Abstract
Objective
To estimate rates of completion of CD4+ lymphocyte testing (CD4 testing) within 12 weeks of testing positive for human immunodeficiency virus (HIV) at a large HIV/AIDS clinic in South Africa, and to identify clinical and demographic predictors for completion.
Methods
In our study, CD4 testing was considered complete once a patient had retrieved the test results. To determine the rate of CD4 testing completion, we reviewed the records of all clinic patients who tested positive for HIV between January 2008 and February 2009. We identified predictors for completion through multivariate logistic regression.
Findings
Of the 416 patients who tested positive for HIV, 84.6% initiated CD4 testing within the study timeframe. Of these patients, 54.3% were immediately eligible for antiretroviral therapy (ART) because of a CD4 cell count ≤ 200/µl, but only 51.3% of the patients in this category completed CD4 testing within 12 weeks of HIV testing. Among those not immediately eligible for ART (CD4 cells > 200/µl), only 14.9% completed CD4 testing within 12 weeks. Overall, of HIV+ patients who initiated CD4 testing, 65% did not complete it within 12 weeks of diagnosis. The higher the baseline CD4 cell count, the lower the odds of completing CD4 testing within 12 weeks.
Conclusion
Patient losses between HIV testing, baseline CD4 cell count and the start of care and ART are high. As a result, many patients receive ART too late. Health information systems that link testing programmes with care and treatment programmes are needed.
ملخص
الغرض
تقدير معدلات اكتمال اختبار الخلايا اللمفاوية CD4+ (اختبار CD4+) خلال 12 أسبوعاً بعد اكتشاف إيجابية اختبار الإصابة بفيروس العوز المناعي البشري (فيروس الأيدز) في عيادة كبرى للأيدز والعدوى بفيروسه في جنوب أفريقيا، وتحديد المنبئات السريرية والديموغرافية لإكمال الاختبار.
الطريقة
اعتُبر في هذه الدراسة أن اختبار CD4+ أصبح مكتملاً فور تلقي المريض نتائج الاختبار. ولتحديد معدل اكتمال الاختبار، راجع الباحثون سجلات جميع المرضى في العيادة الذين تبين إيجابيتهم لاختبار فيروس الأيدز بين كانون الأول/يناير 2008 حتى شباط/فبراير 2009. وحدد الباحثون المنبئات لاكتمال الاختبار عن طريق تحوف لوجستي متعدد المتغيرات.
الموجودات
من 416 مريضاً كان اختبار فيروس الأيدز لديهم إيجابياً، بدأ 84.6% منهم إجراء اختبار CD4+ خلال الحدود الزمنية للدراسة. ومن هؤلاء المرضى، كان 54.3% مؤهلين على الفور لتلقي العلاج بمضادات الفيروسات القهقرية لأن عدد خلايا CD4+ كان أقل من أو يساوي 200 لكل مكرولتر، ولكن 51.3% فقط من المرضى في هذه الفئة أكملوا اختبار CD4+ خلال 12 أسبوعاً من ظهور نتائج اختبار فيروس الأيدز. ومن بين غير المؤهلين على الفور للعلاج بمضادات الفيروسات القهقرية (لأن عدد خلايا CD4+ لديهم كان أكبر من 200 لكل مكرولتر)، أكمل 14.9% فقط اختبار CD4+ خلال 12 أسبوعاً. وإجمالياً، من المرضى الإيجابيين لفيروس الأيدز الذين بدؤوا اختبار CD4+ ، لم يكمل 65% منهم الاختبار خلال 12 أسبوعاً من التشخيص. كلما ارتفع الخط القاعدي لعدد خلايا CD4+، قلت فرص إكمال اختبار CD4+ خلال 12 أسبوعاً.
الاستنتاج
هناك فقدان مرتفع للمرضى بين اختبار فيروس الأيدز، والخط القاعدي لعدد خلايا CD4+ وبدء الرعاية والمعالجة بمضادات الفيروسات القهقرية. ونتيجة لذلك، يتأخر كثير من المرضى في تلقي المعالجة بمضادات الفيروسات. وهناك حاجة لنظم معلومات صحية تربط بين برامج إجراء الاختبار وبرامج الرعاية والمعالجة.
