Abstract
Elastin is an essential component of vertebrate arteries that provides elasticity and stores energy during the cardiac cycle. Elastin production in the arterial wall begins midgestation but increases rapidly during the last third of human and mouse development, just as blood pressure and cardiac output increase sharply. The aim of this study is to characterize the structure, hemodynamics, and mechanics of developing arteries with reduced elastin levels and determine the critical time period where elastin is required in the vertebrate cardiovascular system. Mice that lack elastin (Eln−/−) or have approximately one-half the normal level (Eln+/−) show relatively normal cardiovascular development up to embryonic day (E) 18 as assessed by arterial morphology, left ventricular blood pressure, and cardiac function. Previous work showed that just a few days later, at birth, Eln−/− mice die with high blood pressure and tortuous, stenotic arteries. During this period from E18 to birth, Eln+/− mice add extra layers of smooth muscle cells to the vessel wall and have a mean blood pressure 25% higher than wild-type animals. These findings demonstrate that elastin is only necessary for normal cardiovascular structure and function in mice starting in the last few days of fetal development. The large increases in blood pressure during this period may push hemodynamic forces over a critical threshold where elastin becomes required for cardiovascular function. Understanding the interplay between elastin amounts and hemodynamic forces in developing vessels will help design treatments for human elastinopathies and optimize protocols for tissue engineering.
Keywords: aorta, blood pressure, ultrasound, elastin, mechanics
decreased elastin in humans is associated with supravalvular aortic stenosis (SVAS, OMIM 185500), an autosomal dominant disease resulting from loss of function mutations in the elastin gene that lead to functional elastin haploinsufficiency and results in aortic stenosis, hypertension, and cardiac failure (27). SVAS can occur alone or as a condition of Williams Syndrome (WS, OMIM 194050), which is caused by a large deletion in chromosome seven that includes the elastin gene. Cardiovascular defects occur in about one-half of WS patients and are often the first symptom in infants and young children. WS infants with cardiovascular abnormalities before 4 mo of age have a poor prognosis (8).
Mice haploinsufficient for elastin (Eln+/−) provide a genetic model for SVAS. High blood pressure is a common component of SVAS and WS and is completely penetrant in Eln+/− mice. Large blood vessels in elastin-insufficient humans and mice show numerous morphological changes, including an increased number of elastin layers (elastic laminae) (26). Elastic laminae are organized circumferentially with alternating layers of smooth muscle cells (SMCs) and collagen fibers into lamellar units. The number of lamellar units is established during development and is regulated by mechanical tension in the wall (41). This unique adaptation in wall structure suggests developmental remodeling in response to altered mechanics and hemodynamics (10, 37). Although human and mouse vessels show similar developmental adaptations, human vessels continue on to pathological remodeling and develop stenoses that lead to cardiac disease. Mouse vessels show functional remodeling with no evidence of stenoses. Eln+/− mice live a normal life span despite smaller diameter vessels and significant hypertension (10, 40). The pathological remodeling of human vessels vs. the functional remodeling of mouse vessels with elastin haploinsufficiency could be caused by differences in the hemodynamic and mechanical forces on the arteries during development. Determining these forces and the resulting arterial structure and function in mice may help design treatments for children and infants with SVAS that encourage functional instead of pathological vessel wall remodeling.
Elastin and other structural matrix proteins, such as collagen, are expressed at high levels during the last third of embryonic development and up to ∼1 mo after birth in mice (22, 28). During this same period, blood pressure and blood flow increase rapidly (19, 23), and arterial diameter and thickness increase significantly (16, 24). The extracellular matrix proteins provide resiliency and strength to the growing vessel wall as the mechanical forces increase. The amount of collagen and the mechanical strength of tissue-engineered vessels can be increased by exposing the vessels to mechanical forces during culture (30). Mimicking developmental conditions by continuous increases in the mechanical forces increases collagen deposition and vessel strength even more (36). Elastin deposition and the assembly of functional elastic fibers in tissue-engineered vessels has been difficult to obtain, perhaps because of the numerous proteins involved in elastic fiber assembly (38) and the complex hemodynamic conditions in developing arteries. Understanding when elastin is required for normal cardiovascular development may help optimize protocols for elastin production in tissue-engineered vessels.
Mice lacking elastin (Eln−/−) die soon after birth with cardiovascular abnormalities, including tortuous, stenotic, stiff arteries and high left ventricular (LV) pressure with low cardiac output (25, 37); therefore, elastin is critical at some earlier point for normal cardiovascular development. The relationships between matrix protein expression, hemodynamics, mechanical forces, and vessel wall remodeling suggest that elastin might only be required for proper cardiovascular function in vertebrates late in development when the mechanical and hemodynamic forces increase beyond a certain threshold. To investigate this possibility, we measured blood pressure, blood flow, cardiac function, and vascular geometry, ultrastructure, and mechanics in embryonic day (E) 18 mice with 0% (Eln−/−), ∼50% (Eln+/−), and 100% [wild type (WT)] elastin. Our findings suggest that elastin plays a minor role in vascular development up until this time and that hemodynamic forces reach a critical threshold between E18 and birth when elastin becomes necessary for normal cardiovascular structure and function.
MATERIALS AND METHODS
Mice.
Timed matings were established for C57BL/6J mice bearing a heterozygous deletion of exon 1 in the elastin gene (Eln+/−) (25). The morning after mating was considered E1, and the embryos were used at E18. All protocols were approved by the Institutional Animal Care and Use Committee, and this investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health.
Blood pressure.
