Left, in Red) and Patients With Schizophrenia or Schizoaffective Disorder (Center, in Blue). Difference map (controls − patients) is shown on the right. Data thresholded at P < .05, corrected. Top row, left medial view; second row, left lateral view; third row, right medial view; fourth row, right lateral view.
Fig. 2.
Scatterplot of Group Differences for Amygdala/Ventral Prefrontal Cortex (vPFC) Functional Connectivity (FC) Regression Parameter Estimates.
For the OFC-seeded network, we observed significant correlations within the inferior frontal gyrus bilaterally, in the left supramarginal and superior frontal gyri, and in right STG and precentral gyrus. FC was lower in patients than controls in the insula, claustrum, STG, and anterior cingulate cortex (BA 24) bilaterally; in the left thalamus and medial frontal gyrus (BA 32); and in the right inferior parietal lobule and postcentral gyrus (see Supplementary Figure 1, Supplementary Table 1). There were no significant group differences in FC for the subgenual anterior cingulate cortex (ACC), supragenual ACC, or DLPFC networks (see Supplementary Figures 2–4, Supplementary Tables 2–4).
Behavioral Data
Mean BPAQ scores are given in table 1. As expected, patients had higher scores than controls on the BPAQ total score, t = 2.25, P = .015, one-tailed. Similar results were obtained when the patient group was limited to only those with a diagnosis of schizophrenia, t = 1.99, P = .026, one-tailed.
Mean LHA scores are given in table 1. As expected, patients trended toward higher scores than controls on the LHA total score. Similar results were obtained when the patient group was limited to only those with a diagnosis of schizophrenia. The LHA and BPAQ scores were highly correlated in both patients (r = .66, P = .001) and controls (r = .63, P = .002).
Relations Between FC and Behavior
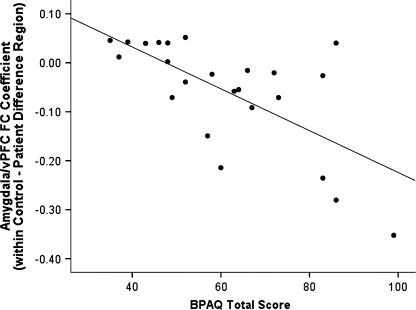
Across groups, amygdala/vPFC FC in the region in which patients and controls differed correlated strongly and negatively with BPAQ scores, r = −.55, n = 45, P = 8.6 × 10−5. This correlation was highly significant in patients, r = −.66, n = 24, P = .0004 (see figure 3; correlations were similar for each hemisphere, rleft = −.44, P = .033; rright = −.56, P = .005). The correlation between BPAQ and amygdala/vPFC FC in the difference region was similar, r = −.68, n = 20, P = .0009, when the 4 patients with schizoaffective disorder were excluded from the analysis. FC data for the left and right difference regions are shown in Supplementary Tables 5 and 6 and Supplementary Figures 5 and 6. Similar results were obtained for number of the LHA total score (rleft = −.24, n = 22, P = .29; rright = −.41, P = .057; rbilateral = −.46, P = .029) and total arrests (rleft = −.34, n = 17, P = .18; rright = −.64, P = .005; rbilateral = −.65, P = .005). Thus, as predicted, higher levels of aggression in patients were correlated with lower amygdala/vPFC FC.
Fig. 3.
Correlation Between Buss Perry Aggression Questionnaire Total Score and Amygdala/Ventral Prefrontal Cortex (vPFC) Functional Connectivity (FC) Regression Parameter Estimates (Group Difference Region) in Patients With Schizophrenia or Schizoaffective Disorder.
The correlation with BPAQ was not significant in controls in the region of group differences for the left or bilateral amygdala seeds (rleft = −.12, n = 21, P = .60; rbilateral = −.14, P = .54) but approached significance for the right-sided seed, r = −.37, P = .10. Correlations with LHA were not significant for right or bilateral seeds (r’s > −.28, n = 21, P’s > .21) but approached significance for the left-sided seed (rleft = −.37, P = .10).
Total scores on the BPAQ were not significantly correlated with FC in OFC control-patient difference regions or with FC associated with supragenual cingulate, subgenual cingulate, or DLPFC regions (there were no group differences for those latter seeds). None of the correlations were significant for either group (P’s > .15).
Partial correlation analyses showed that the relationship between total BPAQ and amygdala/vPFC FC regression coefficients in patients remained significant even after accounting for age. Furthermore, neuroleptic medication dosage did not account for this relationship for the patient group.
