Abstract
ATP-binding cassette transporters (ABC transporters) utilize the energy of ATP hydrolysis to translocate an unusually diverse set of substrates across cellular membranes. ABCA4, also known as ABCR, is a ~250 kDa single-chain ABC transporter localized to the disk margins of vertebrate photoreceptor outer segments. It is composed of two symmetrically organized halves, each comprising six membrane-spanning helices, a large glycosylated exocytoplasmic domain located inside the disk, and a cytoplasmic domain with an ATP-binding cassette. Hundreds of mutations in ABCA4 are known to cause impaired vision and blindness such as in Stargardt disease as well as related disorders. Biochemical and animal model studies in combination with patient analyses suggest that the natural substrate of ABCA4 is retinylidene-phosphatidylethanolamine (N-retinylidene-PE), a precursor of potentially toxic diretinal compounds. ABCA4 prevents accumulation of N-retinylidene-PE inside the disks by transporting it to the cytoplasmic side of the disk membrane where it can dissociate, allowing the released all-trans-retinal to enter the visual cycle. The pathogenesis of diseases caused by mutations in ABCA4 is complex, comprising a loss-of-function component as well as photoreceptor stress caused by protein mislocalization and misfolding.
1 Introduction to ABC Transporters
ATP-binding cassette transporters (ABC transporters) utilize the energy of ATP hydrolysis to unidirectionally translocate a diverse set of substrates, ranging from ions to lipids and peptides, across cellular membranes (Higgins 1992). These ubiquitous integral membrane proteins are present in all living organisms and constitute one of the largest classes of proteins (Kos and Ford 2009; Linton and Higgins 2007). ABC transporters can function as either importers or exporters, moving their substrates in or out of the cytoplasm, respectively (Dawson et al. 2007). As a result, these proteins participate in a great variety of biological processes that involve active transport of substances across extracellular or intracellular membranes, for example, in nutrient uptake and drug resistance.
Despite a generally low sequence identity, all ABC transporters share the same architecture (Kos and Ford 2009; Linton and Higgins 2007; Rees et al. 2009). A minimum of four domains is required for functional activity: two transmembrane domains (TMDs) and two nucleotide-binding domains (NBDs), also known as ATP-binding cassettes. TMDs are responsible for binding substrate and forming the translocation path, whereas NBDs provide energy for transport by hydrolyzing ATP to ADP. Besides these four core domains, some ABC transporters have additional elements that may be fused to TMDs or NBDs, for instance, extracellular domains located across the membrane from NBDs. The quaternary organization of ABC transporters varies. In prokaryotes, different domains are often expressed as separate polypeptide chains that associate to form a functional transporter, whereas many eukaryotic members constitute a single amino acid sequence (full transporters) or comprise two symmetrical polypeptide chains (half transporters).
2 Human ABC Transporters
To date, 49 ABC transporters have been identified in the human genome. These are organized into seven subfamilies (ABCA to ABCG) based on gene structure, amino acid sequence and phylogenetic analysis (Vasiliou et al. 2009). Subfamily A is composed of 12 proteins. Although the substrates for most of its members have yet to be identified, indirect evidence indicates that these proteins are likely to be involved in lipid transport in different organs and cell types. All members of this subfamily are large full transporters organized in two topologically similar halves and ranging from 1,543 (ABCA10) to 5,058 (ABCA13) residues in size. A distinctive feature of family A members is the presence of a large extracellular domain in the N-terminal half of the sequence. Mutations in several ABCA genes have been linked to inherited diseases such as Tangier disease (defects in ABCA1) (Zarubica et al. 2007) and harlequin type ichthyosis (defects in ABCA12) (Akiyama et al. 2005). The focus of this review is ABCA4, an ATP-binding cassette transporter predominantly found in photoreceptor cells. Defects in the ABCA4 gene lead to Stargardt disease and may also be implicated in several other severe visual disorders.
3 ABCA4 and Vision Diseases
Stargardt disease is an autosomal recessive form of juvenile macular degeneration with an estimated prevalence of 1 in 10,000 (Paskowitz et al. 2006; Walia and Fishman 2009; Weleber 1994). It is usually diagnosed within the first two decades of life and leads to progressive irreversible loss of central vision and delayed dark adaptation. At the histological level, degeneration of photoreceptors and the underlying retinal pigmented epithelium (RPE) occurs within and near the macula. The reason for the death of RPE cells, which are responsible for maintenance of photoreceptors and phagocytosis of their aging outer segments, is believed to be the accumulation of an age-related pigment lipofuscin containing various toxic by-products of the visual cycle (Sparrow et al. 2009). In particular, elevated levels of diretinoid-pyridinium-ethanolamine (A2E), the ultimate product of condensation of two molecules of all-trans-retinal and one molecule of phosphatidylethanolamine (PE), and its precursors were found in ocular tissues from Stargardt patients (Rozet et al. 1998). Degeneration of photoreceptors seems to be secondary to the loss of the RPE.
Although A2E has been widely accepted as the major harmful component of lipofuscin, other compounds have been proposed to have toxic effects on photore-ceptors and the RPE (Sparrow et al. 2009). In particular, studies of the Abca4−/− Rdh−/− mice revealed that all-trans-retinal, a precursor of A2E, is directly involved in acute light-induced retinopathy (Maeda et al. 2009a, b). From this viewpoint, formation of A2E may in fact represent a way of detoxification of photoreceptors by reducing the concentration of free all-trans-retinal following an intense photobleach.
In 1997, the gene defective in Stargardt disease was identified and found to encode the ABC transporter, ABCA4 (also known as ABCR) (Allikmets et al. 1997a, b). ABCA4 mutations have also been found in several other visual disorders including fundus flavimaculatus (currently considered a form of Stargardt disease) (Rozet et al. 1998), cone-rod dystrophy (Hamel 2007), and autosomal recessive retinitis pigmentosa (Martinez-Mir et al. 1998). It has also been proposed that individuals carrying mutations in ABCA4 may have a higher risk of developing age-related macular degeneration (AMD) (Allikmets et al. 1997a; Mata et al. 2001; Rozet et al. 1998), although other studies argue against this link (Schmidt et al. 2003).
4 Molecular View of ABCA4
4.1 Primary Structure
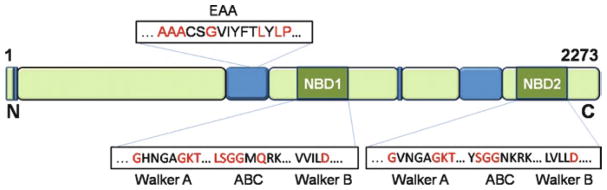
To date, the amino acid sequences of ABCA4 from nine different species have been published. These include proteins from human, crab-eating macaque, cow, mouse, rat, dog, African and Western clawed frogs, and puffer fish. Alignment of these sequences with ClustalW2 (Larkin et al. 2007) shows high similarity among ABCA4 proteins, with identity scores ranging from 62% (human vs. puffer fish) to 97% (human vs. macaque). All these primary structures feature a number of highly conserved sequence motifs mostly located in the NBDs, which are used to identify members of the ABC transporter superfamily (Fig. 1). Specifically, the ABC transporter signature motif, ‘LSGGQ’, is present in both NBD1 and NBD2, although it is reduced to ‘SGG’ in NBD2. Nucleotide-binding domains also have Walker A and B motifs typical of ATP-processing enzymes. The Walker A motif is ‘GXXXXGKT’ (‘X’ is any residue) and the Walker B motif is ‘hhhhD’ (‘h’ is any hydrophobic residue). Interestingly, another ABC transporter motif, ‘EAA’, is located in the membrane-spanning region of the N-terminal half of ABCA4. This long motif has a sequence of ‘EAAXXXGXXXXXXXIXLP’, with some variations among individual proteins (Mourez et al. 1997). Importantly, it represents a signature of ABC importers, where it contributes to the interface between the transmembrane helices and the NBD (Rees et al. 2009). The presence of the ‘EAA’ motif in ABCA4 may be of interest because it is generally assumed that all eukaryotic ABC transporters are exporters. Curiously, the ‘EAA’ motif is absent in the symmetrically organized C-terminal half of ABCA4.
