Abstract
Pregnancy-associated plasma protein-A (PAPP-A) increases local IGF-I bioavailability through cleavage of inhibitory IGF binding protein (IGFBP)-4 in a variety of systems, including the cardiovascular system. To test the hypothesis that expression of PAPP-A promotes the development of atherosclerotic lesions, we generated transgenic mice that express human PAPP-A in arterial smooth muscle. Four founder lines were characterized for transgenic human PAPP-A mRNA and protein expression, IGFBP-4 protease activity, and tissue specificity. In study I, apolipoprotein E knockout (ApoE KO) mice, a well-characterized mouse model of atherosclerosis, and ApoE KO mice expressing the human PAPP-A transgene at relatively high levels (ApoE KO/Tg) were fed a high-fat diet. At harvest, aortas were dissected and opened longitudinally for en face staining of lipid-rich lesions. Lesion area was increased 3.5-fold in aortas from ApoE KO/Tg compared with ApoE KO mice (P < 0.001), but no significant difference was seen in lesion number. In study II, replacement of PAPP-A expression in arterial smooth muscle of double ApoE KO/PAPP-A KO mice resulted in a 2.5-fold increase in lesion area (P = 0.002), without an effect on lesion number. PAPP-A transgene expression was associated with a significant increase in an IGF-responsive gene (P < 0.001), suggesting increased local IGF-I action. We therefore conclude that expression of human PAPP-A localized to arterial smooth muscle accelerates lesion progression in a mouse model of atherosclerosis. These data provide further evidence for the importance of PAPP-A in the cardiovascular system and suggest PAPP-A as a potential therapeutic target in the control of atherosclerosis.
Keywords: atherosclerosis
pregnancy-associated plasma protein-A (PAPP-A) is a metalloproteinase of the metzincin superfamily that increases local IGF bioavailability through cleavage of inhibitory IGF binding protein (IGFBP)-4 in many systems in vitro, and a similar function in vivo has been proposed (7, 8, 38). In particular, PAPP-A appears to be an important regulatory component in the cardiovascular system. PAPP-A is expressed by arterial smooth muscle cells and, to a lesser extent, by endothelial cells (5, 11, 13, 43). The most potent stimulators of PAPP-A expression in these vascular cells are the proinflammatory, proatherogenic cytokines, IL-1β and TNF-α (11, 13). In vivo, we have found that marked increases in PAPP-A expression are associated with neointimal formation in pig coronary arteries following balloon angioplasty (5) and with unstable plaque from patients who died suddenly of cardiac causes (3). The latter study has been confirmed by others and has led to the possible use of elevated serum PAPP-A as a marker of acute coronary syndromes (2, 15, 20, 29, 46). Moreover, we have found that genetic ablation of PAPP-A in mice results in resistance to the development of neointimal hyperplasia as an acute injury response (41) and to the progression of atherosclerosis as a chronic injury response (21). Thus diminished local IGF-I action as a consequence of reduced PAPP-A activity could have clinical relevance (9). However, the question of whether PAPP-A is pro- or antiatherosclerotic has been debated (16).
To further test the hypothesis that PAPP-A is a major player in adverse vascular responses, we generated and characterized transgenic mice expressing human PAPP-A in arterial smooth muscle under the control of a minimal SM22-α promoter (28). We show that these animals exhibit accelerated lesion development in a mouse model of atherosclerosis.
MATERIALS AND METHODS
All procedures involving mice were approved by Mayo Clinic's Institutional Animal Care and Use Committee and complied with the standards stated in the Guide for the Care and Use of Laboratory Animals.
Transgene construct.
The pcDNA3.1 construct with the cytomegalovirus (CMV) promoter driving expression of full-length human PAPP-A has been described previously (34). This construct was digested with NruI and HindIII to release the CMV promoter. The remaining PAPP-A vector was then blunt-ligated with a 445-bp SM22-α promoter insert that had been excised with KpnI and XhoI from a pGL3-basic vector (provided by Dr. Robert Simari, Mayo Clinic). This minimal SM22-α promoter has been shown to have more specific targeted expression to large arterial vessels (28). Transfection of rat arterial smooth muscle cells (A7r5; American Type Culture Collections, Manassas, VA) with this construct (designated pSM22-α-PA) using TransFast reagents (Promega, Madison, WI) resulted in high expression of human PAPP-A as measured by UltraSensitive PAPP-A ELISA, kindly provided by Diagnostic Systems Laboratories (Webster, TX). Expression of human PAPP-A could not be detected when transfecting rat osteoblastic cells with pSM22-α-PA. Subsequently, a variant of the pSM22-α-PA construct was created by deleting bp −256 to −249 (5′-GGCCGCCC-3′) of the SM22-α promoter using QuikChange (Stratagene, LaJolla, CA) and 5′-GGTTTTTCCC/TCAGCACCGCCCCGCCCC-3′ and 5′-GCGGTGCTGA/GGGAAAAACCTTTTATGGGCA-3′ as forward and reverse primers, respectively, where / indicates site of deletion. The deletion was verified in the resulting mutated expression construct (pΔSM22-α-PA) by sequencing. Wamhoff et al. (47) had shown that this 8-bp G/C-rich cis element in the SM22-α promoter is responsible for turning off downstream transcription during atherosclerotic plaque development and that deletion of this repressor element allowed SM22-α promoter to be constitutively active. Effective expression of human PAPP-A in transfected rat arterial smooth muscle cells was confirmed for the mutated construct.