To further specify the location of the BPAQ/FC relationship for the amygdala-seeded network, we entered the demeaned BPAQ score in a voxelwise analysis on the amygdala/vPFC data for patients, with the search space limited only to the region in which patients had lower FC than controls. Scores on the BPAQ were significantly correlated with the regression coefficient for this contrast in an 865-voxel cluster with cluster maxima at (−8, 20, 1) that was significant at P = 2.6 × 10−5, corrected, and a 373-voxel cluster at (19, 57, −2), P = .016, corrected.
Discussion
We found that FC between the amgydala and vPFC is disrupted in medicated patients with schizophrenia and schizoaffective disorder. Moreover, in patients, amygdala/vPFC FC showed a robust inverse relationship with self-reported aggression, especially with the right-seeded network, such that higher levels of aggressive attitudes were associated with a reduced positive, trending toward negative, FC between amygdala and vPFC. In addition, in patients, the results were consistent across 2 other measures of aggression, albeit less strongly in the case of the LHA. On balance, then, the results support the idea that amygdalofrontal functional disconnectivity is associated with aggression and antisocial behavior. These correlational results were specific to amygdala/vPFC FC because they were not found for resting-state FC derived from seeds in OFC, supra- or subgenual cingulate, or bilateral DLPFC.
These results are consistent with increasing evidence that ventral prefrontal regions involved in social cognition are dysfunctional in schizophrenia.31,51 Recent work, including that from our laboratory, has shown that aggression is associated with abnormalities in this region. For example, in men with schizophrenia, we found that abnormal right ventral prefrontal white matter integrity was associated with increased impulsivity and aggression.10 These effects were specific to ventral prefrontal regions because they were not observed in more dorsal frontal regions. Volumetric abnormalities in OFC also have been associated with aggression.37,38
The reduced FC seen in the amygdala/vPFC network is not simply a reflection of a general reduction in FC in schizophrenia. Although FC was reduced in networks associated with OFC seeds, such was not the case for networks based in the subgenual ACC, supragenual ACC, or DLPFC.
Our results also contrast with findings that reduced FC between amygdala and anterior cingulate has been associated with depression,52 suggesting that different patterns of amygdalar disconnectivity result in different symptomatologies. Similarly, abnormalities in FC between the amygdala and DLPFC have been found in response to facial expressions of anger presented during scanning in patients with schizophrenia.53 The developmental pattern of these connections is not known, but they may reflect cortical immaturity in schizophrenia, as has been proposed.54
The current study has a number of limitations. The region of reduced connectivity lies in a region prone to magnetic susceptibility artifacts. However, such artifact might diminish the likelihood of detecting group differences. Second, the amygdala is a small and heterogenous structure that does not comprise a single functional unit. However, the ROI chosen for the current article was based on that used by Stein et al40 in their anatomically based effective connectivity study of the amygdala. Moreover, at the current resolution afforded by fMRI, it is difficult to study functional subunits. This remains a target for future work. Another issue that is of relevance is the unconstrained nature of the resting state. We should point out in this regard that Fransson55 examined so-called task-unrelated thoughts (concerning inner speech, imagery, planning for the future, episodic memory, and task-unrelated attention) and found that these were correlated with neither activation during a working memory task nor resting-state activation in the DMN. Moreover, the unconstrained nature of the task would presumably add noise to the results, weakening statistical power. This stands in contrast to the strong effects commonly observed in such studies.48,56 Most significantly in this regard, our group has shown short- and long-term test-retest reliability in the moderate to high range for FC during rest.57 Finally, patient participants all had chronic schizophrenia or schizoaffective disorders and were all on antipsychotic medication. However, medication dosages did not account for our group differences in voxel-based analyses or for the relationship between decreased FC and increased aggression. Moreover, in a subanalysis in which patients with schizoaffective disorder were omitted, the correlation between total BPAQ scores and amygdala/vPFC FC regression parameter estimates in the group difference region was almost identical to that in the larger analysis.
The current findings show that FC between amygdala and frontal regions are disrupted in schizophrenia and add to a growing literature suggesting abnormalities in both those regions. The significant correlation between reduced amygdala/vPFC FC in patients and aggression is consistent with our prior DTI findings in schizophrenia.10 These results point toward the potential merits of employing multimodal assessments of structural and functional frontolimbic connectivity in patients with schizophrenia in future studies.
Supplementary Material
Supplementary tables 1–6 and figures 1–6 are available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institutes of Health (RO1 MH64783 to M.J.H., R37 MH49334 to D.C.J.); National Alliance for Research on Schizophrenia and Depression (to F.X.C.); gifts from Linda and Richard Schaps and Jill and Bob Smith and Taubman Foundation (to F.X.C.).