Fig.1.
Diagram depicting the location of highly conserved motifs specific for ABC transporters in the primary structure of ABCA4. Predicted transmembrane regions are shown in blue. See text for detailed description of the various motifs
4.2 Localization
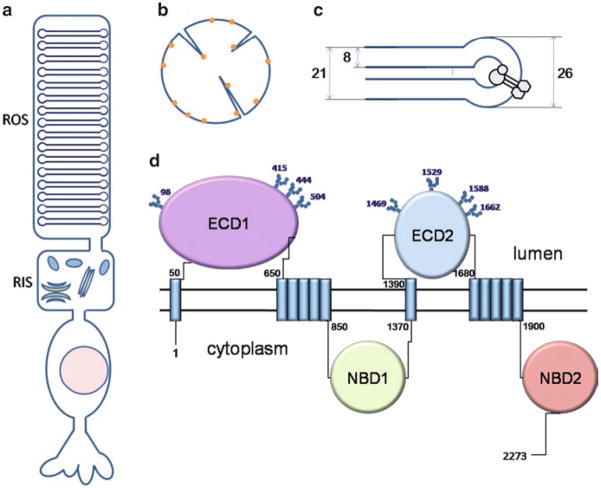
About 30 years ago, an electron microscopy study by Papermaster et al. (1978) of immunochemically labeled frog photoreceptors localized “a large intrinsic membrane protein” to rod outer segments. The rod outer segment (ROS) is a specialized compartment of the rod cell harboring hundreds of flattened closed membrane structures called disks, that is connected to the rest of the rod cell with a cilium (Fig. 2a). ABCA4 was shown to be situated in the rims and incisures of these disks (Fig. 2b, c). A homologous protein was later identified in bovine rod outer segments (Molday and Molday 1979) and shown to have the same localization (Illing et al. 1997). The “rim protein” was cloned and classified as a member of the ATP transporter superfamily based on its sequence homology (Allikmets et al. 1997b; Azarian and Travis 1997; Illing et al. 1997). For some time it was believed that mammalian ABCA4 was specific to rod cells (Illing et al. 1997; Sun and Nathans 1997). However, the presence of ABCA4 in foveal and peripheral cone cells was later demonstrated by immunohistochemistry and western blot analysis (Molday et al. 2000). This finding agrees well with another early study by Papermaster et al. (1982) showing that ABCA4 localized to the margins of cone outer segment lamellae in frogs.
Fig. 2.
Localization and topology of ABCA4. (a) Schematic representation of a rod cell. ROS: rod outer segment; RIS: rod inner segment. (b) Top view of a ROS disk. Molecules of ABCA4 are denoted by yellow dots. (c) Side view of a cross-section of a disk. ABCA4 is localized to the rim of the disk. Numbers represent dimensions of a mouse disk in nm. (d) Topology of human ABCA4. Known glycosylation sites are shown with squares. Numbers are positions of the corresponding residues in the primary structure. ECD1, ECD2: exocytoplasmic domains 1 and 2, respectively. NBD1, NBD2: nucleotide-binding domains 1 and 2, respectively
The reason for such a restricted localization of ABCA4 within the ROS disks is still unclear. A possible explanation is that the distribution of this protein is dictated by the size of its extracellular domains (also called exocytoplasmic domains 1 and 2, or ECD1 and ECD2) located in the disk lumen (Fig. 2c). Indeed, assuming a spherical shape of the larger ECD1, which comprises about 600 residues, the approximate volume of this domain calculated based on the average partial specific volumes of amino acids (Harpaz et al. 1994) is about 8 nm3. A rough estimate of its diameter is therefore 5–6 nm, which is greater than most values for the distance between membranes of the same disk published to date (0–5 nm, depending on the species (Nickell et al. 2007)), but presumably less than the inner diameter of the rim (Fig. 2c). It was proposed that ABCA4 might play a structural role in maintaining the shape of the disk and connecting it to the plasma membrane of ROS (Roof and Heuser 1982), but this hypothesis did not receive experimental support.
So far, structural and functional studies of ABCA4 have focused on the protein expressed in the retina. But it needs to be noted that several studies detected lower levels of ABCA4 expression in the brain (Bhongsatiern et al. 2005; Tachikawa et al. 2005). For example, the presence of ABCA4 mRNA in the lateral ventricles of the rat brain was revealed by in situ hybridization, and the expression of the protein in the choroid plexus was shown by western blotting (Bhongsatiern et al. 2005). In a more recent study, quantitative real-time PCR analysis confirmed ABCA4 mRNA expression in the brains of several mammals, including humans, with the highest level of expression observed in mice (Warren et al. 2009).
4.3 Insights into Topology, Structure and Posttranslational Modifications
Four regions containing transmembrane helices can be identified in the primary structure of ABCA4 based on hydropathy plots (Fig. 1). Two single transmembrane helices are located at approximate positions 24–46 and 1370–1392 (human protein), delineating the beginning of the N-terminal and C-terminal halves of the protein, respectively (Fig. 2d). The following exocytoplasmic domains 1 (600 residues, N-terminal half) and 2 (300 residues, C-terminal half) are situated in the disk lumen, as demonstrated by glycosylation studies (Bungert et al. 2001; Illing et al. 1997; Molday and Molday 1979). In each half of the protein, the exocytoplasmic domain is succeeded by the second hydrophobic region presumably comprising five transmembrane helices. Thus, the total number of transmembrane helices in ABCA4 is most likely 12, in agreement with what was shown for many other ABC transporters (Rees et al. 2009). Each hydrophobic region is followed by a large soluble domain (520 and 370 residues, respectively) located on the cytoplasmic side of the disk membrane. These cytoplasmic regions contain NBDs and are therefore responsible for ATP hydrolysis.
Despite much experimental effort, knowledge of the structural features of ABCA4 is minimal. The main source of full-length ABCA4 for structural and functional studies has been rod outer segments of bovine retina (Ahn and Molday 2000; Ahn et al. 2000; Illing et al. 1997; Sun et al. 1999), where this ~250 kDa protein is present at a molar ratio of about 1:120 to rhodopsin. After isolation of ROS by the well developed sucrose gradient method, ROS membranes are solubilized in detergent and the protein is usually purified to homogeneity in one step by using immunoaffinity chromatography with the Rim3F4 monoclonal antibody (Illing et al. 1997). In a limited number of cases, ABCA4 was also isolated from human post-mortem retinas (Bungert et al. 2001). In addition, many studies have used recombinant human or bovine ABCA4 (89% sequence identity) transiently expressed in mammalian cells, where these proteins localized to the endoplasmic reticulum or intracellular vesicular structures (Ahn et al. 2000, 2003; Sun et al. 2000; Zhong et al. 2009). It is generally assumed that native and recombinant ABCA4 proteins have identical or at least similar properties, but because of limited yields, no thorough studies have been done to assess the folding and structure of ABCA4 obtained from different sources. Moreover, because most studies assume that this protein is a monomer, possible oligomeric states have not been studied. It has been shown, however, that the closely related ABCA1 transporter forms dimers and may undergo a transition to higher oligomers during the catalytic cycle (Trompier et al. 2006).
