Abstract
Cardiac diseases persistently increase the contractility demands of cardiac myocytes, which require activation of the sympathetic nervous system and subsequent increases in myocyte Ca2+ transients. Persistent exposure to sympathetic and/or Ca2+ stress is associated with myocyte death. This study examined the respective roles of persistent β-adrenergic receptor (β-AR) agonist exposure and high Ca2+ concentration in myocyte death. Ventricular myocytes (VMs) were isolated from transgenic (TG) mice with cardiac-specific and inducible expression of the β2a-subunit of the L-type Ca2+ channel (LTCC). VMs were cultured, and the rate of myocyte death was measured in the presence of isoproterenol (ISO), other modulators of Ca2+ handling and the β-adrenergic system, and inhibitors of caspases and reactive oxygen species generation. The rate of myocyte death was greater in TG vs. wild-type myocytes and accelerated by ISO in both groups, although ISO did not increase LTCC current (ICa-L) in TG-VMs. Nifedipine, an LTCC antagonist, only partially prevented myocyte death. These results suggest both LTCC-dependent and -independent mechanisms in ISO induced myocyte death. ISO increased the contractility of wild type and TG-VMs by enhancing sarcoplasmic reticulum function and inhibiting sarco(endo)plasmic reticulum Ca2+-ATPase, Na+/Ca2+ exchanger, and CaMKII partially protected myocyte from death induced by both Ca2+ and ISO. Caspase and reactive oxygen species inhibitors did not, but β2-AR activation did, reduce myocyte death induced by enhanced ICa-L and ISO stimulation. Our results suggest that catecholamines induce myocyte necrosis primarily through β1-AR-mediated increases in ICa-L, but other mechanisms are also involved in rodents.
Keywords: Ca2+ signaling, β2-adrenergic receptor, mouse heart, necrosis, β2a-subunit
cardiovascular diseases that persistently increase the contractile demands (diseases such as hypertension and myocardial infarction) on the heart induce a progressive increase in the death of ventricular myocytes (VMs), and this can initiate heart failure (HF) and drive its progression (18, 25). The factors that initiate myocyte death in these hearts are still not clearly defined and are the topic of this study.
Hearts subjected to pathological hemodynamic stress must increase their Ca2+-mediated force generation to maintain cardiac output, and this requires activation of the sympathetic reflex responses. Sympathetic neurohormones enhance myocyte contractility, in large part by binding to β-adrenergic receptors (β-AR), which activate protein kinase A (PKA) signaling cascades (28). A principle PKA target is the L-type Ca2+ channel (LTCC), and its phosphorylation increases channel activity and Ca2+ influx. This increased Ca2+ influx loads the sarcoplasmic reticulum (SR) with extra Ca2+ and increases SR Ca2+ release, culminating in an increase in myocyte contractility (5). While this increase in myocyte Ca2+ produces the force required to meet the excessive contractile demands, it can also activate processes that cause cardiac dysfunction, such as pathological hypertrophy (21). Persistent activation of Ca2+ and adrenergic signaling cascades in cardiovascular disease is now clearly linked to activation of myocyte death signaling (17, 34, 42). How each contributes to cell death signaling is complicated by the fact that β-adrenergic signaling has profound effects on Ca2+ handling. Therefore, the respective contributions of increased Ca2+ influx through LTCCs, increased Ca2+ handling per se, and non-Ca2+ related β-adrenergic target protein activation on cell death are still not clearly defined and are the topic of this research.
Catecholamine signaling in the heart is complex, in part because of multiple AR subtypes with unique signaling partners. For example, β1- and β2-AR subtypes are known to have distinct effects on cardiac contraction and myocyte apoptosis (1, 35). β1-AR overstimulation can cause myocyte necrosis and/or apoptosis, while β2-AR activation can induce cardioprotective, prosurvival signaling (2, 37). The present study also explored the role of persistent activation of β-AR subtypes in cell death and survival.
In human heart disease, Ca2+ and adrenergic signaling are disrupted (14, 27). The LTCCs are in a highly active gating mode with increased open probability (10), and this contributes to abnormal Ca2+ influx and possibly to myocyte death signaling. Persistent activation of β-ARs causes disruption of β1- and β2-signaling cascades, and these changes are also linked to increased myocyte death (17, 38). It is not clear if adrenergic-mediated cell death is caused by the resultant increase in LTCC activity and increased Ca2+ influx through the LTCC (ICa-L), or if other factors are involved (13).
In the present experiments, we used myocytes in which we induced a persistent increase in Ca2+ influx through the LTCC (by expressing the β2a-subunit of the LTCC). The transgenic (TG) β2a-subunit causes increases in LTCC open probability, in much the same way as PKA-mediated channel phosphorylation in HF, but does not require PKA. Therefore, we could test the effects of increased Ca2+ influx on myocyte death without having to expose the myocyte to catecholamines. Another interesting feature of TG myocytes is that the ICa-L is not increased with catecholamine exposure. This allowed us to test the effects of chronic catecholamine exposure on cell death signaling without inducing an increase in Ca2+ influx.
The present experiments show that persistent increases in Ca2+ through the LTCC (TG myocytes) are sufficient to induce myocyte death without requiring exposure to β1-sympathetic agonists. β1-adrenergic agonists increased myocyte death in wild-type (WT) and TG myocytes, primarily by increasing Ca2+ influx. β1-adrenergic signaling also induced myocyte death via ICa-L-independent mechanisms that involved the SR and the Na+/Ca2+ exchanger (NCX). These studies suggest that persistent exposure of cardiac myocytes to β1-adrenergic agonists primarily causes their death by excessive increases in those Ca2+ handling processes that normally mediate physiological increases in myocyte contractility.
MATERIALS AND METHODS
Mice.
WT (FVB/N) and TG mice were used in these studies. As described previously (23, 31), a TG mouse line with cardiac-specific and inducible low overexpression (3.1-fold) of LTCC β2a-subunit was created. Mice between 4∼5 mo of age were used. All animal protocols were approved by the Animal Care and Use Committee of Temple University. All animals received humane care in compliance with University standards and “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH publication 85–23, revised 1996).