Pregnant mothers were anesthetized with 1.5% isoflurane. Body temperature was maintained with a feedback-controlled heating pad, and heart rate was continuously monitored by ECG. The uterus was externalized and placed on moistened gauze. The exposed embryos were warmed by a heat lamp and moistened with warm saline. Each embryo remained in its embryonic sack and was gently manipulated into position for measurements. LV pressure was measured in each embryo with a 30-gauge needle connected to a pressure transducer (Uniflow; Baxter). A parasternal long-axis view of the LV was monitored by ultrasound (Vevo 770, 55-MHz probe; Visualsonics) as the needle was advanced in the LV chamber with the injection arm of the imaging platform, as previously described for neonatal mice (37). LV pressure was recorded for several minutes, and then the needle was withdrawn and heart function was monitored for several minutes after withdrawal. The blood pressure measurements took 7–10 min/embryo, and an entire litter could be measured without any evidence of distress in the mother or the embryos.
The fluid-filled pressure system does not have the dynamic response necessary to acquire the complete waveform at the high heart rates of E18 mice, but a damped pressure waveform was acquired and mean LV pressure and heart rate were measured over five cardiac cycles. Assuming that the true LV diastolic pressure is zero, the measured mean pressure is equal to one-half the systolic pressure (37).
Echocardiography.
Mothers and embryos were prepped as described for the blood pressure studies and imaged with the same ultrasound system. Different groups of mice were used because it was not possible to perform both pressure and echocardiography studies on the same animal. Procedures for ultrasound imaging of embryonic mice were modified from Phoon (31). Aortic diameters were measured from m-mode images of the ascending aorta. LV diameters, fractional shortening, and mass were determined from two-dimensional parasternal short-axis images at the level of the papillary muscles using standard equations. Aortic velocity-time integral, cardiac output, and heart rate were calculated from suprasternal Doppler and two-dimensional images at the aortic valve using Visualsonics software. Because of limitations with positioning the embryos, not all views were obtained for each embryo. Imaging usually took 15–20 min/embryo and was stopped at the first sign of distress in the mother or the embryos. Distress was usually noted by slow embryonic heart rates 1–1.5 h after externalization of the uterus.
Morphology, ultrastructural analysis, and DNA amount.
Each mouse was killed and weighed, and the chest was opened to image the aorta and major arteries for length and morphology information before removal of the heart and aorta. The heart was washed with physiological saline, blotted, and weighed (wet weight). Normalized total heart weight was calculated by dividing the heart weight (mg) by the body weight (g). For ultrastructural analysis, the proximal ascending aorta was fixed in 2.5% glutaraldehyde/0.1 M sodium cacodylate, sequentially stained with osmium tetroxide, tannic acid, and uranyl acetate, and then dehydrated and embedded in PolyBed 812 (Polysciences). Thin sections (60 nm) were counterstained with uranyl acetate and lead citrate, examined on a Zeiss 902 electron microscope, and imaged with Kodak EM film. Two aortas were imaged for each genotype, and several grids were examined for each aorta. For DNA analysis, aortas were cut between the heart and innominate artery and frozen at −80°C until use. DNA was isolated using standard proteinase K methods and quantified using PicoGreen (Invitrogen) and a fluorescent plate reader.
Mechanical testing and unloaded dimensions.
Mechanical testing was performed on the ascending aorta using a pressure and force arteriograph (Danish Myotechnology). The ascending aorta was mounted in the test system at its unloaded length and preconditioned for three cycles. After preconditioning, the intravascular pressure was increased from 2.5 to 40 mmHg in steps of 2.5 mmHg (5 s/step) for three cycles. The mechanical tests were not started at 0 mmHg because it was difficult to ensure that the test system returned to 0 mmHg before each cycle, and the application of any negative pressure caused the aorta to collapse. For the first protocol, the force either remained constant or decreased with increased pressure, implying that the aorta was either at or below its in vivo length (17). If the force decreased, the aorta was stretched 2%, and the protocol was repeated. After testing, the aorta was removed and cut into two to three rings, ∼0.2 mm thick. The rings were placed in physiological saline solution, and unloaded dimensions and circumferential residual strain, as characterized by the opening angle (OA), were measured (37, 40).
The in vivo length protocol (17), where the longitudinal force remained approximately constant with increasing pressure from 2.5 to 40 mmHg, was used for all analyses. Compliance was calculated as the average percent change in outer diameter for each 2.5-mmHg pressure step. For the compliance calculations at the lowest and highest points (2.5 and 40 mmHg, respectively), only the diameter change above or below that point was used, as necessary. Pressure, longitudinal force, and outer diameter from the in vivo length protocol were converted to stress and stretch ratios as described previously (37, 40). The incremental elastic modulus was calculated as the incremental slope of the stress-stretch ratio curve for each pressure step (4, 7). Aortas from all genotypes showed constant force with increasing pressure when stretched an average of 2% from their unloaded lengths; hence, 1.02 was used as the stretch ratio when calculating the deformed inner diameter for the stretch ratio and stress calculations.
Statistics.
Outliers that were greater than three standard deviations from the mean were not included in the genotype comparisons. One Eln+/− mouse was eliminated from the pressure data and two WT, two Eln+/−, and one Eln−/− mice were eliminated from the mechanical test data as outliers. One-way ANOVA followed by Tukey's post hoc test were used to determine statistical differences using SPSS software (SPSS). P < 0.05 was considered significant.
RESULTS
Absent or reduced elastin does not significantly increase blood pressure in E18 mice.