The N-terminal and C-terminal moieties of ABCA4 may interact with each other. This was established by comparing the catalytic and nucleotide-binding properties of individually expressed and co-expressed halves of the transporter in mammalian cells (Ahn et al. 2003). It should be noted, however, that the observations made in this study may result in part from protein misfolding.
The results of an early study suggested that ABCA4 may be phosphorylated in a light-dependent manner (Szuts 1985). This possible modification still needs to be explored, especially because it has been shown that phosphorylation of other ABC transporters can regulate transport activity (Noe et al. 2001; See et al. 2002) or mediate degradation (Kolling and Losko 1997; Martinez et al. 2003).
4.4 Structural Features of Individual Domains
Structural features of the exocytoplasmic domains of ABCA4 have not been extensively studied. These domains do not show significant sequence similarity to known proteins, except for other closely related ABCA transporters. An early work showed that bovine and frog ABCA4 are glycosylated and that binding of the Concanavalin A lectin requires disruption of the disk membranes, suggesting that the modified residues are located in the disk lumen (Molday and Molday 1979). In a subsequent study, eight N-linked glycosylation sites were identified in the two exocytoplasmic domains by systematically mutating the predicted sites in human recombinant ABCA4 expressed in COS-1 cells (Fig. 2d) (Bungert et al. 2001). Deglycosylated ABCA4 displayed only a slight decrease in molecular weight as determined by SDS-PAGE, indicating that its sugar chains are small (Azarian and Travis 1997; Bungert et al. 2001; Illing et al. 1997). It was also shown that ECD1 and ECD2 of bovine ABCA4 are linked to each other by at least one disulfide bond, as determined by trypsin digestion under reducing and non-reducing conditions (Bungert et al. 2001). The biological roles of the exocytoplasmic domains of ABCA4 are unknown. In closely related ABCA1 the corresponding “largest extra-cellular loops” seem to be responsible for interacting with apolipoprotein A-I (Fitzgerald et al. 2002). It is therefore possible that the exocytoplasmic domains of ABCA4 may be involved in interactions with other proteins.
The two cytoplasmic domains of human ABCA4 have been successfully expressed in E. coli and isolated from the soluble fraction of cell lysates as well as refolded from insoluble inclusion bodies (Biswas 2001; Biswas and Biswas 2000; Biswas-Fiss 2006; Suarez et al. 2002). Fluorescence anisotropy measurements suggest that these two domains interact in a nucleotide-dependent manner with dissociation constants in the sub-nanomolar range (Biswas-Fiss 2006). A conventional ATP-binding cassette (or NBD) of about 200 residues in size constitutes a part of each cytoplasmic domain. The structural and functional properties of the remaining ~320 (cytoplasmic domain 1) and ~170 (cytoplasmic domain 2) residues are unclear, although in some ATP transporters these regions carry out regulatory functions (Gerber et al. 2008; Kashiwagi et al. 1995). Mutagenesis studies have demonstrated that a conserved ‘VFVNFA’ motif located at the C-terminus of cytoplasmic domain 2 is essential for correct folding of ABCA4 (Zhong et al. 2009).
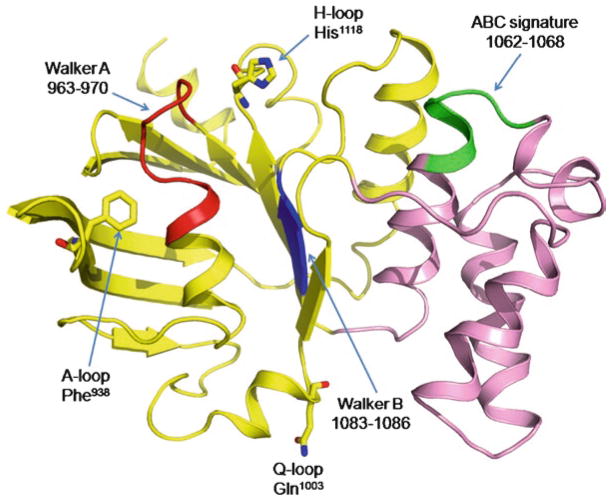
NBDs are the only regions of ABC transporters that are structurally highly conserved. Thus, some insights into the structure of NBDs of ABCA4 can be gained through homology modeling (Cideciyan et al. 2009; Molday et al. 2009) (Fig. 3). Similar to the NBDs of many other ABC transporters, these domains are organized in two distinct halves, a RecA-like subdomain, which is universal for many ATPases, and a smaller helical domain, unique for ABC transporters (Davidson and Chen 2004). The RecA subdomain houses the Walker A and Walker B motifs that participate in binding and coordination of the nucleotide and magnesium atom. Two conserved single-residue motifs, the H-loop and the A-loop, are also important for correct binding of the substrate. Another one-residue motif, the Q-loop, is located at the border of the RecA-like and helical domains. In addition to contacting the γ-phosphate of ATP, this loop presumably couples the energy-producing NBDs to the ligand-binding TMDs. It has been firmly established that dimerization of NBDs is essential for substrate translocation by ABC transporters (Davidson and Chen 2004; Kos and Ford 2009; Linton and Higgins 2007; Rees et al. 2009). When in the dimeric state, the ABC signature motif, situated in the helical subdomain of each NBD, participates in formation of the ATP-binding site along with the Walker A motif of the partner NBD.
Fig. 3.
Cartoon representation of a homology model of NBD1 obtained using SWISS-MODEL server (Kiefer et al. 2009) and refined by energy minimization with the NAMD Molecular Dynamics Simulator (Phillips et al. 2005). The crystal structure of the ABC transporter ATP-binding protein from Thermotoga maritima (Protein data bank ID 1VPL, 33.3% sequence identity) was used as a template (Cideciyan et al. 2009). The RecA-like subdomain is yellow; the helical domain is pink. Walker A, Walker B and ABC signature motifs are red, blue and green, respectively. Single-residue motifs are shown as sticks. The figure was prepared with Pymol (http://pymol.sourceforge.net/)
5 Biological Role of ABCA4
5.1 Identification of Substrate: Biochemical Evidence
Several biochemical studies have been performed to determine the natural substrate of ABCA4. It should be noted, however, that an assay that directly measures active transport across a membrane, as well as reveals the direction of that transport, has yet to be developed. Without such an assay, the reported biochemical evidence is based on the observation that binding of the substrate stimulates the ATPase activity of many ABC transporters. A seminal work measuring the ATP hydrolysis rate of bovine ABCA4 reconstituted in liposomes in the presence of 43 different compounds revealed that isomers of retinal had a two- to fivefold stimulatory effect, whereas all-trans-retinol and all-trans-retinoic acid showed lower activation efficiencies (Sun et al. 1999). Among other tested substances, amiodarone, digitonin, dehydroabietylamine, and 2-tert-butylanthroquinone demonstrated the same level of activation as retinal. Further kinetic analyses, however, suggested that retinal is a transported substrate, whereas the other stimulatory compounds may act as allosteric effectors (Sun et al. 1999). Subsequent studies have focused exclusively on the stimulatory effect of retinal-related compounds. In particular, it was shown that a naturally occurring reversible conjugate of all-trans-retinal and phosphatidyletha-nolamine, N-retinylidene-PE, increases the rate of ATP hydrolysis threefold (Ahn et al. 2000). Furthermore, a solid phase assay revealed that the ratio between the bound N-retinylidene-PE and ABCA4 is approximately 1:1 and, most importantly, that ATP could release N-retinylidene-PE and all-trans-retinal from the protein, while ADP and AMP-PNP were far less effective in this regard (Beharry et al. 2004). Based on these studies, it has been accepted that biochemical evidence points to retinal and N-retinylidene-PE as transported substrates of ABCA4 (Molday 2007; Molday et al. 2009; Sullivan 2009; Vasiliou et al. 2009). Of note, both the basal and the stimulated ATPase activities of ABCA4 varied significantly from study to study and among protein preparations, displaying a strong dependence on detergent type, the presence and composition of lipids and the presence of a reducing agent among other factors (Ahn et al. 2000; Sun et al. 1999).