Cardiac VM isolation and culture.
Adult mouse VMs were isolated and cultured according to the methods described previously (23–24, 32, 43) with modifications. Briefly, animals were anesthetized with pentobarbital sodium (120 mg/kg), and hearts were digested via retrograde perfusion of a Tyrode solution containing collagenase (290 U/ml) and salts (in mM): 0.02 CaCl2, 10 glucose, 5 HEPES, 5.4 KCl, 1.2 MgCl2, 150 NaCl, 2 sodium pyruvate, 5 taurine, 10 2,3-butanedione monoxine (BDM), pH 7.4. After 6- to 8-min digestion, the ventricles were minced, and isolated VMs were equilibrated in Tyrode solutions serially supplemented with 125–250 μmol/l CaCl2 (10 min for each step). Then VMs were centrifuged down, and the pellet was resuspended in a culture medium: MEM (Invitrogen, Carlsbad, CA) containing Hanks' buffered salt solution, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.55 μg/ml transferring, 2 mM glutamin, 4 mM NaHCO3, and 2 mg/ml bovine serum albumin. Cells were plated in cell culture dishes coated with 10 μg/l laminin at a density of 50 rod-shaped cells/mm2 and cultured at 37°C in a 5% CO2 incubator for 1 h to allow attachment. Then the culture medium was changed. VMs were cultured in the absence of BDM, because it has profound effects on Ca2+ handling and multiple phosphorylation-dependent signaling cascades (24), which are the focus of the present study. Myocytes in culture were not paced, because we found that an inconsistent percentage of the myocytes responded to field stimulation, and long-term pacing might change the composition of the culture medium by hydrolyzing hydrogen peroxide (H2O2).
Neonatal rat VMs (NRVMs) were isolated using the method described in detail previously (26), which were used as positive controls for stimulation of apoptosis and reactive oxygen species (ROS) generation induced by H2O2.
Adult mouse myocyte viability in culture.
Only rod-shaped adult myocytes retain functional and morphological integrity. It has been shown that rod-shaped myocytes in culture are negative for annexin V, propidium iodide staining, and Trypan blue. Therefore, the percentage of rod-shaped myocytes has been used widely as an index of myocyte viability (45). In our study, only healthy, rod-shaped myocytes (length-to-width ratio > 3:1) with clear sarcomere structures were counted. The myocyte viability rate is calculated as number of rod-shaped myocytes/total number of rod- and round-shaped myocytes. Trypan blue staining was also applied to determine myocyte survival. Cells in tissue culture dishes were stained for 10 min at room temperature with Trypan blue solution at a final concentration of 0.04% (wt/vol). Cells with Trypan blue positive staining were considered dead (9). Our preliminary experiments showed that the percentage of Trypan blue excluding myocytes was not different than the percentage of rod-shaped myocytes.
Measurements of myocyte contractility, intracellular calcium transients, and ICa-L.
All of these experiments were performed with freshly isolated single rod-shaped myocytes with clear sarcomeric cross striations. In brief, myocytes were placed in a heated chamber (35°C) on a stage of an inverted microscope (Nikon Eclipse 200) and perfused with Tyrode solution containing 1 mM CaCl2. Myocytes were loaded with fluo 4-AM to measure Ca2+ transients (intracellular Ca2+ concentration, [Ca2+]i), and myocyte fractional shortening was measured with edge detection (video edge detection, VED-104, Crescent Electronics, Sandy, UT).
Whole cell ICa-L were measured in Na+- and K+-free solutions at 35°C using techniques described in detail previously (23). Membrane potential was controlled by an Axoclamp 2 voltage-clamp amplifier in discontinuous mode (7 kHz) (Axon Instruments). The amplifier was controlled by pCLAMP 10.0 software, and data were acquired by a Digidata 1320. Data were analyzed offline with Clampfit 10.0 software (Molecular Devices).
ROS analysis.
ROS production was examined by 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA, Invitrogen, Carlsbad, CA). Myocytes were washed with Ca2+- and Mg2+-free PBS, H2DCFDA (10 μM) was then added to myocytes at 37°C for 15 min in the dark. Cells were washed and analyzed by a fluorescence microscope or a confocal microscope with excitation at 488-nm and emission at 530-nm wavelengths (19). As a positive control, the ROS production in NRVMs is induce by H2O2.
Drug administration.
Nonselective β-AR agonist isoproterenol (ISO, 0.1 μM) was used to induce myocyte death. A β1-AR agonist dobutamine (DOB, 1 μM), a β2-AR agonist zinterol (ZIN, 1 μM), a β1-AR antagonist CGP-20712A (CGP, 1μM) and a β2-AR antagonist ICI 118551 (ICI, 0.5 μM) were used to induce β-AR subtype activation or inhibition. To explore the role of Ca2+ regulation in myocyte death, an LTCC antagonist nifedipine (10 μM), a Ca2+/calmodulin-dependent kinase II (CaMKII) inhibitor (KN-93, 1 μM), a sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) inhibitor (thapsigargin, 1 μM), a NCX inhibitor (KB-R7943, 10 μM, Tocris, Ellisville, MO), and an intracellular Ca2+ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl) ester (BAPTA-AM, 10 μM) were used. To elucidate the roles of ROS and nitric oxide synthase (NOS) in cultured mouse myocyte death, cells were treated with an antioxidant catalase-polyethylene glycerol (CAT-PEG; 50 U/ml) plus a superoxide dismutase-PEG (SOD-PEG; 250 U/ml), a xanthine oxidase inhibitor (allopurinol, 100 μM), a NAD(P)H oxidase inhibitor (apocynin, 100 μM), and an NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 100 μM). To determine the involvement of caspases in cultured mouse myocyte death, a caspase-3 inhibitor, Z-Asp(O-Me)-Glu(O-Me)-Val-Asp(O-Me) fluoromethyl ketone (z-DEVD-FMK, 20 μM), and a caspase-9 inhibitor, Z-Leu-Glu(O-Me)-His-Asp(O-Me) fluoromethyl ketone trifluoroacetate salt hydrate (z-LEHD-FMK, 20 μM), were used. As positive control for testing the effect of ROS scavengers and caspase inhibitors, H2O2 was used to stimulate ROS production (25 μM, 1 h) and to induce apoptosis (10 μM, 12 h) in cultured NRVMs. The concentration of each drug, which offered maximal protection from myocyte death, was determined in preliminary experiments. All reagents were purchased from Sigma Chemical (St Louis, MO), unless otherwise stated.