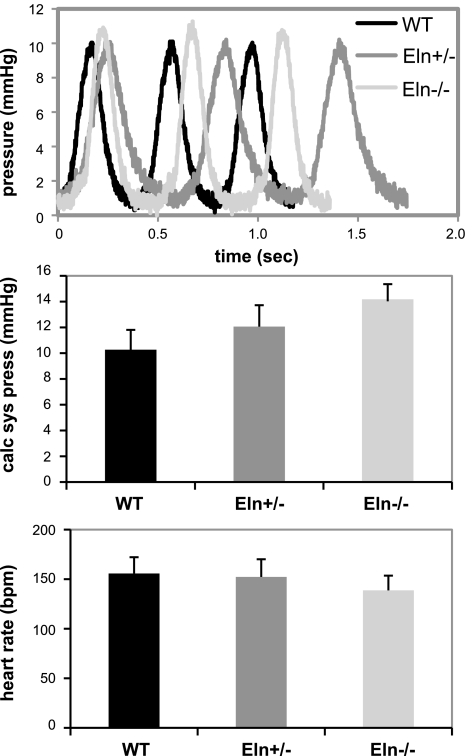
LV pressures were measured in WT, Eln+/−, and Eln−/− E18 mice to determine how elastin levels influence fetal blood pressure. Our data show that reduced elastin has no significant effect on blood pressure at this stage. The calculated systolic LV pressure and heart rate are similar for all three genotypes, although there is a trend toward increased pressure with reduced elastin amounts (Fig. 1). This is in contrast to the significant differences just 3 days later at birth when the LV pressure in Eln+/− mice is 25% higher and in Eln−/− mice is 200% higher than WT animals (37). We are not aware of any published blood pressure measurements in E18 mice, but the pressures for all genotypes in this study are between the ranges previously measured for E13.5 (19) and neonatal (15, 18, 37) mice.
Fig. 1.
Left ventricular (LV) pressure is not significantly different between wild-type (WT) mice, mice that have approximately one-half the normal level of elastin (Eln+/−), and mice that lack elastin (Eln−/−) at embryonic day (E) 18. Top: representative pressure waveforms; middle: calculated systolic pressures; bottom: average heart rates. Data are means ± SE; n = 7–8 mice/genotype.
Cardiovascular function is not impaired by reduced elastin amounts at E18.
High-frequency ultrasound was used to determine if reduced elastin affects cardiovascular function at E18. All three genotypes have approximately the same aortic diameter at systole, but the change in diameter between systole and diastole is approximately three times less in Eln−/− aorta than WT or Eln+/− (Table 1). Despite the stiff aorta, cardiac function parameters in Table 1 are normal in Eln−/− mice, and the parameters for all genotypes are similar to previous studies on late embryonic mice (13, 35, 43). There are no significant differences between the heart rates in Table 1 and the heart rates measured during the pressure studies in Fig. 1. The ultrasound data for WT mice at E18 compare favorably with our previously measured values for postnatal day (P) 1 mice (37); the aortic diameter, LV mass, heart rate, and cardiac output increase with developmental age, as expected. In Eln−/− mice, the cardiac function compared with WT declines dramatically between E18 and P1.
Table 1.
E18 Eln−/− mice have a less compliant aorta but normal cardiac function
| WT | Eln+/− | Eln−/− | |
|---|---|---|---|
| m-Mode | |||
| AoIDs, mm | 0.42 ± 0.05 | 0.45 ± 0.05 | 0.45 ± 0.02 |
| AoIDd, mm | 0.36 ± 0.05 | 0.38 ± 0.05 | 0.43 ± 0.02* |
| AoDC, % | 16.0 ± 1.8 | 16.6 ± 1.8 | 5.3 ± 1.0* |
| Two dimensional | |||
| LVIDd, mm | 0.90 ± 0.07 | 0.92 ± 0.09 | 0.88 ± 0.08 |
| FS, % | 39 ± 5 | 42 ± 5 | 41 ± 4 |
| LVM, mg | 2.8 ± 0.5 | 2.8 ± 0.4 | 2.7 ± 0.4 |
| Doppler | |||
| AoVTI, mm | 15.4 ± 6.3 | 19.3 ± 3.9 | 18.3 ± 3.6 |
| CO, ml/min | 0.3 ± 0.3 | 0.4 ± 0.2 | 0.4 ± 0.2 |
| HR, beats/min | 131 ± 35 | 132 ± 36 | 162 ± 48 |
| n | 5 | 8 | 4 |
Values are means ± SD; n, no. of mice. Eln−/−, mice that lack elastin; Eln+/−, mice that have about one-half the normal level of elastin. Shown are aortic inner diameter at systole (AoIDs) and diastole (AoIDd) and the percent change between systole and diastole (AoDC) from m-mode ultrasound images;
left ventricle (LV) inner diameter at diastole (LVIDd), fractional shortening (FS), and LV mass (LVM) from 2-dimensional ultrasound images; and aortic velocity-time integral (AoVTI), cardiac output (CO), and heart rate (HR) from Doppler ultrasound images.
P < 0.05 for Eln−/− compared with wild-type (WT) and Eln+/−.
The ultrasound data is limited by the axial resolution of the probe (30 μm) and the difficulty in obtaining optimal images in embryonic mice. Note that the axial ultrasound resolution is less than the average diameter change in the Eln−/− aorta and approximately one-half the diameter change of WT and Eln+/− aortas. We were careful to follow the aortic wall throughout the cardiac cycle, and, if there were any uncertainties about the wall boundary, we assumed that the diameter at diastole was less than the diameter at systole. Despite these limitations, our in vivo data show that there are significant differences in aortic compliance and no detectable differences in heart function between Eln−/− and WT or Eln+/− mice.