The nucleotide specificity of ABCA4 and the roles of the two NBDs have been matters of debate. It was shown that purified native ABCA4 bound ATP and GTP with similar affinities (Illing et al. 1997) and that the detergent-solubilized full-length protein had ATPase and GTPase activities of about 200 nmol/min/mg (Ahn et al. 2000). Based on functional studies of individually expressed N- and C-terminal halves of the protein, it was also suggested that only NBD2 possesses the nucleotidase activity, while NBD1 has a tightly bound ADP molecule in the active site and does not participate in transport (Ahn et al. 2003). In contrast, the results of a mutagenesis study of full-length ABCA4 expressed in mammalian cells argue that both NBDs are active, but have distinct functions: NBD1 is responsible for basal ATPase activity, whereas NBD2 produces the retinal-stimulated increase in activity (Sun et al. 2000). Finally, a series of papers devoted to analyzing the individual cytoplasmic domains expressed in E. coli demonstrated that NBD2 is strictly specific for ATP (Biswas and Biswas 2000), and NBD1 is a general ribonucleotidase that prefers CTP as a substrate (Biswas 2001). It was also suggested that NBD2 may have an inhibitory effect on NBD1 within full-size ABCA4 (Biswas-Fiss 2006).
5.2 Proposed General Model of Transport
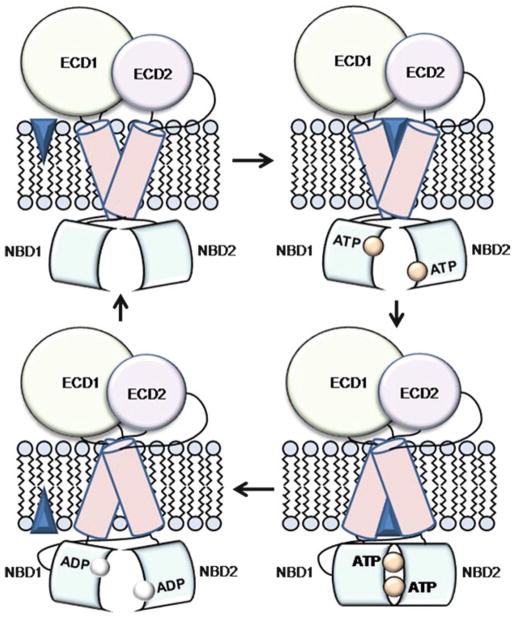
Several working models of transport have been suggested for ABCA4 that mostly differ with respect to the roles of NBDs, as described above (Molday 2007; Molday et al. 2009; Sullivan 2009; Sun et al. 2000). The proposed mechanism of transport is based on the ‘alternating access’ model, established for smaller ABC transporters (Kos and Ford 2009; Linton and Higgins 2007; Rees et al. 2009).
Accumulated biochemical evidence suggests all-trans-retinal and N-retinylidene -PE as the substrates but provides no clues about the direction of transport. The assumption that ABCA4 translocates the substrate from the luminal to the cytoplasmic side of the ROS disk is thus based on the well established logistics of the visual cycle in rods (Fig. 4), according to which all-trans-retinal undergoes reduction to all-trans-retinol by all-trans-retinol dehydrogenase (atRDH) residing on the cytoplasmic side, and is then transported to the cells of the retinal pigment epithelium (RPE) for further processing. Therefore, ABCA4 is currently believed to be an importer, which makes this protein unique among known eukaryotic ABC transporters. The ‘alternating access’ model suggests that the TMDs can form two different binding sites for the substrate (Fig. 5). In importers, the high-affinity site is located across the membrane from NBDs. Binding of the substrate to this site supposedly increases the affinity of NBDs for ATP. Upon binding ATP, NBDs come in close contact to form a dimer with the two nucleotide molecules positioned at its interface. This movement induces a conformational transition in TMDs that leads to the closure of the high-affinity substrate binding site and to the translocation of the substrate molecule to the low-affinity site located on the cytoplasmic side of the membrane. Hydrolysis of ATP then separates the NBDs and promotes dissociation of the ADP molecules, thus completing the transport cycle. In the absence of substrate, the transporter undergoes cycles of slow ATP hydrolysis by the individual NBDs, resulting in the basal ATPase activity.
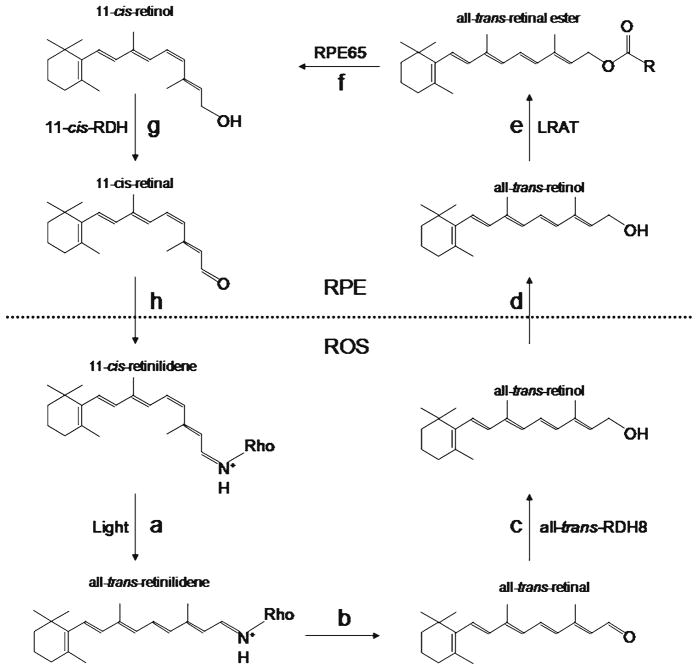
Fig. 4.
Transformations of the chromophore in the visual cycle. Light absorption isomerizes 11-cis-retinal to all-trans-retinal (a), followed by dissociation of all-trans-retinal from rhodopsin (b). All-trans-retinal is converted to all-trans-retinol by all-trans-retinol dehydrogenase 8 located on the cytoplasmic side of the disk membrane (c). All-trans-retinol is then transported to the RPE (d) and esterified by lecithin:retinol acyl transferase (e). Rpe65 isomerase converts all-trans-retinal ester to 11-cis-retinol (f ). 11-cis-Retinol dehydrogenase oxidizes 11-cis-retinol to 11-cis-retinal (g). Finally, 11-cis-Retinal is transported back to the ROS where it reassociates with opsin (h)
Fig. 5.