Statistics.
Data in the text and Figs. 1–7 are reported as means ± SE. Statistical analyses were performed using ANOVA with Student-Newman-Keuls post hoc test or Student's t-tests (Graphpad Prism). A P value of ≤0.05 was considered significant. In this study, n is the number of cells examined, and N is the animal number or times of cell cultures.
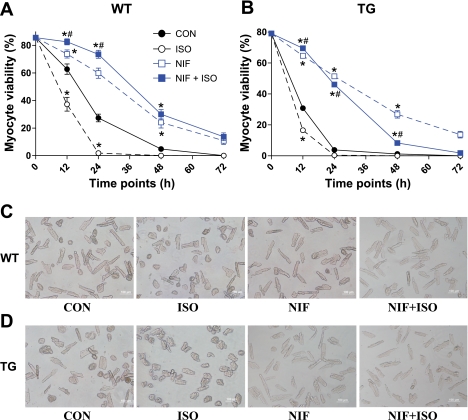
Fig. 1.
Trangenic (TG) myocyte (B) viability was significantly reduced compared with wild-type (WT) myocytes (A), and isoproterenol (ISO) treatment enhanced myocyte death in both groups. Nifedipine (NIF) increased survival of both WT and TG myocytes. ISO + NIF treatment increased myocyte viability compared with NIF alone in WT and initially in TG myocytes. Values are means ± SE; N = 7. C: representative images of WT myocytes at 12 h. *Significant difference vs. control (CON). #Significant difference, ISO + NIF vs. NIF. D: representative images of TG myocytes at 12 h. ISO is a nonselective β-adrenergic receptor (AR) agonist; NIF is an L-type Ca2+ channel (LTCC) antagonist.
Fig. 7.
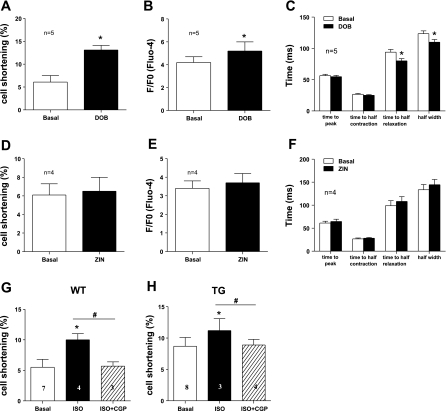
Myocyte contractility: [Ca2+]i transients after treatments was increased by β1-AR activation, but not by β2-AR activation. A and B: WT myocyte fractional shortening and [Ca2+]i transients, respectively, before and after β1-AR agonist DOB treatment. C: [Ca2+]i transient kinetics before and after DOB treatment. D and E: comparison of WT myocyte fractional shortening and [Ca2+]i transients, respectively, before and after β2-AR agonist ZIN treatment. F: [Ca2+]i transient kinetics before and after ZIN treatment. G and H: β1-AR antagonist CGP blunted ISO-induced increase of myocyte contractility in WT and TG mice, respectively. Values are means ± SE. *P < 0.05 vs. basal. #P < 0.05 vs. ISO.
RESULTS
WT and TG myocytes die with time in culture.
The percentage of rod-shaped VMs was not different between WT (85.6 ± 4.6%) and TG (79.1 ± 6.5%) myocytes at the beginning of the culture. After 12 h in culture, myocyte viability decreased in both WT and TG groups (WT 62.6 ± 3.4% and TG 30.1 ± 3.6%, N = 7). The greater decrease in the TG myocytes indicates the involvement of the LTCC (31). A central role for Ca2+ influx via the LTCC in the death of these cultured myocytes was confirmed by nifedipine treatment, which increased myocyte viability and negated the difference between WT and TG VMs at 12 h (Fig. 1). Ca2+ influx through the LTCC could involve so-called window current or from myocytes with spontaneous action potentials in myocytes not paced. This issue was not pursued in the present study. However, even in the presence of nifedipine, the number of viable myocytes continued to decline after 12 h of culture, suggesting LTCC-independent mechanisms of death in these cultured mouse myocytes.
ISO increases death of WT and TG myocytes.
Treating VMs with ISO significantly reduced the viability in both WT and TG myocytes at 12 h, but the reduction of myocyte viability was more pronounced in the TG group (WT 33.2 ± 1.7% vs. TG 16.6 ± 1.4%, N = 7). Nifedipine fully blocked ISO-induced death in WT myocytes and largely blunted ISO-induced TG myocyte death. Importantly, ISO + nifedipine treatment led to a significant increase in myocyte viability (compared with nifedipine alone) in both WT and TG mice (Fig. 1). However, ISO caused myocyte viability to decrease after 12 h of exposure, even with nifedipine, suggesting that ISO-induced myocyte death involves both LTCC-dependent and -independent mechanisms.
ICa-L in TG VMs does not respond to ISO.