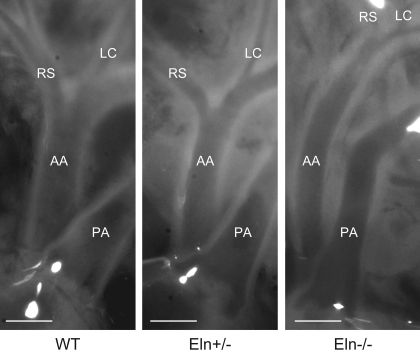
Lack of elastin is associated with longer, thicker vessels at E18.
In vivo and ex vivo dimensions and residual strain of the aorta were examined to determine the effects of elastin amount on vessel morphology and geometry (Table 2). Reduced elastin causes lengthening of the major arteries in both adult (4) and neonatal (37) mice. At E18, there is no significant lengthening in Eln+/− aorta, but the in vivo length is significantly longer in Eln−/− aorta compared with WT and Eln+/− (Fig. 2 and Table 2). The absence of elastin causes SMC hypertrophy and medial thickening until the Eln−/− vessel becomes occluded soon after birth (26, 37). At E18, there is significant thickening of the aortic wall, but the vessel is still patent. The residual strain as measured by the OA is not significantly different between genotypes, although there is a trend toward reduced OA with reduced elastin levels. By P1, the OA is significantly smaller in Eln−/− aorta compared with WT (37). Our range of OA in these studies is consistent with measured OA for the thoracic aorta in young mice (15). We also measured body and heart weights of each animal. Consistent with the echocardiography results (Table 1), there are no significant differences in the normalized total heart weight between genotypes.
Table 2.
E18 Eln−/− mice have a longer, thicker aorta but no signs of cardiac hypertrophy
| WT | Eln+/− | Eln−/− | |
|---|---|---|---|
| Body wt, g | 0.85 ± 0.10 | 0.82 ± 0.11 | 0.81 ± 0.16 |
| NTHW, mg/g | 5.8 ± 0.9 | 6.0 ± 0.7 | 5.7 ± 1.0 |
| L, mm | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.7 ± 0.2* |
| OD, μm | 320 ± 39 | 299 ± 26 | 313 ± 36 |
| T, μm | 57 ± 10 | 62 ± 11 | 73 ± 10* |
| OA, degrees | 135 ± 16 | 116 ± 25 | 110 ± 25 |
| n | 7–18 | 6–26 | 5–19 |
Values are means ± SD. E, embryonic day; NTHW, normalized total heart weight; L, in vivo aortic length; OD, unloaded aortic outer diameter; T, unloaded aortic thickness; OA, aortic opening angle. There are low n values for OA, high n values for BW and NTHW, and intermediate n values for other measurements.
P < 0.05 for Eln−/− compared with WT and Eln+/−.
Fig. 2.
E18 Eln−/− ascending aorta (AA) is significantly longer than WT and Eln−/−. Pressure was not controlled in these images; therefore, diameters cannot be directly compared. Pulmonary artery (PA), right subclavian (RS), and left carotid (LC) arteries are labeled. The heart is at the bottom of each image. Scale bars = 300 μm.
SMC organization appears similar at E18, regardless of elastin amounts.
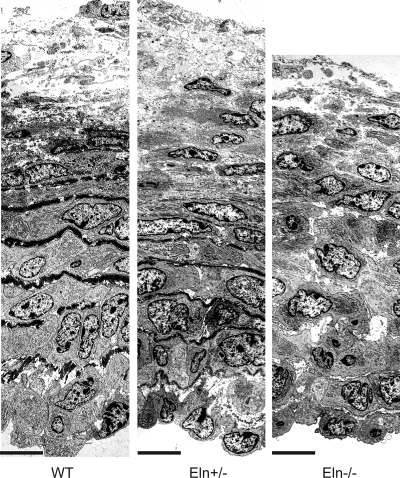
The effect of reduced elastin amounts on the ultrastructure of the embryonic aortic wall in WT and elastin-insufficient animals was assessed by electron microscopy (EM). At E18, all genotypes have approximately eight layers of circumferentially arranged SMCs (Fig. 3). In WT and Eln+/− aortas, the SMC layers alternate with elastic laminae. The central laminae are the thickest and most complete, whereas those closest to the intima and adventitia are thinner and discontinuous. The elastic laminae appear thinner in Eln+/− aorta than WT. Unlike newborn and adult mice, there are not an increased number of laminae in Eln+/− aorta compared with WT (26, 37). In Eln−/− aorta, the SMCs are mostly arranged circumferentially and have close cell-cell contacts. There are a few areas in the center of the wall where the cell-cell contacts have been disrupted and abundant proteoglycans, which do not take up the EM stain, have been deposited. The relatively organized appearance of the SMCs in E18 Eln−/− aorta is different from the disorganized appearance of the hyperproliferating SMCs in newborn Eln−/− aorta (25, 37). We also measured DNA content in each aorta, and, consistent with the equivalent cell number suggested by the EM images, the DNA amount is not significantly different between genotypes. There are 35.6 ± 4.8, 37.9 ± 4.2, and 43.1 ± 8.1 ng DNA in WT, Eln+/−, and Eln−/− aortas, respectively. By P1, there is significantly more DNA in the Eln−/− aorta, and DNA increases with age, as expected (37).
Fig. 3.
At E18, smooth muscle cells (SMCs) in all genotypes are organized in mostly circumferential layers. Shown are representative electron microscopic (EM) images of the proximal ascending aorta with the lumen at the bottom of the image. Elastic laminae are thinner in Eln+/− aorta and absent in Eln−/− aorta, but the SMC arrangement looks similar to WT. Scale bars = 11 μm.