General model of the ABCA4 transport cycle. The substrate is shown as a blue diamond. See text for details
5.3 Abca4 Knockout Mice
Abca4−/− mice (Weng et al. 1999) do not fully reproduce the phenotypes of Stargardt disease and age-related macular degeneration, and some of the described phenotypes were highly exaggerated. These animals exhibit healthy photoreceptors that, under ordinary lighting conditions, show no degradation during their life time. A mild retinal degeneration, however, was induced by exposure to 10,000 lux fluorescent light for 1 h (Maeda et al. 2008), indicating that Abca4−/− animals may be more vulnerable to light damage under more extreme conditions. The initial study also suggested that delayed dark adaptation, another symptom of Stargardt disease, was present in Abca4−/− mice, and that it was initiated by delayed clearance of all-trans-retinal, which is known to re-associate with opsin and trigger the phototrans-duction cascade (Weng et al. 1999). In contrast, others have demonstrated that these animals adapt to the dark even faster than the wild-type mice (Pawar et al. 2008). Likewise, the rates all-trans-retinal clearance in Abca4−/− and wild-type mice were found comparable by other investigators (Maeda et al. 2008). Hence, the Abca4 knockout mice do not represent a precise model of the human macular degeneration. Notably, a phenotypic response very similar to human AMD and Stargardt disease has been recently observed in double-knockout mice lacking both Abca4 and all-trans-retinol dehydrogenase 8 (Rdh8) (Maeda et al. 2009a–c).
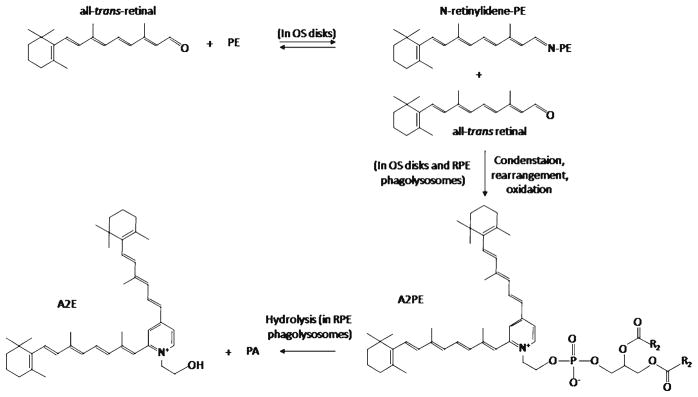
Despite the lack of retinal degeneration in Abca4 deficient mice, biochemical analyses of retinoid compounds and lipids from ocular tissues revealed alterations consistent with the proposed role of ABCA4 as the transporter of all-trans-retinal and/or N-retinylidene-PE. Thus, these animals exhibited elevated levels of PE and N-retinylidene-PE in the retina (Mata et al. 2000; Weng et al. 1999). Moreover, A2E, the ultimate product of condensation of all-trans-retinal and N-retinylidene-PE (Fig. 6), and its potentially toxic photoreactive products accumulated in the cells of RPE, accompanied by formation of lipofuscin pigment granules (Mata et al. 2000, 2001; Radu et al. 2004). Importantly, accumulation of A2E and its precursors was found to be strongly light dependent, as Abca4−/− mice raised in total darkness did not exhibit these compounds (Mata et al. 2000).
Fig. 6.
Formation of A2E, a potentially harmful side product of the visual cycle. In disks of photoreceptor outer segments, all-trans-retinal can spontaneously and reversibly react with PE to form N-retinylidene-PE. N-Retinylidene-PE can condense with a second molecule of all-trans-retinal, which, after rearrangement and oxidation, leads to formation of di-retinoid-pyridinium-phosphati-dylethanolamine (A2PE). Under acidic conditions in phagolysosomes, A2PE is hydrolyzed to yield di-retinoid-pyridinium-ethanolamine (A2E) and phosphatidic acid (PA)
5.4 Proposed Role of ABCA4 in the Visual Cycle
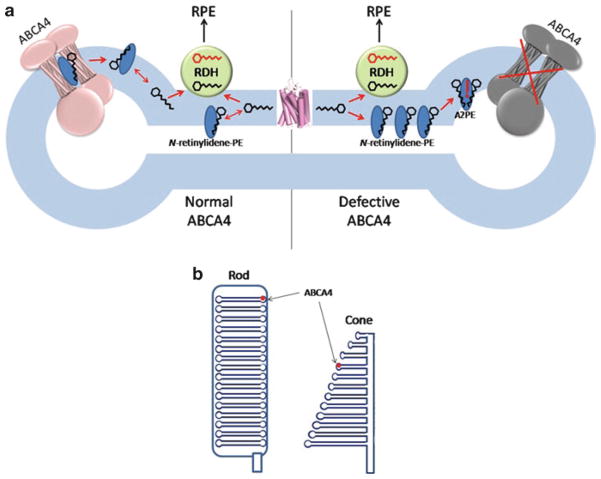
The results obtained from biochemical studies, characterization of Abca4−/− mice, and analyses of patients with Stargardt disease have allowed the creation of a conceptual scheme delineating the possible role of ABCA4 in rod cells (Fig. 7a) (Molday 2007; Molday et al. 2009). Absorption of light by rhodopsin leads to isomerization of 11-cis-retinal to all-trans-retinal, which is then released into the disk membrane. The following step in the visual cycle is reduction of all-trans-retinal to all-trans-retinol by all-trans-retinol dehydrogenase. All-trans-retinol dehydrogenase 8 (RDH8), which is located outside of the disk, is responsible for the majority of this activity (Maeda et al. 2007). It has been suggested that ABCA4 may accelerate the clearance of all-trans-retinal by translocating it from the luminal to the cytoplasmic side of the disk membrane, but it is not clear if such a hydrophobic substance needs assistance in crossing this lipid bilayer, since it has been shown that retinoids undergo rapid spontaneous transfer between liposomes as well as ROS (Ho et al. 1989; Rando and Bangerter 1982). In addition, ABCA4-mediated transport is relatively slow, with the highest published Vmax value for ATP hydrolysis being 673 nmol/min/mg (Ahn et al. 2000), equivalent to ~3 enzymatic cycles per second. This may be inadequate for efficient all-trans-retinal clearance after strong photobleaching. Moreover, given its hydrophobicity and lack of electric charge, all-trans-retinal will probably tend to return to the central part of the membrane unless it is directly passed to RDH8. A much more attractive substrate for ABCA4 is N-retinylidene-PE (Fig. 6). This reversible adduct of all-trans-retinal and PE forms spontaneously and cannot cross the membrane by itself. It has been shown that after a 45% photobleach, about 24% of all-trans-retinal is present in the form of N-retinylidene-PE in wild-type mouse retinas (Mata et al. 2000), whereas in dark-adapted wild-type retinas this fraction reaches nearly 100% (Weng et al. 1999). Hence, the role of ABCA4 could be to flip N-retinylidene-PE to the cytoplasmic side of the disk membrane, where it can dissociate and allow all-trans-retinal to reenter the visual cycle. By preventing accumulation of N-retinylidene-PE inside the disk, ABCA4 also would reduce the reaction of N-retinylidene-PE with the second molecule of all-trans-retinal, leading to formation of di-retinoid-pyridinium-phosphatidylethanolamine (A2PE) (Eldred and Lasky 1993; Mata et al. 2000). Aged ROS disks are continuously shed and undergo phagocytosis and degradation in phagolysosomes of adjacent RPE cells. In acidic phagolysosomes, A2PE is hydrolyzed to yield A2E (Fig. 6), a major component of lipofuscin that cannot be metabolized further. Because of this, patients with impaired ABCA4 activity progressively accumulate large quantities of A2E in the RPE. This accumulation proceeds faster in the macular region of the retina because of high concentrations of cones in the fovea and rods in the parafovea belt. A2E can have several negative effects on RPE cells, including generation of reactive oxygen species (Jang et al. 2005; Radu et al. 2004), impairment of lysosomal degradative functions (Holz et al. 1999), and by acting at high concentrations as a cationic detergent to perturb biological membranes (Eldred and Lasky 1993). Death of RPE cells leads to the loss of photoreceptors and, therefore, decreased central vision.