Generation of transgenic mice.
pΔSM22-α-PA, with bacterial vector sequences removed, was linearized and purified before pronuclear microinjection by the Mayo Clinic Transgenic Core Facility. Embryos from FVB NHSD mice (Harlan, Indianapolis, IN) were used for injection. Positive founders for transgene integration were identified using two complementary PCR strategies targeting ΔSM22-α and human PAPP-A sequences and confirmed by Southern blot. Eight of these demonstrated germ-line transmission when crossed with wild-type (WT) FVB mice. Subsequent breeding and post-harvest genotyping was performed using 5′-ATGATCCATGAGATTGGTCA-3′ and 5′-TATGAAAGCCACAGGTGTCA-3′ as forward and reverse primers with product size of 199 bp.
Primary smooth muscle cell cultures.
Primary smooth muscle cells were isolated from mouse aorta (AoSMC) as described previously (41). Briefly, the aortic arch and descending aorta were collected from WT and PAPP-A transgenic (Tg) mice, and the vessels were incubated in digestion medium (HBSS containing 0.25 mg/ml soybean trypsin inhibitor, 1 mg/ml collagenase, 2 mg/ml BSA, and 15 mmol/l HEPES) for 1 h. The vessels were washed in HBSS, and the adventitial and endothelial layers were dissected away microscopically. Vessels were then minced with a sterile razor blade and incubated in digestion medium plus 0.125 mg/ml elastase for 1 h. Cells were filtered using a 0.1-mm cell strainer, washed twice with HBSS, and plated in DMEM containing 10% FBS and penicillin-streptomycin (100 U/ml and 100 μg/ml, respectively). For experiments, AoSMCs were used between passages 3 and 7.
IGFBP-4 protease assay.
Cell-free IGFBP-4 proteolysis was assayed as previously described (27). Conditioned media (25 μl) from WT and PAPP-A Tg AoSMCs were incubated at 37°C for 6 h with 125I-IGFBP-4 (10,000 cpm) in the absence or presence of 5 nmol/l recombinant IGF-II (Bachem, Torrance, CA). IGF-II facilitates PAPP-A-mediated IGFBP-4 proteolysis in the assay by binding IGFBP-4 and likely changing its conformation (26, 37). Reaction products were separated by SDS-PAGE and visualized by autoradiography. Films were scanned with an UltroScan XL laser densitometer, and absorbance curves were integrated using GelScan XL software (Pharmacia LKB Biotechnology, Piscataway, NJ). Proteolytic activity, in terms of the percent loss of the intact radiolabeled IGFBP-4 band in the presence IGF-II, was classified as high (>95%), medium (51–95%), low (5–50%), or absent (<5%).
Atherosclerosis model.
PAPP-A Tg mice were crossed with apolipoprotein E (ApoE)-deficient mice, the latter being an established mouse model of atherosclerosis (30, 36, 39, 42). These ApoE KO mice were on a mixed C57BL/6 and 129 genetic background (21). The ApoE genotype was determined by PCR screening using The Jackson Laboratory protocol (http://jaxmice.jax.org). For study I (overexpression), offspring from this mating that were heterozygous for the ApoE gene and positive for high level PAPP-A transgene expression were then intercrossed to produce the following experimental genotypes: mice lacking the ApoE gene (ApoE KO), ApoE KO mice expressing the PAPP-A transgene (ApoE KO/Tg), and mice with normal ApoE alleles without (WT) and with PAPP-A transgene expression (Tg). For study II (rescue), mice that were heterozygous for ApoE and PAPP-A were crossed with mice that were heterozygous for ApoE and PAPP-A alleles and positive for PAPP-A transgene expression to produce double ApoE/PAPP-A KO (KO/KO) mice and KO/KO mice that express human PAPP-A in arterial smooth muscle (KO/KO/Tg). Littermates from cross-breedings were used in both studies to minimize genetic background variation within experiments. Males and females housed separately up to 5 per cage were fed a Western-style diet [21% by weight (42% of calories) fat and 0.15% by weight cholesterol (Harland Tekland)] starting at 7 wk of age. After 10 or 20 wk on diet, depending on the study, the mice were anesthetized and blood was collected from the retro-orbital plexus. The heart and aorta were perfused with 20 ml PBS containing 20 mmol/l 2,6-Di-tert-butyl-4-methylphenol and 2 mmol/l EDTA at the rate of 1 ml/min. After perfusion, the aorta was dissected to the iliac bifurcation, and fat and connective tissue were removed. The heart and aortic arch were separated from the aorta and placed in 10% buffered formalin for histology. The remaining aorta was opened longitudinally and pinned in place on black wax for en face analysis. Lipid-rich regions were stained with Sudan IV, as described (16). Images were captured using a Nikon SMZ80 dissecting scope, a Nikon DMX200F camera, and Nikon Act-1 software v2.62 (Nikon, Melville, NY). Image analysis was performed using Adobe Photoshop software v6.0.1 (Adobe Systems). Lesion area was calculated for each animal as a percentage of total aortic area, and the lesion number was expressed in terms of plaques per 50 mm of aorta, as described previously (21).