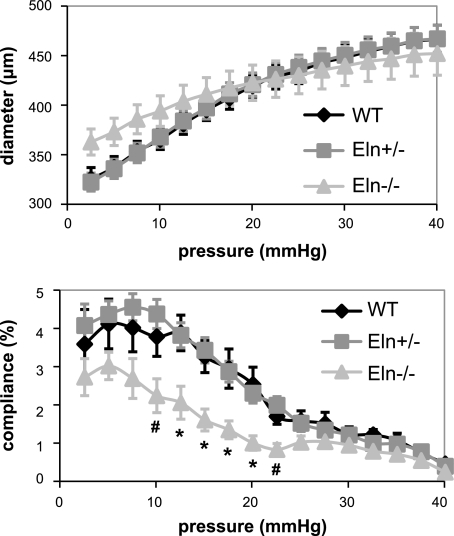
Vessels without elastin have reduced compliance at E18.
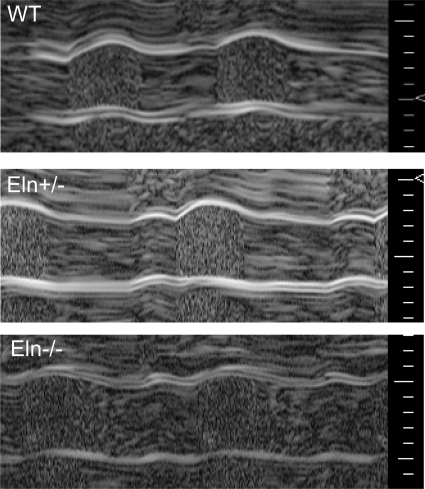
The in vitro mechanical behavior of the aorta was examined to determine how the vessel diameter and compliance change with pressure when elastin levels are decreased. In the absence of elastin, the E18 aorta behaves like a rigid tube with little change in diameter with pressure and significantly reduced compliance compared with WT and Eln+/− aortas (Fig. 4). At the in vivo length, the axial forces remain approximately constant with pressure and are not significantly different between genotypes (data not shown). The reduced compliance at E18 is similar to the reduced compliance in P1 Eln−/− aorta (37). The ex vivo compliance data were confirmed in vivo using m-mode ultrasound (Fig. 5 and Table 1). Discrepancies between the absolute diameter values of the in vivo and in vitro data may be caused by the reduced resolution of the ultrasound probe (30 μm) compared with that of our pressure and force arteriograph (3 μm). Also, the loading rate of the in vitro studies is necessarily ∼20 times slower than the in vivo loading rate, and there may be viscoelastic effects that decrease the measured in vitro diameter. Viscoelastic effects are negligible in adult arteries (11) but may be more significant in embryonic arteries. Finally, the comparisons are made using the in vitro diameter at the calculated systolic pressure, which was obtained under isoflurane anesthesia. Isoflurane has been shown to reduce blood pressure; therefore, the actual blood pressure and diameters may be slightly larger.
Fig. 4.
E18 Eln−/− aorta has reduced compliance in vitro. Shown is average outer diameter (top) and compliance (bottom) vs. pressure. P < 0.05 for Eln−/− compared with WT and Eln+/− (*) and Eln−/− compared with Eln+/− (#). Data are means ± SE; n = 9 WT, 17 Eln+/−, and 6 Eln−/−.
Fig. 5.
E18 Eln−/− aorta has reduced compliance in vivo. Representative m-mode ultrasound images of the ascending aortic wall in each genotype are shown. Scale is marked in 100-μm increments.
Mechanical properties in vessels without elastin.
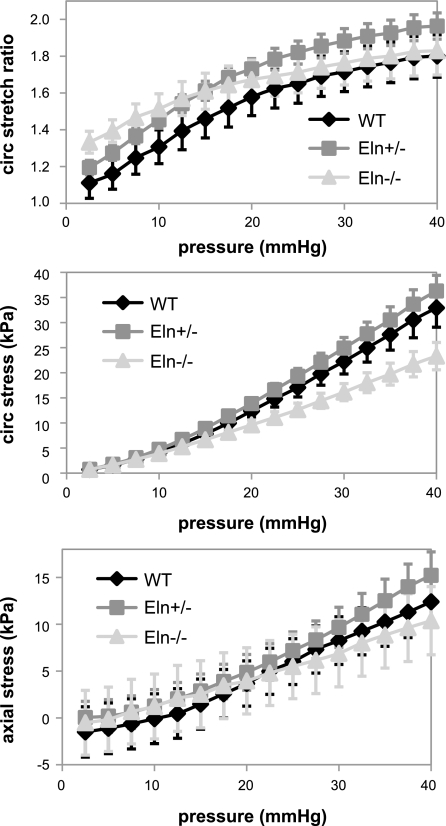
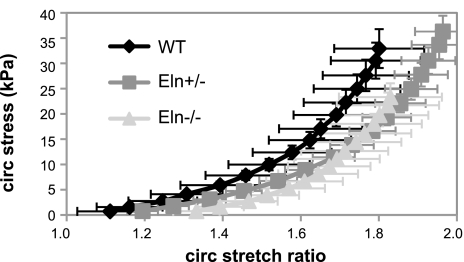
The pressure-diameter data do not account for initial differences in vessel geometry between the three genotypes. To compare mechanical properties that are independent of the starting geometry, the circumferential stretch ratio and circumferential and axial stresses were calculated from the unloaded dimensions and the in vitro mechanical test data. The circumferential stretch ratio, circumferential stress, and axial stress at each pressure were not significantly different between genotypes (Fig. 6). This is in contrast to our previous data for newborn mice where the Eln+/− aorta has significantly higher stretch ratios than WT and the Eln−/− aorta has significantly reduced circumferential stresses compared with WT and Eln+/− at the same pressure (37). Figure 7 shows that the circumferential stress-stretch ratio curves shift to the right with decreasing elastin amounts, as in adult animals (4), but the shift is not significant at E18. Consistent with the similar stress-stretch ratio curves, the incremental elastic modulus is not significantly different between genotypes (data not shown). We are unaware of published mechanical studies on embryonic mouse arteries with which to compare our data. However, our E18 and P1 data predict that the physiological circumferential stresses and incremental elastic modulus increase with developmental age, which is consistent with findings in mechanical studies of postnatal arterial development in mice (15).