Fig. 7.
(a) Illustration of the proposed biological role of ABCA4. The left part of the diagram represents a ROS disk with a functional ABCA4, whereas the right part represents a ROS disk with an inactive ABCA4. Hexagons with carbon backbone denote all-trans-retinal (black) and all-trans-retinol (red). RDH: all-trans-retinol dehydrogenase 8. RPE: retinal pigment epithelium. See text for the description of the mechanism. (b) Schematic representations of rod and cone outer segments. Cone disks are open structures
5.5 Unresolved Issues
The above described scheme constitutes the best model for the function of ABCA4 available to date. However, several unresolved issues indicate that the biological role of this protein may be more complex and that the current transport model may lack important steps. In particular, it is known that N-retinylidene-PE is unstable and exists in equilibrium with all-trans-retinal and PE (Ahn et al. 2000). The suggested scheme assumes that RDH8, the main all-trans-retinol dehydrogenase of the photoreceptor outer segment, quickly reduces all-trans-retinal to all-trans-retinol on the cytoplasmic side of the disk membrane, thereby shifting the equilibrium towards dissociation of N-retinylidene-PE. However, the RDH8-mediated reduction of all-trans-retinal is unexpectedly slow, and likely to be effective in clearing all-trans-retinal only at high illumination intensities (Palczewski et al. 1994). If this observation is taken into account, then considerable amounts of A2PE may well form on the outer side of disks.
Another concern arises from important morphological differences between rods and cones (Fig. 7b). Unlike closed ROS disks surrounded by a plasma membrane, cone disks are open structures with contiguous intradiscal and extracellular spaces (Mustafi et al. 2009). It has been shown by electron microscopy that ABCA4 (then known as “a large integral membrane protein”) is present in the margins of cone disks (Papermaster et al. 1982). Given that NBDs of an ABC transporter must be located inside the cell and assuming that the function of ABCA4 is the same in all types of photoreceptors, it follows that in cones it should transport N-retinylidene-PE from outside of the cell into the cytoplasm. The visual cycle in cones is poorly understood, and the rationale for such a translocation needs to be clarified. One explanation is that in cones N-retinylidene-PE forms on the extracellular side of the membrane and needs to be transported inside the cell for detoxification.
As of now, the function of ABCA4 in the brain is unknown. It has been speculated that it may have an impact on retinoid modulation of central nervous system function (Kim et al. 2008), but it should be noted that, except for in the eye, retinoids are usually present in tissues as retinyl esters, retinol and retinoic acid rather than as retinal. However, retinol and retinoic acid were found to be poor substrates for ABCA4 (Sun et al. 1999).
6 ABCA4 Mutations and Autosomal Recessive Macular Degeneration
Disease-associated alleles of Abca4 are unusually heterogeneous, with about 400 mutations described so far, of which most represent missense substitutions (Allikmets 2000; Lewis et al. 1999). In addition, the most frequent alleles are found in less than 20% of patients, making the search for correlations between individual mutations and disease severity more difficult. Moreover, establishing a reliable method for estimating disease severity represents an additional challenge (Cideciyan et al. 2009).
Mutations are evenly distributed throughout the primary structure of ABCA4. Several studies focused on investigating the effect of amino acid substitutions and deletions on activity of the full-length protein (Sun et al. 2000; Zhong et al. 2009) and its individual cytoplasmic domains (Biswas and Biswas 2000; Biswas-Fiss 2003, 2006; Suarez et al. 2002). Some of these substitutions were found to have a pronounced effect on the ATPase activity of the NBDs (Biswas and Biswas 2000; Biswas-Fiss 2003, 2006; Suarez et al. 2002; Sun et al. 2000), whereas others resulted in reduced expression levels (Sun et al. 2000; Zhong et al. 2009). Establishing the effects of many mutations is currently impossible because of the lack of a suitable transport assay, since only those mutations that alter ATPase activity can be described.
In light of ABCA4 involvement in several visual disorders, a model was proposed in which the severity of the disease in any given individual is inversely correlated with the residual activity of the mutant ABCA4 proteins (Shroyer et al. 1999; van Driel et al. 1998). Recent studies have shown that the residual function hypothesis is oversimplified. In particular, a study in transgenic frogs revealed that some of the mutants retained ABCA4 in the inner segments of their photoreceptors, indicating that protein mislocalization can contribute to the severity of the disease (Wiszniewski et al. 2005). Furthermore, it was recently demonstrated by statistical analysis of data collected on a large cohort of patients over a long period of time that several individuals with two missense or splicing mutations developed much more severe phenotypes than those with two truncating mutations (Cideciyan et al. 2009). This result clearly contradicts the residual function model, according to which truncations represent the most detrimental mutations because they prevent protein expression. Therefore, models involving genotype-phenotype correlations should account for the negative effects of ABCA4 misfolding and mislocalization along with reduced activity.
7 Conclusions
ABCA4 is a member of the superfamily of ATP-binding cassette transporters expressed primarily in vertebrate photoreceptors, where it localizes to the rims of outer membrane disks (rods) and evaginations (cones). A combined effort including biochemical, clinical and animal model studies has highlighted its role in clearance of all-trans-retinal from the disk membranes after photoexcitation of rhodopsin. The most probable substrate of ABCA4 is N-retinylidene-PE, a product of the reaction of all-trans-retinal with phosphatidylethanolamine. If not removed from disk membranes, N-retinylidene-PE can further react with a second molecule of all-trans-retinal to form potentially harmful diretinal compounds. During the process of disk shedding, these compounds, of which the best studied is A2E, accumulate in the cells of RPE, which ultimately leads to RPE cell death and concomitant degeneration of photoreceptors.
Despite impressive progress that has been achieved in understanding the function of ABCA4 in vision, a number of important problems remain. Creation of a transport assay is critical for verification of the proposed substrates and determination of the direction of transport for ABCA4. The role of ABCA4 in brain awaits resolution. Finally, biochemical and structural studies should be undertaken to gain insights into the mechanism of ABCA4-mediated substrate translocation and its regulation.
Acknowledgments
This work was supported by the National Institutes of Health (NIH grants EY09339, P30 EY11373, and EY08123). We thank members of the Palczewski laboratory for their helpful comments.