Histology and immunohistochemistry.
A minimum of four cross sections of aorta (5 μm) were stained for macrophages (Mac-3 or Rat IgG1 isotype control; BD Pharmingen, San Diego, CA) smooth muscle actin (SMA, Spring Bioscience, Fremont, CA; or Rat IgG1 isotype control) and human PAPP-A (monoclonal antibody; Claus Oxvig, Aarhus University, Aarhus, Denmark), as described previously (3, 21).
RNA isolation and quantitative PCR.
RNA was isolated from tissues and cultured cells using the RNase mini kit (Qiagen). One microgram of RNA from each sample was reverse-transcribed with Taqman reverse-transcription reagents, random hexamers, and Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA).
Quantitative PCR reactions were conducted using the primer sequences either previously published (21) or listed below and the iCycler iQ Detection System. Amplification plots were analyzed with iCycler iQ Detection System analysis software v3.0.6070 (Bio-Rad, Hercules, CA). Gene expression was normalized to mouse ribosomal protein L19 as an internal control (21). The overall sequence identity between murine and human PAPP-A is 91% (44). Primer sets were specifically selected and validated to amplify only one or the other: human PAPP-A, 5′-TGACGCGCGAGCAGGTGG-3′ forward and 5′-CCGTCGTGGCCCGTCAGC-3′ reverse or mouse PAPP-A, 5′-CGGCTTCCTGTTGGAC-3′ forward and 5′-CAATCTGTTGCCAGCTCA-3′ reverse.
Akt phosphorylation.
Mouse AoSMC were incubated overnight in media containing 0.1% FBS and then washed and treated without and with 10 nM IGF-I (LongR3-IGF-I; GroPep, Adelaide, Australia). After 15- and 30-min treatments, cells were lysed in the presence of phosphatase inhibitors in 4× sample buffer containing 100 mM dithiothreitol with sonication (3 × 5-s burst). These samples were processed by SDS-PAGE and immunoblotted with Akt antibodies as described previously (10).
Miscellaneous.
Body composition was measured using the EchoMRI-900 body composition analyzer (Echo Medical Systems, Houston, TX). Total cholesterol was measured in the clinical chemistry laboratory at the Mayo Clinic, and serum IGF-I was measured using a mouse IGF-I ELISA kit generously provided by ImmunoDiagnostic Systems (Fountain Hills, AZ).
Data analysis.
ANOVA and post hoc t-tests were used to compare means of multiple groups. Means were considered significant at P < 0.05, and values presented are means ± SE.
RESULTS
Characterization of PAPP-A transgenic mice.
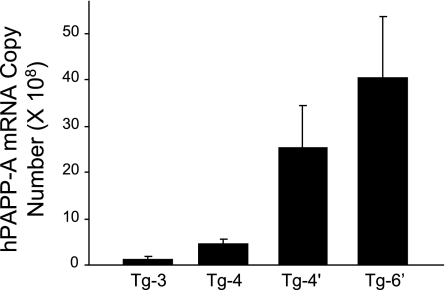
Pronuclear injections resulted in 22 mice testing positive for the human PAPP-A gene, of which 10 showed germ-line transmission. Two of these founders died early of causes unrelated to their genotype, and eight were further characterized. Tg-1 died of unknown causes before any F1 generation was produced. Tg-2 was discontinued as it showed variability in offspring analyses. Aortic SMC cultures from the remaining six Tg lines were analyzed for human PAPP-A mRNA and protein expression and IGFBP-4 proteolytic activity in the culture medium. Based on these results (Table 1), Tg-3, Tg-4, Tg-4′, and Tg-6′ were subjected to further analyses. Tissue specificity of transgene expression was assessed by comparing the ratio of transgene (human) PAPP-A mRNA expression with endogenous (mouse) PAPP-A mRNA expression (Fig. 1). There was detectable human PAPP-A mRNA expression in all the tissues tested; however, except for aorta, bladder, and uterus, the levels were very low. In tissues with high endogenous PAPP-A expression, e.g., kidney, bladder, and uterus, the impact of the human PAPP-A would be minimal. In contrast, the preferential expression in the aorta, where the high transgene expression is coupled with low endogenous PAPP-A, would be more likely to impact vascular physiology. Based on real-time RT-PCR analysis for human PAPP-A expression in aortas from F2 and F3 generations of the different transgenics (Fig. 2), Tg-3 and Tg-4 were classified as low-to-moderate expressers and Tg-4′ and Tg-6′ were classified as high expressers.