Résumé
Objectif
Estimer les taux de réalisation du test de lymphocytes CD4+ (test de CD4) dans les 12 semaines qui suivent un test positif au virus de l'immunodéficience humaine (VIH) dans une grande clinique VIH/SIDA d’Afrique du Sud, et identifier des variables cliniques et démographiques explicatives de cette réalisation.
Méthodes
Dans notre étude, le test de CD4 est considéré comme achevé lorsqu’un patient retire les résultats de l'analyse. Afin de déterminer le taux de réalisation du test de CD4, nous avons examiné les dossiers cliniques de l’ensemble des patients ayant eu un test positif au VIH entre janvier 2008 et février 2009. Nous avons identifié des variables explicatives de la réalisation par une régression logistique multivariable.
Résultats
Sur 416 patients testés positifs au VIH, 84,6% ont commencé un test de CD4 au cours de la période d’étude. Parmi ces patients, 54,3% ont été immédiatement éligibles pour une thérapie antirétrovirale (TARV) du fait d’une numération CD4 ≤ 200 cellules/µl, mais seulement 51,3% des patients dans cette catégorie ont achevé un test CD4 dans les 12 semaines qui ont suivi le test VIH. Parmi ceux non immédiatement éligibles au TARV (CD4 > 200 cellules/µl), 14,9% seulement ont achevé un test de CD4 dans les 12 semaines. Globalement, parmi les patients VIH+ ayant commencé un test de CD4, 65% ne l'ont pas achevé dans les 12 semaines qui ont suivi le diagnostic. Plus la numération cellulaire CD4 de base a été élevée, moins les chances d'achever un test de CD4 dans les 12 semaines ont été importantes.
Conclusion
Les pertes de patients entre le test VIH, la numération cellulaire CD4 de base et le début des soins et du TARV sont élevées. En conséquence, beaucoup de patients reçoivent le TARV trop tard. Des systèmes de renseignements sur la santé mettant en relation les programmes de test avec les programmes de soins et de traitement sont nécessaires.
Resumen
Objetivos
Calcular las tasas de finalización del análisis de linfocitos CD4 (análisis de CD4) en las 12 semanas tras las pruebas positivas para el virus de la inmunodeficiencia humana (VIH) en una importante clínica de tratamiento del VIH/SIDA en Sudáfrica, e identificar las variables independientes clínicas y demográficas para su finalización.
Métodos
En nuestro estudio, el análisis de CD4 se dio por finalizado una vez que el paciente estuvo en posesión de los resultados de la prueba. Para determinar la tasa de finalización del análisis de CD4 se revisaron las historias clínicas de todos los pacientes que dieron positivo en las pruebas del VIH entre enero de 2008 y febrero de 2009. Las variables independientes de finalización se identificaron mediante una regresión logística multivariable.
Resultados
De los 416 pacientes que dieron positivo en la prueba del VIH, el 84,6% inició los análisis de CD4 en el periodo de tiempo del estudio. De estos pacientes, el 54,3% fue apto de forma inmediata para el tratamiento antirretroviral (TAR) porque el recuento de linfocitos CD4 era ≤ 200/μl, si bien únicamente el 51,3% de los pacientes de esta categoría finalizó el análisis de CD4 en las 12 semanas siguientes a la prueba del VIH. De los pacientes que no fueron inmediatamente aptos para el TAR (linfocitos CD4 > 200/μl), sólo el 14,9% finalizó el análisis de CD4 en 12 semanas. En términos generales, el 65% de los pacientes VIH+ que iniciaron el análisis de CD4 no lo finalizó en las 12 semanas siguientes al diagnóstico. Cuanto mayor fue el recuento inicial de linfocitos CD4, menor fue la probabilidad de finalizar los análisis de CD4 en las 12 semanas.
Conclusión
La pérdida de pacientes entre las pruebas del VIH, el recuento inicial de linfocitos CD4 y el inicio de la asistencia y del TAR es elevada, por lo que muchos pacientes reciben el tratamiento antirretroviral demasiado tarde. Se requieren sistemas de información sanitaria que enlacen los programas de análisis con los programas de atención y tratamiento.