References
- Ahn J, Molday RS. Purification and characterization of ABCR from bovine rod outer segments. Methods Enzymol. 2000;315:864–879. doi: 10.1016/s0076-6879(00)15887-2. [DOI] [PubMed] [Google Scholar]
- Ahn J, Wong JT, Molday RS. The effect of lipid environment and retinoids on the ATPase activity of ABCR, the photoreceptor ABC transporter responsible for Stargardt macular dystrophy. J Biol Chem. 2000;275:20399–20405. doi: 10.1074/jbc.M000555200. [DOI] [PubMed] [Google Scholar]
- Ahn J, Beharry S, Molday LL, Molday RS. Functional interaction between the two halves of the photoreceptor-specific ATP binding cassette protein ABCR (ABCA4). Evidence for a non-exchangeable ADP in the first nucleotide binding domain. J Biol Chem. 2003;278:39600–39608. doi: 10.1074/jbc.M304236200. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K, Sasaki R, Sawamura D, Shimizu H. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R. Simple and complex ABCR: genetic predisposition to retinal disease. Am J Hum Genet. 2000;67:793–799. doi: 10.1086/303100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997a;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997b;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- Azarian SM, Travis GH. The photoreceptor rim protein is an ABC transporter encoded by the gene for recessive Stargardt’s disease (ABCR) FEBS Lett. 1997;409:247–252. doi: 10.1016/s0014-5793(97)00517-6. [DOI] [PubMed] [Google Scholar]
- Beharry S, Zhong M, Molday RS. N-retinylidene-phosphatidylethanolamine is the preferred retinoid substrate for the photoreceptor-specific ABC transporter ABCA4 (ABCR) J Biol Chem. 2004;279:53972–53979. doi: 10.1074/jbc.M405216200. [DOI] [PubMed] [Google Scholar]
- Bhongsatiern J, Ohtsuki S, Tachikawa M, Hori S, Terasaki T. Retinal-specific ATP-binding cassette transporter (ABCR/ABCA4) is expressed at the choroid plexus in rat brain. J Neurochem. 2005;92:1277–1280. doi: 10.1111/j.1471-4159.2004.02941.x. [DOI] [PubMed] [Google Scholar]
- Biswas EE. Nucleotide binding domain 1 of the human retinal ABC transporter functions as a general ribonucleotidase. Biochemistry. 2001;40:8181–8187. doi: 10.1021/bi0106686. [DOI] [PubMed] [Google Scholar]
- Biswas EE, Biswas SB. The C-terminal nucleotide binding domain of the human retinal ABCR protein is an adenosine triphosphatase. Biochemistry. 2000;39:15879–15886. doi: 10.1021/bi0015966. [DOI] [PubMed] [Google Scholar]
- Biswas-Fiss EE. Functional analysis of genetic mutations in nucleotide binding domain 2 of the human retina specific ABC transporter. Biochemistry. 2003;42:10683–10696. doi: 10.1021/bi034481l. [DOI] [PubMed] [Google Scholar]
- Biswas-Fiss EE. Interaction of the nucleotide binding domains and regulation of the ATPase activity of the human retina specific ABC transporter, ABCR. Biochemistry. 2006;45:3813–3823. doi: 10.1021/bi052059u. [DOI] [PubMed] [Google Scholar]
- Bungert S, Molday LL, Molday RS. Membrane topology of the ATP binding cassette transporter ABCR and its relationship to ABC1 and related ABCA transporters: identification of N-linked glycosylation sites. J Biol Chem. 2001;276:23539–23546. doi: 10.1074/jbc.M101902200. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Swider M, Aleman TS, Tsybovsky Y, Schwartz SB, Windsor EA, Roman AJ, Sumaroka A, Steinberg JD, Jacobson SG, Stone EM, Palczewski K. ABCA4 disease progression and a proposed strategy for gene therapy. Hum Mol Genet. 2009;18:931–941. doi: 10.1093/hmg/ddn421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Annu Rev Biochem. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- Dawson RJ, Hollenstein K, Locher KP. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol Microbiol. 2007;65:250–257. doi: 10.1111/j.1365-2958.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald ML, Morris AL, Rhee JS, Andersson LP, Mendez AJ, Freeman MW. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J Biol Chem. 2002;277:33178–33187. doi: 10.1074/jbc.M204996200. [DOI] [PubMed] [Google Scholar]
- Gerber S, Comellas-Bigler M, Goetz BA, Locher KP. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science. 2008;321:246–250. doi: 10.1126/science.1156213. [DOI] [PubMed] [Google Scholar]
- Hamel CP. Cone rod dystrophies. Orphanet J Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz Y, Gerstein M, Chothia C. Volume changes on protein folding. Structure. 1994;2:641–649. doi: 10.1016/s0969-2126(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Ho MT, Massey JB, Pownall HJ, Anderson RE, Hollyfield JG. Mechanism of vitamin A movement between rod outer segments, interphotoreceptor retinoid-binding protein, and liposomes. J Biol Chem. 1989;264:928–935. [PubMed] [Google Scholar]
- Holz FG, Schutt F, Kopitz J, Eldred GE, Kruse FE, Volcker HE, Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272:10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J Biol Chem. 2005;280:39732–39739. doi: 10.1074/jbc.M504933200. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Endo H, Kobayashi H, Takio K, Igarashi K. Spermidine-preferential uptake system in Escherichia coli. ATP hydrolysis by PotA protein and its association with membrane. J Biol Chem. 1995;270:25377–25382. doi: 10.1074/jbc.270.43.25377. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Weickert CS, Garner B. Role of ATP-binding cassette transporters in brain lipid transport and neurological disease. J Neurochem. 2008;104:1145–1166. doi: 10.1111/j.1471-4159.2007.05099.x. [DOI] [PubMed] [Google Scholar]
- Kolling R, Losko S. The linker region of the ABC-transporter Ste6 mediates ubiquitination and fast turnover of the protein. EMBO J. 1997;16:2251–2261. doi: 10.1093/emboj/16.9.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos V, Ford RC. The ATP-binding cassette family: a structural perspective. Cell Mol Life Sci. 2009;66:3111–3126. doi: 10.1007/s00018-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, Lupski JR, Leppert M, Dean M. Genotype/Phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR, in Stargardt disease. Am J Hum Genet. 1999;64:422–434. doi: 10.1086/302251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton KJ, Higgins CF. Structure and function of ABC transporters: the ATP switch provides flexible control. Pflugers Arch. 2007;453:555–567. doi: 10.1007/s00424-006-0126-x. [DOI] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci USA. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Golczak M, Maeda T, Palczewski K. Limited roles of Rdh8, Rdh12 and Abca4 on all-trans-retinal clearance in mouse retina. Invest Ophthalmol Vis Sci. 2009a;50(11):5435–5443. doi: 10.1167/iovs.09-3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009b;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Maeda A, Matosky M, Okano K, Roos S, Tang J, Palczewski K. Evaluation of potential therapies for a mouse model of human age-related macular degeneration caused by delayed all-trans-retinal clearance. Invest Ophthalmol Vis Sci. 2009c;50:4917–4925. doi: 10.1167/iovs.09-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LO, Agerholm-Larsen B, Wang N, Chen W, Tall AR. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzalez-Duarte R, Balcells S. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- Mata NL, Weng J, Travis GH. Biosynthesis of a major lipofuscin fluorophore in mice and humans with ABCR-mediated retinal and macular degeneration. Proc Natl Acad Sci USA. 2000;97:7154–7159. doi: 10.1073/pnas.130110497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata NL, Tzekov RT, Liu X, Weng J, Birch DG, Travis GH. Delayed dark-adaptation and lipofuscin accumulation in abcr+/− mice: implications for involvement of ABCR in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1685–1690. [PubMed] [Google Scholar]