β1-Adrenergic agonists increase the ICa-L in normal VMs (39), and our data suggest that excessive Ca2+ entry through this channel can cause myocyte death induced by persistent β-adrenergic stimulation. Our study design also is predicated on the supposition that the association of β2a with the LTCC causes channel activation similar to that caused by ISO and eliminates any additional ISO effects on ICa-L. ISO increased ICa-L in WT myocytes (maximal ICa-L amplitude: basal −11.2 ± 3.9 pA/pF vs. ISO −18.4 ± 2.9 pA/pF, n = 7, P < 0.05) and shifted the voltage dependence of ICa-L activation to more negative voltages [half-activation potential (V0.5) of activation: basal −5.7 ± 2.3 mV vs. ISO −21.9 ± 3.2 mV, n = 7, P < 0.05] (Fig. 2). In TG myocytes, ICa-L was increased (maximal ICa-L amplitude: WT −11.2 ± 3.9 pA/pF vs. TG −21.1 ± 1.9 pA/pF, n = 7, P < 0.05), and the voltage dependence of activation was shifted to negative voltages (V0.5 of activation: WT −5.7 ± 2.1 mV vs. TG −29.0 ± 2.8 mV, n = 7, P < 0.05) by β2a overexpression. These data show that β2a causes ISO-like effect on ICa-L. ISO exposure did not increase ICa-L amplitudes (maximal ICa-L amplitude: basal −21.1 ± 1.9 pA/pF vs. ISO −22.3 ± 2.4 pA/pF, n = 5) or change the voltage dependency of ICa-L activation in TG myocytes (V0.5 of activation: basal −29.0 ± 2.8 mV vs. TG −26.5 ± 1.5 mV, n = 5). These findings suggest that ISO enhanced the death of TG myocytes by an LTCC-independent mechanism.
Fig. 2.
L-type Ca2+ current (ICa-L) lost responsiveness to ISO in TG myocytes. A and B: representative traces of ICa-L before and after the application of ISO in WT and TG myocytes, respectively. C: comparison of peak ICa-L before and after ISO treatment in WT and TG myocytes. ISO had no effect on TG myocytes. D: voltage-current relationships of ICa-L in WT or TG myocytes before and after ISO treatment. E and F: voltage dependence of ICa-L activation and average half-activation potential (V0.5), respectively, in WT and TG myocytes before and after ISO. Values are means ± SE. G/Gmax, normalized conductance. *P < 0.05 vs. WT basal.
The SR can act as an LTCC-independent Ca2+ source for β-adrenergic induced myocyte death.
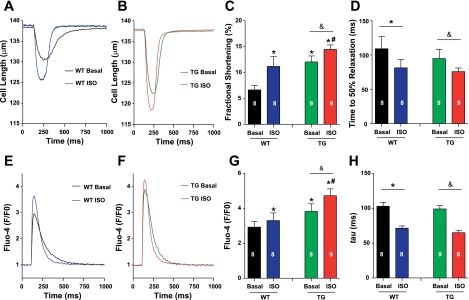
Myocyte death induced by β-adrenergic stimulation is known to involve alterations in SR Ca2+ handling, with SR Ca2+ overload thought to be centrally involved (16). However, these effects could be entirely explained by the ability of catecholamines to increase ICa-L. Since ISO did not increase ICa-L in TG myocytes, we were able to explore its ICa-L-independent effects on myocyte death. The effects of ISO on myocyte contraction and [Ca2+]i transients were measured in both WT and TG myocytes. As shown in Fig. 3, in both WT and TG myocytes, ISO increased the amplitude of cell shortening (WT: basal 7.8 ± 1.0% vs. ISO 10.7 ± 0.7%, n = 11, P < 0.05; TG: basal 12.1 ± 1.2% vs. ISO 14.5 ± 0.9%, n = 10, P < 0.05) and [Ca2+]i transients (WT: basal 2.9 ± 0.3 vs. 3.3 ± 0.4, n = 11, P < 0.05; TG: basal 3.8 ± 0.4 vs. 4.7 ± 0.4, n = 9, P < 0.05). These data show that ISO can enhance Ca2+ handling even when it does not increase ICa-L. These effects could result from an increase in SR Ca2+ uptake, storage, and release, secondary to the ability of PKA to phosphorylate phospholamban and reduce its inhibitory effect on SERCA (27). The increased rate of myocyte relaxation and [Ca2+]i decay in both WT and TG myocytes after ISO supports an increased SERCA activity. These data suggest that the enhanced myocyte Ca2+ handling, especially an increased SR Ca2+ content, could cause myocyte death, even when ICa-L is not increased by ISO. Consistent with this hypothesis, a specific SERCA inhibitor (thapsigargin, 1 μM) reduced the myocyte death produced by ISO (Fig. 4) in WT myocytes. In TG myocytes, thapsigargin reduced myocyte death under control conditions, but did not prevent the robust myocyte death in these myocytes after ISO exposure. This could be due to very high cytosolic Ca2+ in TG myocytes because of their increased basal Ca2+ influx via the LTCC and reverse-mode NCX (see below).
Fig. 3.
The effects of ISO on myocyte contractility, cytosolic calcium concentration ([Ca2+]i), and transients in WT and TG mice. A and B: representative examples of myocyte contraction in WT and TG myocytes, respectively, before and after ISO treatment. C and D: average effects of ISO on the amplitude and time to 50% relaxation of myocyte fractional shortening, respectively, in WT and TG mice. E and F: representative traces of myocyte [Ca2+]i transients in WT and TG myocytes, respectively, after ISO treatment. G and H: average amplitude of [Ca2+]i transients and the time constant (tau) for Ca2+ transient decay, respectively, in WT and TG myocytes after ISO treatment. Values are means ± SE (WT, n = 8 and TG, n = 9). F/F0, relative fluorescence. *P < 0.05 vs. WT basal; #P < 0.05 vs. WT ISO; &P < 0.05 vs. TG basal.
Fig. 4.