Table 1.
hPAPP-A expression in aortic smooth muscle cell cultures from WT and PAPP-ATg mice
| hPAPP-A mRNA | hPAPP-A | Proteolytic Activity | |
|---|---|---|---|
| WT | 0 | 0 | Low |
| Tg-3 | 2,110 ± 8 | 14 ± 0.1 | High |
| Tg-4 | 1,790 ± 146 | 22 ± 1.1 | High |
| Tg-4′ | 1,970 ± 392 | 6 ± 0.3 | High |
| Tg-5 | 98 ± 7 | 2 ± 1.5 | Medium |
| Tg-6′ | 7,080 ± 620 | 20 ± 0.3 | High |
| Tg-7 | ND | 1 ± 0.6 | Medium |
Values are means ± SE. Human pregnancy-associated plasma protein-A (hPAPP-A) mRNA are copies per nanogram mRNA. hPAPP-A is milli international units per 100,000 cells. Proteolytic activity is cell-free inhibitory IGF binding protein (IGFBP-4) proteolysis; see materials and methods. WT, wild-type; Tg, transgene; ND, not determined.
Fig. 1.
Tissue specificity of pregnancy-associated plasma protein-A (PAPP-A) transgene expression. Results are presented as the ratio of transgene (human) PAPP-A expression to endogenous (mouse) PAPP-A expression in aorta (A), bladder (B), heart (H), kidney (K), liver (L), and uterus (U). The dotted line indicates human/mouse PAPP-A expression = 1.0. Results from transgene (Tg)-3 and Tg-4′ are shown. Similar results were seen with the other 2 founder lines.
Fig. 2.
PAPP-A transgene expression levels in aorta of founder transgenic mice. Data are means ± SE of n = 4 mice. hPAPP-A, human PAPP-A.
Body weights of the four PAPP-A Tg mice and WT FVB mice measured up to 16 wk of age are shown in Fig. 3. There was no difference in the growth curves of any of the PAPP-A Tg lines compared with their WT littermates. No human PAPP-A could be detected in serum from PAPP-A Tg mice. Thus PAPP-A expression targeted to arterial smooth muscle appears to be highly localized and with minimal, if any, systemic effects.
Fig. 3.
Growth curves of male (A) and female (B) wild-type (WT) and PAPP-A Tg mice. Data are means of n = 10–13 mice for PAPP-A Tg groups and n = 45 for WT. The SE is not reported for clarity of presentation.
Effect of PAPP-A expression on the development of atherosclerosic lesions.
The breeding scheme to generate ApoE KO, ApoE KO/Tg, WT, and Tg mice for study I (overexpression) is described in materials and methods. The high expresser, Tg-6′, was used for this study. Mice were fed a high-fat, Western-style diet beginning at 7 wk of age. Characteristics of the mice after 10 or 20 wk on diet are presented in Table 2. Mice of all four genotypes gained weight on the high-fat diet. Cholesterol levels were similarly elevated in ApoE KO and ApoE KO/Tg mice and were 10-fold elevated relative to WT and Tg mice. EchoMRI analysis also indicated similar body composition. Serum IGF-I levels were not significantly different in ApoE KO/Tg (467 ± 31 μg/l) and ApoE KO (374 ± 86 μg/l) mice. Lesions in the descending aorta to the iliac bifurcation were stained with Sudan IV and evaluated for lesion area and number (Fig. 4A). As shown in Fig. 4B, ApoE KO/Tg mice 10 wk on diet had a 3.5-fold increase in lesion area compared with ApoE KO mice (P < 0.001). There was no significant difference in lesion number between ApoE KO/Tg and ApoE KO mice (Fig. 4C). Lesions were barely detectable in WT and Tg mice even after 20 wk on the diet. In these and subsequent experiments, the data for male and female mice within each group are combined, since we found no differences relating to sex. Sections of the ascending aorta from ApoE KO and ApoE KO/Tg mice were stained for macrophages and smooth muscle actin. A representative image is presented in Fig. 5. There were no significant differences in macrophage area whether expressed as absolute area or percent of plaque area, the latter being 50.3 ± 5.35% and 45.2 ± 5.76% in ApoE KO and ApoE KO/Tg mice, respectively. Similarly, there were no significant differences in smooth muscle actin staining in ApoE KO and ApoE KO/Tg plaque.
Table 2.