- Molday RS. ATP-binding cassette transporter ABCA4: molecular properties and role in vision and macular degeneration. J Bioenerg Biomembr. 2007;39:507–517. doi: 10.1007/s10863-007-9118-6. [DOI] [PubMed] [Google Scholar]
- Molday RS, Molday LL. Identification and characterization of multiple forms of rhodopsin and minor proteins in frog and bovine rod outer segment disc membranes. Electrophoresis, lectin labeling, and proteolysis studies. J Biol Chem. 1979;254:4653–4660. [PubMed] [Google Scholar]
- Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791:573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourez M, Hofnung M, Dassa E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 1997;16:3066–3077. doi: 10.1093/emboj/16.11.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Engel AH, Palczewski K. Structure of cone photoreceptors. Prog Retin Eye Res. 2009;28:289–302. doi: 10.1016/j.preteyeres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe J, Hagenbuch B, Meier PJ, St-Pierre MV. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology. 2001;33:1223–1231. doi: 10.1053/jhep.2001.24171. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Jager S, Buczylko J, Crouch RK, Bredberg DL, Hofmann KP, Asson-Batres MA, Saari JC. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Zorn MA, Kraehenbuhl JP. Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol. 1978;78:415–425. doi: 10.1083/jcb.78.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster DS, Reilly P, Schneider BG. Cone lamellae and red and green rod outer segment disks contain a large intrinsic membrane protein on their margins: an ultrastructural immunocytochemical study of frog retinas. Vision Res. 1982;22:1417–1428. doi: 10.1016/0042-6989(82)90204-8. [DOI] [PubMed] [Google Scholar]
- Paskowitz DM, LaVail MM, Duncan JL. Light and inherited retinal degeneration. Br J Ophthalmol. 2006;90:1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar AS, Qtaishat NM, Little DM, Pepperberg DR. Recovery of rod photoresponses in ABCR-deficient mice. Invest Ophthalmol Vis Sci. 2008;49:2743–2755. doi: 10.1167/iovs.07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kale L, Schulten K. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Mata NL, Bagla A, Travis GH. Light exposure stimulates formation of A2E oxiranes in a mouse model of Stargardt’s macular degeneration. Proc Natl Acad Sci USA. 2004;101:5928–5933. doi: 10.1073/pnas.0308302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR, Bangerter FW. The rapid intermembraneous transfer of retinoids. Biochem Biophys Res Commun. 1982;104:430–436. doi: 10.1016/0006-291x(82)90655-6. [DOI] [PubMed] [Google Scholar]
- Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: integral membrane components. J Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozet JM, Gerber S, Souied E, Perrault I, Chatelin S, Ghazi I, Leowski C, Dufier JL, Munnich A, Kaplan J. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet. 1998;6:291–295. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Postel EA, Agarwal A, Allen IC, Jr, Walters SN, De la Paz MA, Scott WK, Haines JL, Pericak-Vance MA, Gilbert JR. Detailed analysis of allelic variation in the ABCA4 gene in age-related maculopathy. Invest Ophthalmol Vis Sci. 2003;44:2868–2875. doi: 10.1167/iovs.02-0957. [DOI] [PubMed] [Google Scholar]
- See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J Biol Chem. 2002;277:41835–41842. doi: 10.1074/jbc.M204923200. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Lewis RA, Allikmets R, Singh N, Dean M, Leppert M, Lupski JR. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res. 1999;39:2537–2544. doi: 10.1016/s0042-6989(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans-retinal: the making of RPE bisretinoids. J Lipid Res. 2009;51(2):247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez T, Biswas SB, Biswas EE. Biochemical defects in retina-specific human ATP binding cassette transporter nucleotide binding domain 1 mutants associated with macular degeneration. J Biol Chem. 2002;277:21759–21767. doi: 10.1074/jbc.M202053200. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Focus on molecules: ABCA4 (ABCR) – an import-directed photoreceptor retinoid flipase. Exp Eye Res. 2009;89:602–603. doi: 10.1016/j.exer.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Nathans J. Stargardt’s ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet. 1997;17:15–16. doi: 10.1038/ng0997-15. [DOI] [PubMed] [Google Scholar]
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem. 1999;274:8269–8281. doi: 10.1074/jbc.274.12.8269. [DOI] [PubMed] [Google Scholar]
- Sun H, Smallwood PM, Nathans J. Biochemical defects in ABCR protein variants associated with human retinopathies. Nat Genet. 2000;26:242–246. doi: 10.1038/79994. [DOI] [PubMed] [Google Scholar]
- Szuts EZ. Light stimulates phosphorylation of two large membrane proteins in frog photoreceptors. Biochemistry. 1985;24:4176–4184. doi: 10.1021/bi00336a054. [DOI] [PubMed] [Google Scholar]
- Tachikawa M, Watanabe M, Hori S, Fukaya M, Ohtsuki S, Asashima T, Terasaki T. Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem. 2005;95:294–304. doi: 10.1111/j.1471-4159.2005.03369.x. [DOI] [PubMed] [Google Scholar]
- Trompier D, Alibert M, Davanture S, Hamon Y, Pierres M, Chimini G. Transition from dimers to higher oligomeric forms occurs during the ATPase cycle of the ABCA1 transporter. J Biol Chem. 2006;281:20283–20290. doi: 10.1074/jbc.M601072200. [DOI] [PubMed] [Google Scholar]
- van Driel MA, Maugeri A, Klevering BJ, Hoyng CB, Cremers FP. ABCR unites what ophthalmologists divide(s) Ophthalmic Genet. 1998;19:117–122. doi: 10.1076/opge.19.3.117.2187. [DOI] [PubMed] [Google Scholar]
- Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3:281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walia S, Fishman GA. Natural history of phenotypic changes in Stargardt macular dystrophy. Ophthalmic Genet. 2009;30:63–68. doi: 10.1080/13816810802695550. [DOI] [PubMed] [Google Scholar]
- Warren MS, Zerangue N, Woodford K, Roberts LM, Tate EH, Feng B, Li C, Feuerstein TJ, Gibbs J, Smith B, de Morais SM, Dower WJ, Koller KJ. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59:404–413. doi: 10.1016/j.phrs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Weleber RG. Stargardt’s macular dystrophy. Arch Ophthalmol. 1994;112:752–754. doi: 10.1001/archopht.1994.01090180050033. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Wiszniewski W, Zaremba CM, Yatsenko AN, Jamrich M, Wensel TG, Lewis RA, Lupski JR. ABCA4 mutations causing mislocalization are found frequently in patients with severe retinal dystrophies. Hum Mol Genet. 2005;14:2769–2778. doi: 10.1093/hmg/ddi310. [DOI] [PubMed] [Google Scholar]
- Zarubica A, Trompier D, Chimini G. ABCA1, from pathology to membrane function. Pflugers Arch. 2007;453:569–579. doi: 10.1007/s00424-006-0108-z. [DOI] [PubMed] [Google Scholar]
- Zhong M, Molday LL, Molday RS. Role of the C terminus of the photoreceptor ABCA4 transporter in protein folding, function, and retinal degenerative diseases. J Biol Chem. 2009;284:3640–3649. doi: 10.1074/jbc.M806580200. [DOI] [PMC free article] [PubMed] [Google Scholar]