Fig. 6.
There are no significant differences in the average circumferential (circ) stretch ratio (top), circumferential stress (middle), or axial stress (bottom) vs. pressure for each genotype. Data are means ± SE; n = 8 WT, 15 Eln+/−, and 6 Eln−/−.
Fig. 7.
The average circumferential stress vs. stretch ratio relationship for E18 aorta does not significantly depend on elastin amount. The slope of this curve at physiological pressure was used to calculate the incremental elastic modulus in Table 3.
DISCUSSION
Cardiovascular development in mice follows a similar progression to humans, but on a compressed time scale. In mice, the heart begins beating at approximated E8.5, and the definitive vascular pattern is established at approximately E14, just when elastin expression begins to increase in the aorta. The systemic and pulmonary circulations separate completely with the closure of the ductus arteriosus a few hours after birth. The total amount of elastin and other matrix gene expression increases concurrently with blood pressure and blood flow from the start of cardiovascular development up until about P30 (39). This relationship suggests that the concentration of matrix components in the artery wall is increased to meet the mechanical demands of the developing cardiovascular system. We studied Eln−/− and Eln+/− mice to determine the time period where elastin is required for normal cardiovascular development and function.
Embryonic cardiovascular function in Eln−/− mice.
In vivo and in vitro data show that the Eln−/− aorta is two to three times less compliant than Eln+/− and WT aortas at physiological pressures, but this does not impair cardiac function. At birth, Eln−/− mice still have a less compliant aorta, but they also have double the LV pressure of WT mice and severely compromized cardiac function (37). Thus, in only 3 days, heart function in Eln−/− mice deteriorates from normal to lethal.
The aorta in E18 Eln−/− mice is longer and thicker than WT aorta. It has been shown that arteries grow axially in response to increased axial strain. Paradoxically, arteries also grow axially and become tortuous in response to decreased axial strain (20). Increased arterial length and subsequent tortuosity are characteristic of adult mice with reduced elastin levels (2, 34) and of other mouse models with elastic fiber defects (14, 29, 42), suggesting that intact elastic fibers are necessary to maintain axial tension and vessel stability. In Eln−/− aorta, the axial tension may be reduced enough that axial growth begins at E18. In Eln+/− aorta, there seems to be enough elastin to maintain axial tension at E18, but by P1 the tension is reduced and axial growth has occurred. The increased thickness in Eln−/− aorta causes a trend toward reduced circumferential stress at high pressures, but this is not significant at E18. By P1, when the LV pressures are high, the increased thickness normalizes the physiological circumferential stress to WT values (37).
Besides the differences in length and thickness, other profound morphological differences in P1 Eln−/− arteries are not present in E18 arteries. P1 arteries show severe tortuosity, with the carotid arteries often forming complete loops in vivo, and the aorta has stenoses and dilations that change the diameter two- to threefold from normal values (37). There are no elastic laminae in Eln−/− aorta, but the SMCs are organized in distinct and mostly circumferentially oriented layers. This is in contrast to the disorganized, overproliferating SMCs in newborn Eln−/− aorta (25, 37). These data show that, although there are cardiovascular changes, the cardiovascular system in mice can develop and function normally without elastin until approximately E18.
It is surprising that E18 mice lacking elastin have normal LV blood pressure and cardiac function despite having a less compliant aorta. While one explanation is that shunting of blood through the patent ductus arteriosus into the pulmonary circulation provides for cardiac unloading in response to higher aortic afterload, our previous studies have shown that the large pulmonary vessels are affected in a similar manner to the systemic vessels in response to elastin insufficiency (34). Hence, these smaller, stiffer pulmonary vessels may not be able to support normal cardiac unloading and could contribute to the effects attributed to the systemic circulation.
Cardiovascular function in Eln+/− mice.
Eln+/− mice with reduced elastin amounts have similar LV pressure, cardiac function and aortic morphology, wall structure, and mechanical behavior to WT at E18. The individual elastic laminae in Eln+/− aorta are ∼50% thinner than WT, but both genotypes have the same number of laminae across the wall. Between E18 and birth, additional laminae are added to the outer aspect of the medial layer in Eln+/− aorta. Newborn Eln+/− animals also have 25% higher LV pressure compared with WT, and the aorta is longer with some differences in mechanical behavior (37). Like Eln−/− mice, one of the first adaptations in the Eln+/− cardiovascular system is an increase in arterial length. Unlike Eln−/− mice, the SMCs are able to functionally adapt the vessel wall structure by adding elastic laminae instead of pathologically proliferating to occlude the vessel lumen. Eln+/− mice live a normal life span despite increased blood pressure, probably because the pressure increase occurs just before or at birth when the cardiovascular system is still developing and matrix proteins are expressed at high levels. As adults, Eln+/− arteries have smaller diameters and thinner walls than WT arteries (40), and Eln+/− mice have increased stroke volume and cardiac output (10). Hence, the majority of the cardiovascular remodeling associated with reduced elastin levels occurs between E18 and P1, but some additional remodeling occurs postnatally.