Characteristics of mice in study I overexpression
| ApoE KO | ApoE KO/Tg | P Value | |
|---|---|---|---|
| 10-wk diet | |||
| Weight gain* | 11.1 ± 1.29 | 14.2 ± 1.91 | 0.201 |
| Cholesterol, mg/dL | 2,603 ± 165 | 2,566 ± 151 | 0.661 |
| %Fat | 37.7 ± 2.06 | 33.5 ± 2.38 | 0.201 |
| WT | Tg | P Value | |
|---|---|---|---|
| 20-wk diet | |||
| Weight gain* | 18.3 ± 2.17 | 16.3 ± 1.77 | 0.553 |
| Cholesterol, mg/dL | 364 ± 31 | 241 ± 26 | 0.011 |
| %Fat | 40.4 ± 4.88 | 36.3 ± 2.38 | 0.426 |
Values are means ± SE of n = 7–13 mice/group. ApoE KO, apolipoprotein E knockout.
Weight gain in grams after indicated weeks on high-fat diet.
Fig. 4.
Study I (overexpression): representative en face Sudan IV-stained aortas (A). Quantitated lesion area (B) and lesion number (C) in aortas from apolipoprotein E knockout (ApoE KO; gray bars) and ApoE KO expressing human PAPP-A transgene (ApoE KO/PAPP-A Tg; black bars) mice after 10 wk on high-fat diet are shown. Results are means ± SE of n = 7–13 mice per group. *Significantly different from ApoE KO, P < 0.001.
Fig. 5.
Macrophage staining of ApoE KO and ApoE KO/Tg aortas.
In our previous study (21), global PAPP-A gene deletion markedly diminished lesion progression in ApoE KO mice fed a high-fat diet for 20 wk. Study II (rescue) addressed the question of whether replacement of the PAPP-A gene specifically in arterial smooth muscle in these double ApoE KO/PAPP-A KO (KO/KO) mice would restore the atherosclerotic phenotype. Low-to-moderate expresser, Tg-3, was used for these experiments. KO/KO and KO/KO/Tg mice (see materials and methods for breeding scheme) were fed a high-fat diet for 20 wk. Cholesterol levels were similarly elevated in KO/KO and KO/KO/Tg mice, and EchoMRI analyses indicated similar body composition (Table 3). Serum IGF-I levels were not significantly different in KO/KO mice and KO/KO/Tg mice. As shown in Fig. 6, A and B, KO/KO/Tg mice showed a 2.5-fold increase in lesion area compared with KO/KO mice (P = 0.002). Thus reinstated expression of PAPP-A in arterial smooth muscle restored atherosclerotic lesion development. There was no significant difference in lesion number between the two groups (Fig. 6C).
Table 3.
Characteristics of mice in study II rescue
| KO/KO | KO/KO/Tg | P Value | |
|---|---|---|---|
| 20-wk diet | |||
| Weight gain* | 15.7 ± 2.70 | 19.0 ± 2.21 | 0.383 |
| Cholesterol, mg/dL | 2,774 ± 379 | 2,092 ± 387 | 0.232 |
| %Fat | 39.9 ± 4.70 | 40.5 ± 2.64 | 0.920 |
| Serum IGF-I, μg/l | 484 ± 96 | 449 ± 38 | 0.723 |
Values are means ± SE of 7 mice/group.
Weight gain in grams after indicated weeks on high-fat diet.
Fig. 6.
Study II (rescue): representative en face Sudan IV-stained aortas (A). Quantitated lesion area (B) and lesion number (C) in aortas from KO/KO (gray bars) and KO/KO/Tg (black bars) mice after 20 wk on high-fat diet are shown. Results are means ± SE of n = 8 mice/group. *Significantly different from KO/KO, P = 0.002.
Effect of PAPP-A on local IGF-I bioavailability.
One approach to assessing bioactivity of IGF-I in vivo is to assay for expression of IGF-I-responsive genes. Because IGF-I has been found to stimulate IGFBP-5 mRNA expression in vitro and in vivo (1, 12, 19, 21, 50), increased IGFBP-5 mRNA expression can be used as an indicator of increased IGF signaling through the IGF-I receptor. In agreement with this we find that AoSMC treated with IGF-I show increased signaling through the IGF-I receptor, as measured by Akt phosphorylation, and also increased IGFBP-5 expression (supplemental Fig. 1). An advantage for in vivo assessment is that IGFBP-5 mRNA levels are upregulated for at least 24 h in response to IGF-I receptor activation in contrast with the transient changes in intracellular signaling intermediates. As shown in Fig. 7, IGFBP-5 mRNA was markedly elevated in aortas from ApoE KO/Tg mice compared with ApoE KO mice (P < 0.001), suggesting that the increased PAPP-A expression results in increased IGF-I bioavailability.
Fig. 7.
IGF binding protein (IGFBP)-5 gene expression in aortas from ApoE KO and ApoE KO/Tg mice. Results are means ± SE; n = 5 ApoE KO and n = 4 ApoE KO/Tg. *Significantly different from ApoE KO, P < 0.001.