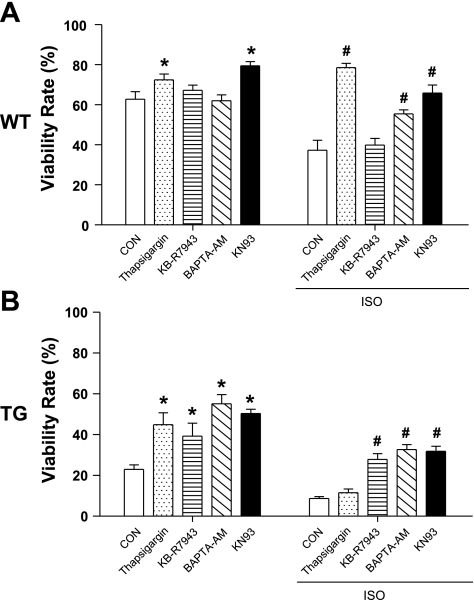
Effects of drugs modulating Ca2+ handlings on myocyte viability, with or without ISO treatment. A: WT myocyte viability at 12 h. B: TG myocyte viability at 12 h. Values are means ± SE; N = 6. *P < 0.05 vs. WT CON; #P < 0.05 vs. TG CON. Thapsigargin, a sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) inhibitor; KB-R7943, a Na+/Ca2+ exchanger (NCX) inhibitor; BAPTA-AM, an intracellular Ca2+ chelator; KN-93, a Ca2+/calmodulin-dependent kinase II (CaMKII) inhibitor.
Reverse-mode NCX activity contributes to Ca2+-mediated myocyte death of TG myocytes.
Rodent VMs have a higher cytoplasmic Na+ concentration ([Na+]) than myocytes from larger mammals (6). This causes rodent myocytes to accumulate Ca2+ via reverse-mode NCX when placed in culture (7). This rodent-specific Ca2+ handling causes these myocytes to be more susceptible to Ca2+ overload-mediated cell death in the nonpaced culture conditions we employed in these studies. To test the contribution of NCX-mediated Ca2+ influx to myocyte death induced by ISO, myocytes were exposed to an NCX inhibitor (KB-R7943), which is known to preferentially inhibit Ca2+ influx via reverse-mode NCX (15). KB-R7943 did not improve survival of WT myocytes under control conditions or after ISO (Fig. 4). KB-R7943 did improve the survival of TG myocytes under both control conditions and after ISO treatment in TG myocytes. These results suggest that Ca2+ influx through reverse-mode NCX was not a major death, inducing Ca2+ influx in WT myocytes. The protective effect of KB-R7943 in TG myocytes suggests that NCX-mediated Ca2+ influx is greater in these myocytes (31). This could result from an increase in NCX abundance or from an increase in NCX activity, resulting from a greater [Na+] in TG myocytes (not studied).
Ca2+ is the cause of death in cultured mouse myocytes.
Our data suggest that Ca2+ influx through the LTCC and the NCX overloads the SR and promotes mouse myocytes to die in culture, and ISO accelerates this process. To further document a central role of cell [Ca2+] in myocyte death, cells were loaded with a Ca2+ chelator, BAPTA-AM. BATPA-AM slowed the death of WT and TG myocytes in control conditions and after ISO (Fig. 4). BAPTA-AM also increased myocyte viability beyond 12 h (data not shown).
CaMKII is a mediator of myocyte death.
CaMKII has been implicated in Ca2+-mediated myocyte death (8). We used a CaMKII inhibitors, KN93, to test the role of CaMKII in WT and TG myocyte death in culture. KN93 significantly preserved the viability of myocytes from both WT and TG mice (Fig. 4).
ROS and caspases are not involved in ISO-induced WT and TG myocyte death.
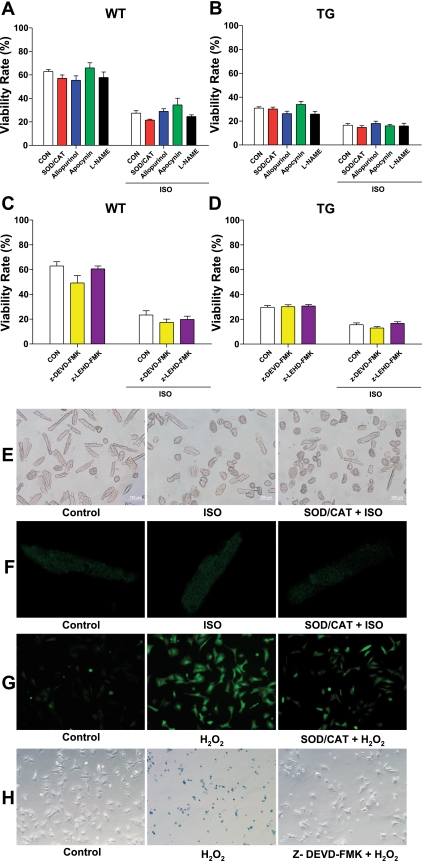
ROS can be released from mitochondria to mediate cell death when there is cellular Ca2+ overload (33). Particularly, the chronic release of ROS appears to derive from the nonphagocytic NAD(P)H oxidase and mitochondria (36). The fluorescence intensity of carboxy-H2DCFDA was measured to indicate intracellular ROS production. Both increasing Ca2+ influx through the LTCC (TG myocytes) and increasing cellular Ca2+ by ISO did not significantly increase myocyte ROS production (Fig. 5). In addition, ROS scavengers (SOD-PEG plus CAT-PEG) did not prevent the myocyte death induced by ISO. The NAD(P)H oxidase inhibitor apocynin, the xanthine oxidase inhibitor allopurinol, as well as NOS inhibitor l-NAME also did not affect myocyte death in both WT and TG groups (Fig. 5). As a positive control, ROS scavengers and inhibitors were proven to be effective in reducing the ROS production induced by H2O2 in NRVMs. These results show that, under our experimental conditions, Ca2+-mediated myocyte death does not involve ROS and NOS.
Fig. 5.