Introduction
Although access to antiretroviral therapy (ART) for human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) in South Africa has increased dramatically since 2004, the majority of patients with HIV infection initiate care and treatment with very low CD4+ lymphocyte (CD4 cell) counts, often after they are already symptomatic and long after they are first eligible for ART. During 2009 in South Africa, eligibility for ART was determined by a CD4 cell count ≤ 200 cells/μl or by World Health Organization (WHO) Stage IV condition.1 A recent analysis in the Free State province showed that more than half of the patients enrolled in the public sector HIV/AIDS treatment programme began ART with a CD4 cell count < 100 cells/μl.2 Similarly, in an analysis at a large public-sector treatment facility in Johannesburg, 53% of a random sample of patients who started ART in 2005 had a baseline CD4 cell count < 100 cells/μl.3 More recent data from the same facility showed that the median CD4 cell count was 98 cells/μl for all patients who started therapy during 2007–2008, with 25% having a CD4 cell count < 34 cells/μl (A Brennan, unpublished data, 2009).
Starting care and treatment for HIV infection and AIDS in patients with a low CD4 cell count imposes unnecessary costs on patients and society.4 Costs to patients include additional morbidity and mortality from HIV- and AIDS-related illnesses and a worse prognosis after initiating ART. Numerous studies have shown that a low CD4 cell count (≤ 100 cells/μl) and WHO Stage IV at the start of treatment are major predictors of mortality.5–7 Immune system recovery after 3 years on ART is also positively correlated with a patient’s CD4 cell count when ART is begun.8 Access to care and treatment among patients with very low CD4 cell counts is associated with additional health care utilization costs to society through outpatient and inpatient services for opportunistic infections and AIDS-related illnesses. A portion of these costs could be avoided through earlier access to HIV care and treatment, since a substantial share of health care is paid for by the government.
For an HIV+ individual to initiate ART as early as eligibility criteria allow, a specific sequence of events must be completed, starting with HIV testing to diagnose the infection, a baseline CD4 cell count and enrolment in a care programme with regular CD4 cell monitoring, and ending with initiation of ART. In recent years access to HIV testing has increased dramatically, and a recent national survey found that more than half of all South African adults have been tested at least once.9 This expansion of testing, however, has not translated into earlier treatment initiation.5–7 One likely reason for this is that people who test positive do not always complete CD4 testing after their HIV test. Because of delays in the completion of CD4 testing, further delays occur in obtaining appropriate care and treatment.
CD4 testing involves two steps. First a blood sample is obtained from the patient at a clinic. In South Africa the blood sample is typically then sent to an external laboratory for analysis, and the results are usually available to the clinic in 1 week. For the purposes of the analysis in this article, CD4 testing was considered complete when a patient actually returned to the clinic and obtained the results. At combined voluntary HIV counselling and testing (VCT) and HIV/AIDS clinics, patients are typically tested for a baseline CD4 cell count on the day they test positive for HIV, although they may return for CD4 testing at a later date. When a stand-alone HIV testing centre does not perform CD4 cell counts (e.g. mobile VCT units and VCT provided through youth centres and social organizations), a patient with HIV infection must go to a referral clinic to initiate and complete CD4 testing.
A first step in solving the problem of post-VCT loss to initiation of appropriate HIV care and treatment is to obtain accurate estimates of the magnitude of the problem in public-sector facilities that serve most South African patients. Because patients who test positive for HIV infection at VCT programmes need to complete CD4 testing to enrol in an appropriate care or treatment programme, the primary objective of this study was to estimate the proportion of walk-in VCT patients who completed CD4 testing within 6 and 12 weeks of testing positive for HIV infection at a large, urban, public-sector, comprehensive HIV/AIDS clinic in South Africa.