DISCUSSION
In this study we demonstrate that targeted expression of PAPP-A to arterial smooth muscle in mice results in enhanced atherosclerotic lesion growth. The minimal 5′-flanking sequence of the SM22-α promoter is sufficient to direct specific expression to arterial smooth muscle cells and not to venous or visceral smooth muscle cells (28). However, SM22-α transcription is markedly reduced in atherosclerotic lesions as are several other smooth muscle cell differentiation marker genes (40, 47). Therefore, for these studies we deleted the responsible G/C-rich cis repressor element in the SM22-α promoter to prevent the decreased SM22-α promoter-driven expression in lesions, as demonstrated by Wamhoff et al. (47). Expression of human PAPP-A in the atherosclerotic lesion (Fig. 2 and supplemental Fig. 2) and in aortic smooth muscle cell cultures, the latter being another model of smooth muscle cell dedifferentiation (35), indicated preserved promoter activity.
In study I (overexpression), a relatively high level of PAPP-A expression in arterial smooth muscle clearly accelerated lesion development in ApoE KO mice on a high-fat diet. The impact was on lesion area (3.5-fold greater in ApoE KO/Tg compared with ApoE KO after 10 wk on diet) but not lesion number, indicating a predominant effect on lesion progression rather than lesion initiation. These data in a targeted gain-of-function model are counterpart to findings in ApoE KO mice that are globally deficient in PAPP-A (21). In that study, double ApoE KO/PAPP-A KO (KO/KO) mice had a 70% reduction in lesion area after 10 wk on a high-fat diet compared with ApoE KO mice with normal PAPP-A gene expression, but with no major differences in lesion number between these mice. Thus PAPP-A appears to promote lesion progression rather than lesion initiation.
Study II (rescue) further tested the hypothesis that local PAPP-A was important for lesion development and showed that restoration of PAPP-A gene expression at a low-to-moderate level in arterial smooth muscle of KO/KO mice increased lesion area 2.5-fold. Again, there was no difference in the number of lesions. Thus, in two different experimental models and with two different PAPP-A transgenic founders, we demonstrated a critical role for vascular cell-derived PAPP-A in the development of atherosclerosis.
The ApoE KO mice in the present study appeared to have less overall lesion development, as assessed by Sudan IV staining of aortic lesions, than the ApoE KO mice in a previous study. This is likely due to differences in genetic background of the mice in the two studies. Dansky et al. (17) showed that ApoE KO mice on a C57BL/6 background were more susceptible to the development of atherosclerosis than ApoE KO mice on a FVB background. However, lesions of both strains could progress to fibroproliferative plaques, albeit at different rates. The important point of our studies is the consistent response to PAPP-A.
Our present and previous data (21) indicate that PAPP-A plays a critical role in atherosclerotic lesion development, at least in part, through enhanced IGF bioavailability in the local vascular environment (circulating IGF-I levels are normal in PAPP-A KO mice). These conclusions also fit very well with studies from the Fagin laboratory using mice with targeted expression to smooth muscle of IGF-I, IGFBP-4, and protease-resistant IGFBP-4 (48, 49, 51). Those studies demonstrated a role for local IGFBP-4 proteolysis in modulating IGF-I action on smooth muscle cells in vivo. Byun et al. (8) reported that PAPP-A enhanced the bioactivity of IGFs in vitro by degrading IGFBP-4, and overexpression of PAPP-A has been shown to increase osteoblast proliferation and myoblast proliferation and differentiation through an IGF-dependent mechanism (24, 38). Taken together, these studies in mice support an underlying mechanism for lesion development based on PAPP-A cleavage of IGFBP-4 and, hence, IGF receptor activation. In addition, Nichols et al. (31) demonstrated that inhibition of IGF-I-mediated signaling decreased atherosclerotic lesion size in pigs on a high cholesterol diet. IGF-I, IGFBP-4, and PAPP-A are significantly upregulated in lesional areas compared with nonlesional areas of ApoE KO aortas, whereas there is no difference in IGF-I receptor expression in the two areas (21). The concept of IGFBP-4 functioning as a local reservoir for IGFs with PAPP-A-mediated IGFBP-4 proteolysis necessary to activate IGF activity (7, 25, 43) was experimentally demonstrated in vivo by Ning et al. (32). Expression of the IGF-I-responsive gene, IGFBP-5, has been used as an in vivo marker of IGF-I receptor-mediated activity (1, 12, 19, 21, 50). We verified in mouse AoSMC cultures that treatment with IGF-I stimulated intracellular signaling and IGFBP-5 expression. In this study, IGFBP-5 mRNA abundance was significantly elevated in aortas from ApoE KO/Tg mice compared with ApoE KO mice. These data are consistent with PAPP-A enhancement of local IGF-I bioavailability. It is of note that Sukhanov et al. (45) found that systemic IGF-I infusion decreased atherosclerotic plaque size ∼30% in ApoE KO mice on a Western-style diet. Although these findings appear to be contrary with our findings of increased plaque with increased PAPP-A (and increased local IGF-I), endocrine and paracrine IGF-I likely have different mechanisms of action on the cardiovascular system.