Elastin and cardiovascular hemodynamics.
In evolution, elastin first appears in the high pressure, pulsatile, closed circulatory system of vertebrates where blood vessels become important components of cardiac function. In jawless vertebrates and invertebrates, the vessel walls are composed of fibrillin and collagen or collagen alone. Fibrillin is the major protein in microfibrils, which have limited elasticity and are associated with elastic fibers in vertebrate tissues (9). Collagen is ∼10 times stiffer than elastin, and in vertebrate vessels it provides strength at high pressures (17). Table 3 summarizes the aortic wall composition for vertebrate and invertebrate species. In invertebrate vessels, fibrillin and/or collagen are arranged in circumferential layers that resemble vertebrate elastic laminae (6). Mechanical tests show that invertebrate arteries behave similarly to vertebrate arteries in their physiological pressure region with nonlinear stress-strain relationships and low hysteresis (energy loss) (6, 9, 33). These vessels are working at low pressures, however, indicating that fibrillin and collagen are only capable of providing the necessary elasticity and strength needed for the low-pressure circulatory system of invertebrate animals.
Table 3.
Comparison of aortic wall composition and hemodynamic parameters in various species and throughout mouse development
| Description | Aortic Wall Composition | Mean P, mmHg | Circ Stress, kPa | T/EL, N/m | Circ Einc, kPa | Ref. No. |
|---|---|---|---|---|---|---|
| Higher vertebrate | elastin, fibrillin, collagen | 80–100 | 100–200 | 1–3 | 400–900 | 12, 39, 41 |
| P60 mouse | 90 | 200 | 1 | 500 | 40 | |
| Lower vertebrate | elastin, fibrillin, collagen | 30–60 | 30–60 | 0.3–0.5 | 300–500 | 12 |
| Jawless vertebrate | fibrillin/collagen | 15–25 | 20–30 | 0.1–0.3 | 30–100 | 6 |
| P1 mouse | 22 | 20 | 0.1 | 100 | 37 | |
| Invertebrate | fibrillin/collagen or collagen only | 5–15 | 2–6 | 0.03–0.1 | 30–100 | 6 |
| E18 mouse | 8 | 5 | 0.03 | 20 | Current work |
P, postnatal day; Mean P, mean arterial pressure; Circ Stress, physiological circumferential stress;T/EL, tension/elastic laminae; Circ Einc, circumferential incremental elastic modulus.
In vertebrates, the aortic wall structure evolved to accommodate pulsatile blood flow and increased overall blood pressure, and additional proteins (fibrillin and then elastin) were added to accommodate the hemodynamic demands. Blood pressure affects the physiological circumferential stress on the artery wall, the tension per elastic laminae, and the incremental elastic modulus of the artery. Table 3 includes a comparison of these parameters throughout evolution, with data for developing mice included below the classification that has the most similar parameter values. A comparison of values shows that the developmental stage in mice, P1, when most of the cardiovascular changes appear with absent or reduced elastin levels has similar parameters to the evolutionary stage, jawless vertebrate, just before elastin is present in the artery wall. At E18, when we find few differences between mice with 100% and 0% elastin, the parameter values are in the range of invertebrates that do not need elastin. Various investigators have emphasized the importance of each of these parameters (circumferential stress, tension/elastic laminae, and incremental elastic modulus) in evolution, development, and remodeling of arteries (2, 5, 6, 12, 17, 41). We emphasize that each one depends on the physiological blood pressure of the animal; therefore, pressure is likely the critical parameter that determines the structure and function of both invertebrate and vertebrate arteries.
We have focused on mechanics and hemodynamics, but it must be pointed out that Eln+/− and Eln−/− vascular phenotypes may be influenced by alterations in signaling pathways linked to elastin or elastic fibers. Sequences within elastin have been shown to interact with, and signal through, cell surface receptors to influence, among other properties, cell movement, proliferation, and gene expression (1, 3, 21). In addition, elastin is assembled on fibrillin-rich microfibrils, which also bind transforming growth factor (TGF)-β family growth factors (32). Hence, the presence or absence of elastin may influence the amount of bound growth factor, which would have consequences for development. It is possible, for example, that Eln+/− vessel structure is a consequence of alterations in growth factor signaling resulting from an increased number of “naked” microfibrils able to bind TGF-β because of a relative absence of elastin compared with WT. Further studies are required to explore the relationship between elastin and growth factor signaling.
In conclusion, as pressure increases throughout development and evolution, the aortic wall composition is altered to withstand the resulting hemodynamic and mechanical forces and to provide optimal cardiovascular function. In WT mice, the LV blood pressure increases 1–2 mmHg/day from E9.5 to E14.5 (19). Our data suggest that pressure continues at this rate through E18 and then jumps significantly by 10–15 mmHg between E18 and birth (37). Our studies on E18 mice with absent or reduced elastin levels show that elastin is not necessary for normal cardiovascular function until the last few days of development when this large pressure increase occurs. At E18, Eln−/− and Eln+/− LV pressure, cardiac function parameters and aortic mechanics, morphology, and ultrastructure are remarkably similar to WT. At birth, LV pressure is increased over WT in both genotypes, and Eln−/− mice have additional changes, including decreased cardiac function and abnormal arterial morphology, ultrastructure, and mechanics (37). This transition suggests that pressure increases beyond the threshold of E18 mice switch the cardiovascular system from one that does not require elastin for normal function to one that does and that pressure regulation may be a viable treatment for cardiovascular defects in human elastinopathies and may help modulate elastin production in tissue-engineered arteries.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-087563 (to J. E. Wagenseil) and HL-53325, HL-74138, and HL-084922 (to R. P. Mecham).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
ACKNOWLEDGMENTS
We thank Dean Li at the University of Utah for providing the Eln+/− mice.