Methods
Study site and population
Themba Lethu Clinic (TLC) is the comprehensive care management and treatment facility for HIV infection and AIDS at Helen Joseph Hospital, a large academic hospital in Johannesburg. This clinic was one of the first public-sector ART roll-out sites in South Africa and remains one of the largest treatment sites in the country. As of May 2009, more than 20 000 patients had initiated ART at the clinic. Services provided by TLC include VCT, CD4 cell testing, pre-ART care and wellness management, tuberculosis care and treatment, ART and adherence support. The clinic is funded by the Gauteng Department of Health with additional support from the United States President’s Emergency Plan for AIDS Relief and the United States Agency for International Development through a South African nongovernmental organization called Right to Care.
This study focused on individuals who are “walk-ins” to TLC for HIV testing. Walk-in patients are those who come to the clinic of their own accord and request an HIV test. Other patients who receive VCT services at the clinic are referred for HIV testing from hospital wards or by other clinics or private physicians because they are already symptomatic. These patients have already experienced delays in access to HIV care and therefore were not the focus of this study.
Walk-in VCT clients receive standard pretest counselling, have an HIV test and then receive post-test counselling that is differentiated according to their test result (positive or negative). If a patient tests positive and states an intention to receive care at TLC (rather than some other facility), the standard procedure is to initiate CD4 testing during the same visit. Patients are then counselled to return in 1 week to collect their CD4 cell count result. The blood sample is sent to a separate laboratory for processing and the CD4 cell count results are available to the clinical staff within 1 week. CD4 testing is considered completed when the patient returns to collect the results.
When patients complete CD4 cell testing, they are enrolled in the general HIV care programme (called the Wellness Program), CD4 cell count results are discussed and future care and treatment plans are reviewed with the patient. Care, wellness and monitoring are initiated if the baseline CD4 cell count is > 250 cells/μl. A similar care and wellness programme as well as a series of ART adherence training activities in preparation for the initiation of ART are started if the baseline CD4 cell count is ≤ 250/μl.
Data collection
We conducted a retrospective record review in June 2009 at TLC to identify all walk-in patients in the VCT programme who tested positive between 1 January 2008 and 28 February 2009. Censoring of the data set as of June 2009 provided a minimum of 12 weeks (1 March through 31 May 2009) for all subjects to have completed CD4 testing after the last date of HIV testing included in the study.
Patients included in the current analysis were identified through the VCT log books used to register patients. For each HIV-positive VCT patient we recorded testing date, sex, age, employment and marital status from the VCT log book, pretest and post-test counselling forms and ART programme medical records. The latter source was used to determine whether a patient initiated and completed CD4 testing after the HIV test date.
Data analysis
At the study site, CD4 cell counts are available within 1 week and patients are requested to return 1 week after their HIV test to collect their CD4 cell count results and complete CD4 cell testing. Waiting for 1, 2 or 3 weeks to complete CD4 testing may have few negative consequences for their health, but a delay of several months or more could involve substantial risks to patients who already have very low CD4 cell counts. To allow for an adequate period to complete CD4 cell testing, we conservatively defined our primary outcome as whether or not a person completed CD4 testing within 12 weeks (84 days) of testing positive for HIV infection. As a secondary analysis, we stratified this time interval into two categories: testing completed within 6 weeks (≤ 42 days) or between 6 and 12 weeks (43–84 days). We used these definitions to estimate the proportion of patients who completed CD4 testing within each period.
We compared baseline demographic characteristics (sex, age, marital status and employment status) of the group of patients who returned to collect their results versus those who did not return within 12 weeks by using simple proportions and medians, where appropriate. We also identified predictors of returning for CD4 cell count results with multivariate logistic regression. All statistical analyses were done with STATA software version 10.1 (STATA Corporation, College Station, United States of America).
The study was approved by the Human Research Ethics Committee (Medical) of the University of Witwatersrand and Institutional Review Board of Boston University’s Medical Campus.