Supplementary Material
Acknowledgments
We thank Raj Sangoi (RT) (R) (MR) for assistance in image acquisition and Daniel S. Margulies for assistance with image processing and analysis.
References
- 1.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 2.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56:367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 3.Buchsbaum MS, Tang CY, Peled S, et al. MRI white matter diffusion anisotropy and PET metabolic rate in schizophrenia. Neuroreport. 1998;9:425–430. doi: 10.1097/00001756-199802160-00013. [DOI] [PubMed] [Google Scholar]
- 4.Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 8.Szeszko PR, Robinson DG, Ashtari M, et al. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33:976–984. doi: 10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- 9.Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160:572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- 10.Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter, aggression and impulsivity in men with schizophrenia. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- 11.Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: a first voxelwise correlational study. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim KO, Ardekani BA, Nierenberg J, Butler PD, Javitt DC, Hoptman MJ. Voxelwise correlational analyses of white matter integrity in multiple cognitive domains in schizophrenia. Am J Psychiatry. 2006;163:2008–2010. doi: 10.1176/appi.ajp.163.11.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162:602–605. doi: 10.1176/appi.ajp.162.3.602. [DOI] [PubMed] [Google Scholar]
- 14.Kumra S, Ashtari M, Cervellione KL, et al. White matter abnormalities in early-onset schizophrenia: a voxel-based diffusion tensor imaging study. J Am Acad Child Adolesc Psychiatry. 2005;44:934–941. doi: 10.1097/01.chi.0000170553.15798.94. [DOI] [PubMed] [Google Scholar]
- 15.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 16.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16:1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 19.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Greicius MD, Kiviniemi V, Tervonen O, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- 23.Liang M, Zhou Y, Jiang T, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Liu Z, Liang M, et al. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- 25.Bluhm RL, Miller J, Lanius RA, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Liang M, Jiang T, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 28.Meyer-Lindenberg A, Poline JB, Kohn PD, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 29.Meyer-Lindenberg AS, Olsen RK, Kohn PD, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- 30.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoptman MJ, Nolan KA. The role of prefrontal abnormalities in schizophrenia. In: Javitt DC, Kantrowitz JT, editors. Schizophrenia. 3rd edn. New York, NY: Springer; 2008. [Google Scholar]
- 32.Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 33.Kubicki M, Westin C-F, Maier SE, et al. Uncinate fasciculus findings in schizophrenia: a magnetic resonance diffusion tensor imaging study. Am J Psychiatry. 2002;159:813–820. doi: 10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volavka J. Neurobiology of Violence. 2nd edn. Washington, DC: APA Publishing; 2002. [Google Scholar]
- 35.Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- 36.Steadman HJ, Mulvey EP, Monahan J, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. 1998;55:393–401. doi: 10.1001/archpsyc.55.5.393. [DOI] [PubMed] [Google Scholar]
- 37.Hoptman MJ, Volavka J, Weiss EM, et al. Quantitative MRI measures of orbitofrontal cortex in patients with chronic schizophrenia or schizoaffective disorder. Psychiatry Res. 2005;140:133–145. doi: 10.1016/j.pscychresns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonucci AS, Gansler DA, Tan S, Bhadelia R, Patz S, Fulwiler C. Orbitofrontal correlates of aggression and impulsivity in psychiatric patients. Psychiatry Res. 2006;147:213–220. doi: 10.1016/j.pscychresns.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Rusch N, Spoletini I, Wilke M, et al. Inferior frontal white matter volume and suicidality in schizophrenia. Psychiatry Res. 2008;164:206–214. doi: 10.1016/j.pscychresns.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Buss AH, Perry M. The Aggression Questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 42.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (SCID-I/P, 11/2002 Revision) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 43.American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 1997;154(suppl):1–63. doi: 10.1176/ajp.154.4.1. [DOI] [PubMed] [Google Scholar]
- 44.Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. San Antonio, TX: Psychological Corporation; 2006. [Google Scholar]
- 45.Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. Toronto: Multi-Health Systems; 1992. [Google Scholar]
- 46.Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–157. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 47.Buss A, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 48.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 51.Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 52.Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 53.Radulescu AR, Mujica-Parodi LR. A systems approach to prefrontal-limbic dysregulation in schizophrenia. Neuropsychobiology. 2008;57:206–216. doi: 10.1159/000151731. [DOI] [PubMed] [Google Scholar]
- 54.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 55.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van E, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shehzad Z, Kelly AMC, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. doi: 10.1093/cercor/bhn256. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.