Inhibition of reactive oxygen species (ROS), nitric oxide synthase (NOS) pathways, and the caspase cascade did not affect ISO-induced WT and TG myocyte death. A and B: WT and TG myocyte viability, respectively, after 12 h of ISO treatment in the presence of ROS scavengers superoxide dismutase (SOD)-polyethylene glycerol (PEG) plus catalase (CAT)-PEG, ROS inhibitor allopurinol and apocynin, and NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME). C and D: WT and TG myocyte viability, respectively after 12 h of ISO treatment in the presence of caspase-3 inhibitor z-DEVD-fmk and caspase-9 inhibitor z-LEHD-FMK. Values are means ± SE. E: representative images of myocyte viability in WT adult mouse cardiomyocytes at 12 h. F: representative images of ROS production in WT adult mouse cardiomyocytes at 12 h, indicated by relative fluorescence intensity of carboxy-H2DCFDA. ISO and SOD-CAT treatment did not significantly alter the ROS production in rod-shaped myocytes. G: representative images of ROS production in neonatal rat ventricular myocytes (NRVMs). SOD-CAT reduced ROS production stimulated with H2O2 by 45.5 ± 6.2%. H: representative images of Trypan blue staining in NRVMs. Caspase-3 inhibitor z-DEVD-fmk reduced NRVM death induced with H2O2 by 55.7 ± 2.4%. N = 3.
Apoptosis is not the mechanism of cell death induced by excessive Ca2+ influx and β-adrenergic stimulation.
Our laboratory has previously shown that excessive Ca2+ influx through the LTCC can induce apoptosis in feline VMs (11). Apoptosis can also contribute to myocyte death induced by β-adrenergic stimulation (18). In this study, caspase-3 or caspase-9 inhibitors (z-DEVD-FMK and z-HEVD-FMK) did not inhibit myocyte death in both WT and TG mice (Fig. 5), although these caspase inhibitors were proven to reduce NRVM apoptosis induced by H2O2 (10 μM, 12 h). These results suggest that myocyte death induced by both excessive Ca2+ influx and β-adrenergic stimulation is probably the result of a form of necrosis, similar to what we have observed under some conditions in vivo (31).
β2-AR stimulation can protect against Ca2+-mediated myocyte death.
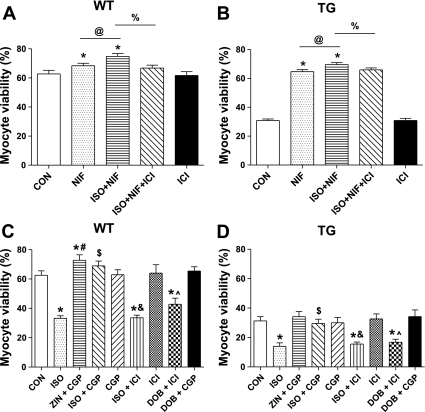
We showed (Fig. 1) that, when myocytes treated with nifedipine were also treated with ISO, the rate of cell death decreased. These results suggest that ISO can have some beneficial effect on cell survival when the LTCC is inhibited. ISO nonselectively binds to and activates both β1-AR and β2-AR and the activation of β2-AR can protect myocytes from different types of stress (2, 12, 37, 44). We found that a β2-AR antagonist (ICI) abolished the beneficial effect of ISO in nifedipine-treated myocytes in both WT and TG myocytes (Fig. 6). In separate experiments, we showed that β2-AR activation (ZIN+CGP or ISO+CGP) caused protection from Ca2+-mediated necrotic cell death. Selective β1-AR activation (ISO+ICI or DOB+ICI) significantly decreased myocyte viability, which was prevented by β1-AR antagonist CGP (Fig. 6). These data support the idea that the activation of β2-AR protects WT and TG myocytes from the Ca2+-mediated death induced by our conditions.
Fig. 6.
β1-AR antagonists abolished ISO-induced myocyte death, while β2-AR stimulation exerted significant protection against myocyte death in WT mice. A and B: for WT and TG myocyte viability, respectively, at 12 h, ISO + NIF treatment caused a greater myocyte viability compared with NIF alone, and the effect was inhibited by the β2-AR antagonist ICI 118551 (ICI; N = 4). C and D: WT and TG myocyte viability, respectively, at 12 h after treatment with β-AR subtype activators and inhibitors (N = 6). Values are means ± SE. ZIN, zinterol, a β2-AR selective agonist; CGP, CGP 20712A, a β1-AR antagonist; ICI is a β2-AR antagonist; DOB, dobutamine, a β1-AR selective agonist; NIF is an LTCC antagonist. *P < 0.05 vs. CON. #P < 0.05, Zin + CGP vs. CGP. &P < 0.05, ISO + ICI vs. ICI. ∧P < 0.05, DOB vs. DOB + CGP. $P < 0.05, ISO + CGP vs. ISO. @P < 0.05, ISO+NIF vs. NIF. %P < 0.05, ISO + NIF + ICI vs. ISO + NIF.
The beneficial effect of β2-AR stimulation could be ascribed to its capability to activate a protective mechanism via β2-AR/Gi coupling and to avoid detrimental β2-AR/Gs coupling. If true, β2-AR stimulation could be independent of myocyte Ca2+ handling. The β1-AR-specific agonist DOB increased myocyte fractional shortening, while β2-AR stimulation with ZIN did not change myocyte contraction and [Ca2+] (Fig. 7). In addition, the positive inotropic effects of ISO were completely abolished by CGP. These data suggest that, in the myocytes used in this study, the positive inotropic effects of β-adrenergic agonists were almost completely mediated through β1-AR.
DISCUSSION
The present study explored the idea that persistent increases in Ca2+ influx through the LTCC are sufficient to induce myocyte death and that chronic β-AR activation causes myocyte death primarily by increasing LTCC-mediated Ca2+ entry. We tested these ideas in mouse myocytes in short-term culture. We took advantage of a TG mouse line with cardiac-specific overexpression of the β2a-subunit of the LTCC, which increases ICa-L without requiring adrenergic agonists. Our experiments showed that mouse myocytes in primary culture succumb to a Ca2+-mediated necrotic cell death that does not involve ROS. In WT myocytes, the Ca2+-mediated death could be inhibited by blocking Ca2+ influx through the LTCC, and in TG myocytes both LTCC and NCX inhibitors reduced the rate of cell death. We showed that β-AR agonists increase the rate of myocyte death, and that this effect primarily results from an enhancement of Ca2+ influx through the LTCC. However, we were able to show that β1-AR stimulation could enhance Ca2+-mediated myocyte death without increasing LTCC-mediated Ca2+ entry in TG myocytes, and this effect was shown to involve PKA-mediated phosphorylation of phospholamban and subsequent increases in SR Ca2+. We also found that β2-AR stimulation had some cardioprotective effects in this system.