Results
All HIV+ patients
A total of 416 walk-in patients tested positive for HIV at the study site during the study period. Each patient was identified as belonging to one of three groups. The first group included 27 patients (6.5%) who had previously tested positive for HIV infection and received CD4 testing at TLC or elsewhere. We had no data to explain why these 27 patients completed another HIV test, and the majority of these repeat testers (20 of 27) were already eligible for ART (CD4 cells ≤ 200/μl) after their previous HIV test. The second group was composed of 37 patients (8.9%) who did not initiate CD4 testing at TLC during the study period and for whom a baseline CD4 cell count was consequently not available. It is possible that some of these patients requested to go elsewhere for further HIV care. The third group, made up of 352 patients (84.6%), initiated CD4 testing at TLC on or after the day of HIV testing, and the remainder of this analysis focuses on this group.
Initiated CD4 cell testing
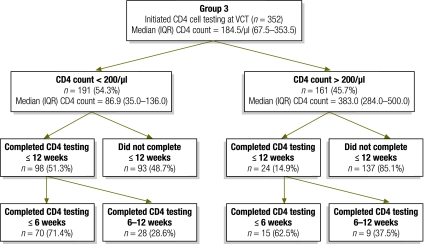
Patients who initiated CD4 testing on or after the day when they tested positive for HIV had a median CD4 cell count of 184.5/μl (interquartile range, IQR: 67.5–353.5). As shown in Fig. 1, 191 (54.3% of 352) had a CD4 cell count ≤ 200/μl and were already eligible for ART. The other 161 (45.7% of 352) had a CD4 cell count > 200/μl and were thus eligible for wellness counselling and pre-ART medical care.
Fig. 1.
Patients with high and low CD4+ lymphocyte counts who completed CD4 testing within 6 or 12 weeks of voluntary HIV counselling and testing, Johannesburg, South Africa, 2008–2009
HIV, human immunodeficiency virus; IQR, interquartile range; VCT, voluntary HIV counselling and testing.
Among the 191 patients eligible for ART at the time of HIV diagnosis, 51.3% completed CD4 testing within 12 weeks and the remaining 48.7% did not (Fig. 1). Among the 161 patients not eligible for ART at the time of HIV diagnosis (CD4 cells > 200/μl), only 14.9% completed CD4 testing within 12 weeks. Among all 352 patients, only 35% completed CD4 testing within 12 weeks of HIV testing.
Outcomes
There were no differences in basic demographic characteristics between those who returned for their CD4 cell count results within 6 weeks, those who returned within 12 weeks, and those who did not return (Table 1). Most patients were women; median age ranged between 36 and 40 years, less than one third were employed and approximately one half were married.
Table 1. Baseline characteristics of patients who tested positive for HIV infection and who did or did not complete CD4 testing within the 12 ensuing weeks, Johannesburg, South Africa, 2008–2009.
| Characteristic | Completed CD4 testing |
Did not complete CD4 testing within 12 weeks (n = 230) |
P-valuea |
|||||
|---|---|---|---|---|---|---|---|---|
| Within 6 weeks (n = 85) |
After 6–12 weeks (n = 122) |
|||||||
| Column A | Column B | Column C | A = B | B = C | A = C | |||
| Males, % | 37.6 | 27.0 | 36.8 | 0.26 | 0.25 | 0.90 | ||
| Median age in years (IQR) | 40.2 (32.3–46.2) | 39.8 (32.6–46.1) | 36.5 (31.2–43.8) | 0.84 | 0.58 | 0.24 | ||
| Married, % | 51.8 | 48.6 | 49.5 | 0.75 | 0.92 | 0.73 | ||
| Employed, % | 21.2 | 32.4 | 23.9 | 0.18 | 0.27 | 0.61 | ||
| Baseline CD4 cell count ≤ 200 cells/μl, % | 82.4 | 75.7 | 40.4 | 0.39 | < 0.01 | < 0.01 | ||
| Median baseline CD4 cell count, cells/μl (IQR) | 77 (34–170) | 163 (123–192) | 263 (103–430) | < 0.01 | < 0.01 | < 0.01 | ||
HIV, human immunodeficiency virus; IQR, interquartile range.
a P-value for test of equality of sample proportions or non-parametric two-sample test of the equality of medians for columns specified.