Although it is unclear from this study what cells are affected by PAPP-A in the atherosclerosis model, it is highly likely that the local PAPP-A/IGFBP-4/IGF system is acting on several cell types in the vasculature, including macrophages (11, 13, 14, 18). Enhanced IGF-I-stimulated lipoprotein uptake by macrophages (23) would be consistent with the observed increase in lipid-rich lesional areas in the Apo E KO/PAPP-A Tg mice. Effects on smooth muscle cell proliferation and migration are also likely (41, 48). Targeted overexpression of IGF-I in smooth muscle resulted in smooth muscle cell hyperplasia, and targeted overexpression of IGFBP-4 induced smooth muscle hypoplasia (48, 49, 51). Along these lines, the proliferation and migration of smooth muscle cells involved in neointimal hyperplasia in response to acute vascular injury was markedly reduced in the absence of PAPP-A (41). Potential effects on vascular cell apoptosis also need to be considered (6, 33).
In conclusion, these findings clearly implicate PAPP-A expression in vascular smooth muscle in promoting lesion progression and could have significant clinical implications since PAPP-A, an extracellular protease, would be an attractive target for therapeutics that would attenuate atherosclerotic lesion development.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-74871 (to C. A. Conover) and a fellowship from The Alfred Benzon Foundation (to M. T. Overgaard).
Present address of M. Nyegaard: Dept. of Haematology, Aalborg Hospital, Sdr Skovvej 15, Aarhus University Hospital, DK-9000, Aalborg, Denmark.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the excellent technical contributions of Jacquelyn Grell, Jessica Mader, and Emily Mason.
REFERENCES
- 1.Adamo ML, Ma X, Ackert-Bicknell CL, Donahue LR, Beamer WG, Rosen CJ. Genetic increase in serum insulin-like growth factor-I (IGF-I) in C3H/HeJ compared with C57BL/6J mice is associated with increased transcription from the IGF-I exon 2 promoter. Endocrinology 147: 2944–2955, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Apple FS, Wu AHB, Mair J, Ravkilde J, Panteghini M, Tate J, Pagani F, Christenson RH, Mockel M, Danne O, Jaffe AS, Committee on Standardization of Markers of Cardiac Damage of the IFCC Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem 51: 810–824, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med 345: 1022–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res 86: 125–130, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bayes-Genis A, Schwartz RS, Lewis DA, Overgaard MT, Christiansen M, Oxvig C, Ashai K, Holmes DR, Jr, Conover CA. Insulin-like growth factor binding protein-4 protease produced by smooth muscle cells increases in the coronary artery after angioplasty. Arterioscler Thromb Vasc Biol 21: 335–341, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest 95: 2266–2274, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 17: 10–18, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A accounts for the insulin-like growth factor (IGF)-binding protein-4 (IGFBP-4) proteolytic activity in human pregnancy serum and enhances the mitogenic activity of IGF by degrading IGFBP-4 in vitro. J Clin Endocrinol Metab 86: 847–854, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Clemmons DR. Modifying IGF1 activity: an approach to treat endocrine disorders, atherosclerosis and cancer. Nat Rev 6: 821–833, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Conover CA, Bale LK, Durham SK, Powell DR. Insulin-like growth factor (IGF) binding protein-3 potentiation of IGF action is mediated through the phosphatidylinositol-3-kinase pathway and is associated with alteration in protein kinae B/Akt sensitivity. Endocrinology 141: 3098–3103, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. Am J Physiol Cell Physiol 290: C183–C188, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Conover CA, Clarkson JT, Bale LK. Effect of glucocorticoid on insulin-like growth factor (IGF) regulation of IGF-binding protein expression in fibroblasts. Endocrinology 136: 1403–1410, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res 18: 213–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conover CA, Harrington SC, Bale LK, Oxvig C. Surface association of pregnancy associated plasma protein-A accounts for its colocalization with activated macrophages. Am J Physiol Heart Circ Physiol 292: H994–H1000, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Consuegra-Sanchez L, Fredericks S, Kaski JC. Pregnancy-associated plasma protein-A (PAPP-A) and cardiovascular risk. Atherosclerosis 203: 346–352, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Conti E, Carrozza C, Capoluongo E, Volpe M, Crea F, Zuppi C, Andreotti F. Insulin-like growth factor-1 as a vascular protective factor. Circulation 110: 2260–2265, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, Shu P, Moore KJ, Breslow JL, Smith JD. Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 19: 1960–1968, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24: 435–444, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Duan C, Hawes SB, Prevette T, Clemmons DR. Insulin-like growth factor-I (IGF-I) regulates IGF-binding protein-5 synthesis through transcriptional activation of the gene in aortic smooth muscle cells. J Biol Chem 271: 4280–4288, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Elesber AA, Lerman A, Denktas AE, Resch ZT, Bunch TJ, Schwartz RS, Conover CA. Pregnancy associated plasma protein-A and risk stratification of patients presenting with chest pain in the emergency department. Int J Cardiol 117: 365–369, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res 100: 1696–1702, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Simoons ML, Zeiher AM, CAPTURE Study Investigators Pregnancy-associated plasma protein-A levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol 45: 229–237, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Hochberg Z, Hertz P, Maor G, Oiknine J, Aviram M. Growth hormone and insulin-like growth factor-I increase macrophage uptake and degradation of low density lipoprotein. Endocrinology 131: 430–435, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Mohan S, Newton J, Rehage M, Tran K, Baylink DJ, Qin X. Pregnancy-associated plasma protein-A regulates myoblast proliferation and differentiation through an insulin-like growth factor-dependent mechanism. J Biol Chem 280: 37782–37789, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laursen LS, Kjaer-Sorensen K, Andersen MH, Oxvig C. Regulation of insulin-like growth factor (IGF) bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol 21: 1246–1257, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 504: 36–40, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, III, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci USA 96: 3149–3153, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Li, Miano JM, Mercer B, Olson EN. Expression of the SM22α promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol 132: 849–859, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund J, Qin QP, Ilva T, Pettersson K, Voipio-Pulkki LM, Porela P, Pulkki K. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation 108: 1924–1926, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 14: 133–140, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Nichols TC, du Laney T, Zheng B, Bellinger DA, Nickols GA, Engleman W, Clemmons DR. Reduction in atherosclerotic lesion size in pigs by αVβ3 inhibitors is associated with inhibition of insulin-like growth factor-I-mediated signaling. Circ Res 85: 1040–1045, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Ning Y, Schuller AGP, Conover CA, Pintar JE. Insulin-like growth factor (IGF) binding protein-4 is both a positive and negative regulator of IGF activity in vivo. Mol Endocrinol 22: 1213–1225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. J Mol Cell Cardiol 33: 1777–1789, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, Sottrup-Jensen L, Conover CA, Oxvig C. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem 275: 31128–31133, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71: 343–353, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Qin X, Byun D, Lau KH, Baylink DJ, Mohan S. Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch Biochem Biophys 379: 209–216, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology 147: 5653–5661, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking Apo E: evaluation of lesional development and progression. J Atheroscler Thromb 14: 141–147, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest 106: 1139–1147, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resch ZT, Simari RD, Conover CA. Targeted disruption of the PAPP-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia following vascular injury. Endocrinology 147: 5634–5640, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Smith JD, Breslow JL. The emergence of mouse models of atherosclerosis and their relevance to clinical research. J Intern Med 242: 99–109, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Smith EP, Kamyar A, Niu W, Wang J, Cercek B, Chernausek SD, Fagin JA. IGF-binding protein-4 expression and IGF-binding protein-4 protease activity are regulated coordinately in smooth muscle during postnatal development and after vascular injury. Endocrinology 142: 4420–4427, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Soe R, Overgaard MT, Thomsen AR, Laursen LS, Olsen IM, Sottrup-Jensen L, Haaning J, Giudice LC, Conover CA, Oxvig C. Expression of recombinant murine pregnancy-associated plasma protein-A (PAPP-A) and a novel variant (PAPP-Ai) with differential proteolytic activity. Eur J Biochem 269: 2247–2256, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, Song YH, Titterington J, Delafontaine P. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 27: 2684–2690, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Thorn EM, Khan IA. Pregnancy-associated plasma protein-A: an emerging cardiac biomarker. Int J Cardiol 117: 370–372, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22α promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 95: 981–988, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest 100: 1425–1439, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Niu W, Witte DP, Chernausek SD, Nikiforov YE, Clemens TL, Sharifi B, Straugh AR, Fagin JA. Overexpression of insulin-like growth factor-binding protein-4 (IGFBP-4) in smooth muscle cells of transgenic mice through a smooth muscle α-actin-IGFBP-4 fusion gene induces smooth muscle hypoplasia. Endocrinology 139: 2605–2614, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Ye P, D′Ercole J. Insulin-like growth factor (IGF-I) regulates IGF binding protein-5 gene expression in the brain. Endocrinology 139: 65–71, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Smith EP, Kuroda H, Banach W, Chernausek SD, Fagin JA. Targeted expression of a protease-resistant IGFBP-4 mutant in smooth muscle of transgenic mice results in IGFBP-4 stabilization and smooth muscle hypotrophy. J Biol Chem 277: 21285–21290, 2002 [DOI] [PubMed] [Google Scholar]