Why do mouse cardiac myocytes die in primary culture?
Our laboratory and others have noted previously that rodent myocytes do not survive for long time periods in primary culture (11, 32). The physiological properties of these myocytes determine their poor ability to survive when removed from the beating heart. In vivo, mouse myocytes are beating at high rates (often in excess of 600 per minute), and much of their Ca2+ efflux occurs during the systolic Ca2+ transient. In the absence of pacing in culture, this aspect of Ca2+ efflux is lost. In addition, these myocytes have intracellular [Na+] that is greater than in larger mammalian myocytes (7), and this promotes Ca2+ entry via the NCX (reverse mode). The reduction in Ca2+ efflux and the increase in Ca2+ influx cause the SR to overload with Ca2+ and are centrally involved in the death of these cells in culture. In the present experiments, we show that Ca2+ influx through the LTCC contributes to the death of mouse myocytes in culture, because increasing Ca2+ influx through these channels (with ISO or overexpression of β2a) exacerbated cell death, and inhibition of Ca2+ influx via these channels significantly prolonged their survival (Fig. 1). Our studies support the idea that, in primary culture, Ca2+ enters mouse myocytes via the LTCC and the NCX, resulting in excess cytoplasmic and SR Ca2+. Spontaneous SR Ca2+ release maintains Ca2+ flux balance by promoting Ca2+ efflux, but appears to activate necrotic cell death. We show that β1-AR stimulation, by enhancing Ca2+ influx via the LTCC and increasing SR Ca2+ uptake, increases SR Ca2+ overload and exacerbates Ca2+-mediated cell death (see discussion below). In most of our conditions, inhibition of SERCA (thapsigargin) reduced the rate of cell death, supporting the conclusions stated above. However, in TG myocytes exposed to ISO, thapsigargin did not have a protective effect. This result suggests that, under these conditions, there was sufficient Ca2+ influx to activate Ca2+-mediated cell death, without requiring spontaneous release.
How does ISO increase the death rate of mouse myocytes in culture?
One goal of the present experiments was to determine how persistent activation of β-AR causes myocyte death. Our results show that the cell death was caused by activation of β1-AR, and that the majority of this effect was via an increase in nifedipine inhibitable Ca2+ influx through the LTCC. β1-AR stimulation can cause myocyte death by activation of the cAMP/PKA pathway (22, 35), the CaMKII pathway (44), or a time-dependent switch from cAMP/PKA to the CaMKII pathway (40). The LTCC is involved in both of these signaling pathways, because both PKA and CaMKII phosphorylate LTCC and increase ICa-L (10). Our results show that LTCC blockers reduced the effect of ISO on cell death, in agreement with previous findings in rat (29) and mouse (3) models, and contrast with others in which LTCC blockers did not affect ISO-induced adult rat myocyte apoptosis (16). We also observed that a component of the ISO-induced myocyte death was independent of the LTCC and was caused by stimulating other aspects of Ca2+ handling and involved the SR (Fig. 4). We also showed that this cell death did not involve ROS and was not due to apoptosis (Fig. 5). Collectively, these findings lead us to the conclusion that persistent catecholamine exposure can cause a necrotic, Ca2+-mediated death in mouse VMs. This cell death occurred in the absence of ischemia or reperfusion conditions.
Why do we see necrosis rather than apoptosis?
Ca2+ and catecholamine stress has been shown to induce both apoptosis (11, 44) and necrosis (16, 31). In the conditions used in our study, we did not find any evidence for apoptosis. It is not entirely clear why different modes of cell death are seen in different experiments (11, 43, 15, 30). We suggest that variation in the magnitude of the Ca2+ stress may explain these seemingly disparate results. We suggest that conditions that produce modest increases in myocyte [Ca2+] are likely to cause apoptosis without necrosis. Conversely, those conditions that produce sufficient increases in SR Ca2+ to cause spontaneous SR Ca2+ release, and spontaneous action potentials (20) might cause necrosis. In this regard, some studies of rodent myocytes that have seen apoptosis have employed BDM, which has multiple effects, including reducing cell Ca2+ (24). Further investigation is needed to clarify these issues.
ROS is not involved in myocyte death induced by the LTCC.
Increased ROS production has been traditionally linked with cell death associated with myocardial infarction or reperfusion injury. The sources of ROS generation in cardiac myocytes include nonphagocytic NAD(P)H oxidase and mitochondria (36, 41). ROS is proposed to be involved in mitochondrial permeability transition pore opening leading to a complete loss of respiratory function of the mitochondrial membrane. In our experiments, the ROS scavengers SOD-PEG and CAT-PEG did not alter the rate of cell death under basal conditions or after exposure to ISO (Fig. 5). The NAD(P)H oxidase inhibitor apocinine and the xanthine oxidase inhibitor allopurinol had no effect on myocyte death either. The ISO effect on myocyte death was also unaltered by NOS inhibitor (l-NAME). These results show that ROS is not responsible for the necrotic death we induced in mouse myocytes.
β2-AR agonists protect from necrotic cell death.
β2-AR stimulation exerts antiapoptotic effects by coupling with Gi protein, which stimulates phosphatidylinositol 3-kinase-protein kinase B, negates the apoptotic signal, and promotes cell survival (2, 12, 45). Our experiments show that β2-AR signaling also produces some protection (albeit for a short time period) from Ca2+-dependent necrotic cell death. The mechanisms responsible for this effect are unclear, but do not appear to be due to a reduction in Ca2+ signaling (Fig. 6).