A substantial majority of patients who completed CD4 testing within 6 weeks (82.4%) or between 6 and 12 weeks (75.7%) after HIV testing had a baseline CD4 cell count ≤ 200 cells/μl. However, 40.4% of all patients who did not complete CD4 testing were also already eligible for ART based on their CD4 cell counts. The baseline median CD4 cell count of 77 cells/μl in patients who completed CD4 testing within 6 weeks was significantly lower (P < 0.01) than the median count of 163 cells/μl in patients who completed testing between 6 and 12 weeks. The median CD4 cell count of 263/μl in patients who did not complete testing within 12 weeks was significantly higher (P < 0.01) than in both of the groups who completed testing (Table 1).
Multivariable analysis (Table 2) showed that the baseline CD4 cell count was negatively associated with the odds of a patient completing CD4 testing. Compared to the group with the lowest baseline CD4 cell count (0–100 cells/μl), those whose baseline CD4 cell count was between 101 and 200 cells/μl had 0.41 times the odds of completing their tests within 6 weeks. Those with higher CD4 cell counts (201–300 cells /μl or > 300 cells/μl) had an even lower odds of completing testing. When we used the 12-week criterion, we found that the odds of patients with baseline CD4 cell counts from 100 to 199 cells/μl completing testing were similar to the odds for those in the lowest CD4 cell count category (< 100 cells/μl), whereas those in the higher categories continued to be substantially less likely to complete testing compared with those having the lowest CD4 cell counts.
Table 2. Predictors of completing CD4 testing in a cohort of walk-in patients who tested positive for HIV infection, Johannesburg, South Africa, 2008–2009.
| Predictor | Completed CD4 cell testing |
||||
|---|---|---|---|---|---|
| Within 6 weeks |
Within 12 weeks |
||||
| Adjusted ORa | 95% CI | Adjusted ORa | 95% CI | ||
| Male (versus female) | 1.03 | 0.58–1.83 | 0.78 | 0.45–1.31 | |
| Age (years) | 1.01b | 0.98–1.04 | 1.01b | 0.98–1.03 | |
| Employed (versus unemployed) | 0.94 | 0.55–1.60 | 0.91 | 0.56–1.49 | |
| Married (versus single) | 0.84 | 0.43–1.65 | 1.13 | 0.62–2.07 | |
| CD4 cell count (cells/μl) | |||||
| 0–100 (reference) | – | – | – | – | |
| 101–200 | 0.41 | 0.22–0.79 | 1.03 | 0.57–1.85 | |
| 201–300 | 0.28 | 0.12–0.63 | 0.43 | 0.21–0.88 | |
| > 300 | 0.07 | 0.03–0.17 | 0.08 | 0.04–0.18 | |
CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio.
a Adjusted ORs based on logistic regression model using all predictors shown.
b Change in the adjusted OR per 1-year increase in age.
Discussion
The UNAIDS/WHO policy statement on HIV testing from 2004 concludes that, “access to integrated prevention, treatment and care services” is a cornerstone of HIV testing scale-up.10 Nevertheless, scale-up of HIV testing since 2004 in South Africa has not resulted in earlier initiation of treatment.2,3,11 At the major urban, public care and treatment site in South Africa involved in this study, 54% of walk-in patients who tested positive for HIV infection were already eligible for ART on the day of testing (CD4 cells ≤ 200/μl), yet 48% of these patients did not complete CD4 testing within 12 weeks and therefore did not obtain appropriate medical care for their illness. Some of these individual are likely to have died, given that some patients who are known to be eligible for ART die before initiating therapy.12
To increase substantially the proportion of patients who initiate ART as soon as they are eligible, a large proportion of patients need to complete CD4 testing before they become eligible (i.e. while CD4 cells are still > 200/μl). Among such patients in the current study, however, 85% did not complete CD4 testing within 12 weeks of being diagnosed as HIV+. Under current conditions, these patients are likely to return for HIV care and treatment at a later date with substantially lower CD4 cell counts.
This study had several limitations. First, we had CD4 cell count data only from the site at which patients were tested for HIV. It is possible that some patients who did not complete CD4 testing at TLC went to another clinic, were tested again for HIV and then completed CD4 testing there. We had no way of tracking HIV+ clients of the VCT who were seen at other clinics. Second, our results are based on data from only one comprehensive HIV care and treatment facility in South Africa, albeit one of the largest HIV clinics in the country. The results show the experience at one site, and caution should be used in generalizing the results to populations of patients walking in for VCT services at other facilities. Finally, we have no information about the reasons why VCT clients did or did not complete CD4 testing.