REFERENCES
- 1.Bax DV, Rodgers UR, Bilek MM, Weiss AS. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. J Biol Chem 284: 28616–28623, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry CL, Germain J, Newman DL, Greenwald SE. Comparative morphological and functional aspects of the aorta of the major vertebrate classes. Lab Anim 8: 279–289, 1974 [DOI] [PubMed] [Google Scholar]
- 3.Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem 280: 40939–40947, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Carta L, Wagenseil JE, Knutsen RH, Mariko B, Faury G, Davis EC, Mecham RP, Ramirez F. Discrete contributions of elastic fiber components to arterial development and mechanical compliance. Athersclero Thromb Vasc Biol 29: 2083–2089, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark ER. Studies on the growth of blood-vessels in the tail of the frog larva-by observation and experiment on the living animal. Am J Anat 23: 37–88, 1918 [Google Scholar]
- 6.Davison IG, Wright GM, DeMont ME. The structure and physical properties of invertebrate and primitive vertebrate arteries. J Exp Biol 198: 2185–2196, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Dobrin PB. Physiology and pathophysiology of blood vessels. In: The Basic Science of Vascular Disease, edited by Sidawy ANSB, DePalma RG. New York, NY: Futura, 1997, chapt. 3, p. 69–105 [Google Scholar]
- 8.Eronen M, Peippo M, Hiippala A, Raatikka M, Arvio M, Johansson R, Kahkonen M. Cardiovascular manifestations in 75 patients with Williams syndrome. J Med Genet 39: 554–558, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faury G. Function-structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathol Biol (Paris) 49: 310–325, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest 112: 1419–1428, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. New York, NY: Springer-Verlag, 1993 [Google Scholar]
- 12.Gibbons CA, Shadwick RE. Functional similarities in the mechanical design of the aorta in lower vertebrates and mammals. Experientia 45: 1083–1088, 1989 [DOI] [PubMed] [Google Scholar]
- 13.Gui YH, Linask KK, Khowsathit P, Huhta JC. Doppler echocardiography of normal and abnormal embryonic mouse heart. Pediatr Res 40: 633–642, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res 100: 738–746, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Guo X, Kassab GS. Axial nonuniformity of geometric and mechanical properties of mouse aorta is increased during postnatal growth. Am J Physiol Heart Circ Physiol 290: H657–H664, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hudlicka O, Tyler K. Growth of vessels during pre- and postnatal development. In: Angiogenesis: The Growth of the Vascular System. London, UK: Academic, 1986, p. 41–66 [Google Scholar]
- 17.Humphrey JD. Cardiovascular Solid Mechanics. New York, NY: Springer-Verlag, 2002 [Google Scholar]
- 18.Ishii T, Kuwaki T, Masuda Y, Fukuda Y. Postnatal development of blood pressure and baroreflex in mice. Auton Neurosci 94: 34–41, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res 93: 857–865, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res 90: 918–925, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Karnik SK, Brooke BS, Bayes-Genis A, Sorensen L, Wythe JD, Schwartz RS, Keating MT, Li DY. A critical role for elastin signaling in vascular morphogenesis and disease. Development 130: 411–423, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Dev Biol 62: 153–188, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Keller BB, MacLennan MJ, Tinney JP, Yoshigi M. In vivo assessment of embryonic cardiovascular dimensions and function in day-10.5 to -145 mouse embryos. Circ Res 79: 247–255, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol 74: 834–841, 1996 [PubMed] [Google Scholar]
- 25.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature 393: 276–280, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102: 1783–1787, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li DY, Toland AE, Boak BB, Atkinson DL, Ensing GJ, Morris CA, Keating MT. Elastin point mutations cause an obstructive vascular disease, supravalvular aortic stenosis. Hum Mol Genet 6: 1021–1028, 1997 [DOI] [PubMed] [Google Scholar]
- 28.McLean SE, Mecham BH, Kelleher CM, Mariani TJ, Mecham RP. Extracellular matrix gene expression in the developing mouse aorta. In: Extracellular Matrices and Development, edited by Miner JH. New York, NY: Elsevier, 2005, p. 82–128 [Google Scholar]
- 29.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415: 171–175, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science 284: 489–493, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Phoon CK. Imaging tools for the developmental biologist: ultrasound biomicroscopy of mouse embryonic development. Pediatr Res 60: 14–21, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ramirez F, Dietz HC. Fibrillin-rich microfibrils: structural determinants of morphogenetic and homeostatic events. J Cell Physiol 213: 326–330, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Shadwick RE. Mechanical design in arteries. J Exp Biol 202: 3305–3313, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Shifren A, Durmowicz AG, Knutsen RH, Faury G, Mecham RP. Elastin insufficiency predisposes to elevated pulmonary circulatory pressures through changes in elastic artery structure. J Appl Physiol 105: 1610–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spurney CF, Leatherbury L, Lo CW. High-frequency ultrasound database profiling growth, development, and cardiovascular function in C57BL/6J mouse fetuses. J Am Soc Echocardiogr 17: 893–900, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA 105: 6537–6542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. Reduced vessel elasticity alters cardiovascular structure and function in newborn mice. Circ Res 104: 1217–1224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81: 229–240, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY, Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol 289: H1209–H1217, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res 20: 99–111, 1967 [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415: 168–171, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Yu Q, Leatherbury L, Tian X, Lo CW. Cardiovascular assessment of fetal mice by in utero echocardiography. Ultrasound Med Biol 34: 741–752, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]