The results in this study contribute to a growing literature that explores the problem of post-VCT loss to initiation of HIV/AIDS care and treatment in South Africa and other sub-Saharan countries.11,13–15 In a semiprivate clinic in Durban with a high prevalence of HIV infection (63% of all patients tested), about 53% of the patients with newly-diagnosed HIV infection did not complete CD4 testing within 8 weeks. In a small community outside Cape Town, 62% of patients who tested positive for HIV infection completed CD4 testing within 6 months of testing positive. Given the relatively long period for completion in this study (24 weeks) and that about 30% of these patients had a CD4 cell count < 200/μl at the time of HIV testing, it remains likely that some of these patients completed CD4 testing as part of their access to other medical care for AIDS-related conditions. An analysis of the data from two towns in central Mozambique showed that approximately half of all patients who tested positive for HIV in multiple testing centres did not initiate CD4 testing within 60 days of their HIV test.14 In a rural area in the northern part of the United Republic of Tanzania, where patients who tested positive for HIV at VCT sites were referred to another clinic in a nearby town about 20 km away, 72% of men and 66% of women completed an initial referral visit to the clinic within 6 months of their HIV test, although it is not known whether these patients actually completed CD4 cell testing.15
Significant improvements are needed to integrate HIV testing into the full continuum of HIV/AIDS care and treatment in South Africa. Before interventions can be implemented and evaluated, however, improved referral systems with a more structured process of patient tracking are needed in all stages of pre-ART medical care and monitoring (i.e. HIV testing, CD4 testing and enrolment in care to initiation of ART). The current system, which is based on separate systems to manage and monitor patients at the VCT and HIV care levels, constrains the monitoring and evaluation of linkages to HIV care.
Patients who test positive for HIV infection must overcome several barriers if they wish to complete CD4 cell testing. We suspect that these obstacles are similar to barriers to treatment adherence in general. Such barriers include (but are not limited to) transport costs and time, loss of earnings, lack of financial resources, stigma, lack of family support and perceived need when individuals are asymptomatic.16–22 Future research must look for ways to overcome these barriers, either directly or through other interventions, to improve post-VCT medical care.
Acknowledgements
The authors thank the staff and management of Themba Lethu Clinic for their assistance with understanding patient flows and patient file management. This analysis could not have been completed without their generous support. The authors thank Melinda Wilson of the United States Agency for International Development for her recognition of the importance of this topic, leadership in securing funding and advice to the study team. The authors also thank the journal reviewer for very helpful comments and suggestions. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the Themba Lethu Clinic, USAID, the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Funding:
Funding for this study was provided by the South Africa Mission of the United States Agency for International Development under the terms of Cooperative Agreement GHSA-00-00020-00, Country Research Activity (G ⁄PHN⁄HN⁄ CS). Matthew Fox was supported by Award Number K01AI083097 from the National Institute of Allergy and Infectious Diseases.
Competing interests:
None declared.
References
- 1.South African National Department of Health. HIV and AIDS and STI Strategic Plan for South Africa, 2007-2011 Johannesburg: Department of Health; 2007. Available from: http://www.doh.gov.za/docs/misc/stratplan-f.html [accessed 17 March 2010]. [Google Scholar]
- 2.Fairall LR, Bachmann M. Effectiveness of antiretroviral treatment in a South African program. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 3.Rosen S, Long L, Sanne I. The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Trop Med Int Health. 2008;13:1005–1015. doi: 10.1111/j.1365-3156.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Revised guidelines for HIV counseling, testing, and referral and revised recommendations for HIV screening of pregnant women. MMWR Morb Mortal Wkly Rep. 2001;50(No. RR-19):1–81. [PubMed] [Google Scholar]
- 5.Lawn SD, Little F, Bekker L-G, Kaplan R, Campbel E, Orrell C, Wood R. Changing mortality risk associated with CD4 cell response to antiretroviral therapy in South Africa. AIDS. 2009;23:335–42. doi: 10.1097/QAD.0b013e328321823f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartlett JG. Update on opt-out testing and initial antiretroviral therapy – CROI 2007 Medscape HIV/AIDS 2007. [Google Scholar]
- 8.Robbins G, Chan E, Spritzler J, Asmuth D, Gandhi R, Rodriguez B, et al. Effect of baseline CD4 cell count on immune reconstitution during combination antiretroviral therapy in ACTG 384. Presented at the: Fourth International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention, Sydney,Australia, 22–25 July2007 [Google Scholar]
- 9.Shisana O, Rehle T, Simbayi L, Zuma Z, Jooste J, Pillay-van-Wyk V, et al. South African national prevalence, incidence, behaviour and communication survey, 2008: a turning tide among teenagers? Cape Town: HSRC Press; 2009. [Google Scholar]
- 10.UNAIDS/WHO policy statement on HIV testing Geneva: United Nations Joint Programme on HIV/AIDS, World Health Organization; 2004.
- 11.April MD, Walensky RP, Chang Y, Pitt J, Freedberg KA, Losina E, et al. HIV Testing Rates and Outcomes in a South African Community, 2001-2006: Implications for Expanded Screening Policies. J Acquir Immune Defic Syndr. 2009;51:310–6. doi: 10.1097/QAI.0b013e3181a248e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassett IV, Wang B, Chetty S, Mazibuko M, Bearnot B, Giddy J, et al. Loss to Care and Death Before Antiretroviral Therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–9. doi: 10.1097/QAI.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losina E, Bassett IV, Giddy J, Chetty S, Regan S, Walensky RP, et al. The “ART” of linkage: early loss to follow up (LTFU) after HIV diagnosis at two PEPFAR sites in Durban, South Africa (Poster TUPE0345). Presented at the: XVIIth International AIDS Conference, Mexico City, Mexico, 3–8 August2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Micek MA, Gimbel-Sherr K, Baptista AJ, Matediana E, Montoya P, Pfeiffer J, et al. Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. JAIDS. 2009;52:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nsigaye R, Wringe A, Roura M, Kalluvya S, Urassa M, Busza J, et al. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009;12:31. doi: 10.1186/1758-2652-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones PS. On a never-ending waiting list: toward equitable access to anti-retroviral treatment? Experiences from Zambia. Health Hum Rights. 2005;8:76–102. doi: 10.2307/4065335. [DOI] [PubMed] [Google Scholar]
- 17.Horne R, Cooper V, Gellaitry G, Date H, Fisher M. Patients' perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45:334–41. doi: 10.1097/QAI.0b013e31806910e3. [DOI] [PubMed] [Google Scholar]
- 18.Kinsler JJ, Wong M, Sayles J, Davis C, Cunningham W. The effect of perceived stigma from a health care provider on access to care among a low-income HIV-positive population. AIDS Patient Care STDS. 2007;21:584–92. doi: 10.1089/apc.2006.0202. [DOI] [PubMed] [Google Scholar]
- 19.Mshana GH, Wamoyi J, Busza J, Zaba B, Changalucha J, Kaluvya S., et al. Barriers to accessing antiretroviral therapy in Kisesa, Tanzania: a qualitative study of early rural referrals to the national programAIDS Patient Care STDS 200620649–57. 10.1089/apc.2006.20.649 [DOI] [PubMed] [Google Scholar]
- 20.Wilson DP, Blower S. How far will we need to go to reach HIV-infected people in rural South Africa? BMC Med. 2007;5:16. doi: 10.1186/1741-7015-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makombe SD, Jahn A, Tweya H, Chuka S, Yu JK, Hochgesang M, et al. A national survey of teachers on antiretroviral therapy in Malawi: access, retention in therapy and survival. PLoS ONE. 2007;2:e620. doi: 10.1371/journal.pone.0000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muula AS, Ngulube T, Siziya S, Makupe C, Umar E, Prozesky H, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa: a systematic review. BMC Public Health. 2007;7:63. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]