In summary, we have shown that increases in Ca2+ influx, if persistent, are sufficient to induce increased necrotic death of isolate mouse VMs. We showed that an increase in LTCC activity (TG myocytes), similar to the type seen in the failing human heart (4, 30), is sufficient to induce myocyte death. We show that, in WT and TG myocytes, β1-AR agonists induce necrotic death by increasing the Ca2+ current and SR Ca2+ uptake and release. β2-AR signaling provides some protection from these effects. Our results suggest that the Ca2+ and adrenergic activity required to produce the increased systolic wall stress in HF makes myocytes prone to necrotic death that would exacerbate cardiac dysfunction and HF. Novel therapies that reduce excess LTCC-mediated Ca2+ influx and β1-AR activation, but promote protective β2-AR signaling, might synergize to reduce myocyte death induced by pathological stress.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants (HL089312, HL033921, and HL091799 to S. R. Houser, and HL088243 to X. Chen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation 110: 1083–1090, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, Xiao RP, Lakatta EG, Talan MI. Cardioprotective and survival benefits of long-term combined therapy with beta2 adrenoreceptor (AR) agonist and beta1 AR blocker in dilated cardiomyopathy postmyocardial infarction. J Pharmacol Exp Ther 325: 491–499, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Balasubramaniam R, Chawla S, Mackenzie L, Schwiening CJ, Grace AA, Huang CL. Nifedipine and diltiazem suppress ventricular arrhythmogenesis and calcium release in mouse hearts. Pflügers Arch 449: 150–158, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Beetz N, Hein L, Meszaros J, Gilsbach R, Barreto F, Meissner M, Hoppe UC, Schwartz A, Herzig S, Matthes J. Transgenic simulation of human heart failure-like L-type Ca2+-channels: implications for fibrosis and heart rate in mice. Cardiovasc Res 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Cardiac Na/Ca exchange function in rabbit, mouse and man: what's the difference? J Mol Cell Cardiol 34: 369–373, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res 57: 897–912, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol 54: 180–187, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee K, Zhang J, Honbo N, Simonis U, Shaw R, Karliner JS. Acute vincristine pretreatment protects adult mouse cardiac myocytes from oxidative stress. J Mol Cell Cardiol 43: 327–336, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Zhang X, Harris DM, Piacentino V, 3rd, Berretta RM, Margulies KB, Houser SR. Reduced effects of BAY K 8644 on L-type Ca2+ current in failing human cardiac myocytes are related to abnormal adrenergic regulation. Am J Physiol Heart Circ Physiol 294: H2257–H2267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res 97: 1009–1017, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res 87: 1172–1179, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Communal C, Colucci WS. The control of cardiomyocyte apoptosis via the beta-adrenergic signaling pathways. Arch Mal Coeur Vaiss 98: 236–241, 2005 [PubMed] [Google Scholar]
- 14.El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Fail Rev 14: 225–241, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Elias CL, Lukas A, Shurraw S, Scott J, Omelchenko A, Gross GJ, Hnatowich M, Hryshko LV. Inhibition of Na+/Ca2+ exchange by KB-R7943: transport mode selectivity and antiarrhythmic consequences. Am J Physiol Heart Circ Physiol 281: H1334–H1345, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolfi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute beta-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem 282: 11397–11409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman DS, Carnes CA, Abraham WT, Bristow MR. Mechanisms of disease: beta-adrenergic receptors–alterations in signal transduction and pharmacogenomics in heart failure. Nat Clin Pract Cardiovasc Med 2: 475–483, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest 115: 565–571, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, Naik TJ, Prasad SV, Ardehali H. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem 284: 2080–2087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houser SR. When does spontaneous sarcoplasmic reticulum Ca(2+) release cause a triggered arrythmia? Cellular versus tissue requirements. Circ Res 87: 725–727, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Houser SR, Margulies KB. Is depressed myocyte contractility centrally involved in heart failure? Circ Res 92: 350–358, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Iwai-Kanai E, Hasegawa K, Araki M, Kakita T, Morimoto T, Sasayama S. Alpha- and beta-adrenergic pathways differentially regulate cell type-specific apoptosis in rat cardiac myocytes. Circulation 100: 305–311, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Jaleel N, Nakayama H, Chen X, Kubo H, MacDonnell S, Zhang H, Berretta R, Robbins J, Cribbs L, Molkentin JD, Houser SR. Ca2+ influx through T- and L-type Ca2+ channels have different effects on myocyte contractility and induce unique cardiac phenotypes. Circ Res 103: 1109–1119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kabaeva Z, Zhao M, Michele DE. Blebbistatin extends culture life of adult mouse cardiac myocytes and allows efficient and stable transgene expression. Am J Physiol Heart Circ Physiol 294: H1667–H1674, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kitsis RN, Mann DL. Apoptosis and the heart: a decade of progress. J Mol Cell Cardiol 38: 1–2, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation 118: 649–657, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehnart SE, Maier LS, Hasenfuss G. Abnormalities of calcium metabolism and myocardial contractility depression in the failing heart. Heart Fail Rev 14: 213–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 93: 896–906, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Maruyama R, Takemura G, Tohse N, Ohkusa T, Ikeda Y, Tsuchiya K, Minatoguchi S, Matsuzaki M, Fujiwara T, Fujiwara H. Synchronous progression of calcium transient-dependent beating and sarcomere destruction in apoptotic adult cardiomyocytes. Am J Physiol Heart Circ Physiol 290: H1493–H1502, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Miriyala J, Nguyen T, Yue DT, Colecraft HM. Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ Res 102: e54–e64, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117: 2431–2444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connell TD, Rodrigo MC, Simpson PC. Isolation and culture of adult mouse cardiac myocytes. Methods Mol Biol 357: 271–296, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4: 552–565, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhaes PJ, Di Virgilio F, Pozzan T. Calcium and apoptosis: facts and hypotheses. Oncogene 22: 8619–8627, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol 189: 257–265, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Sorescu D, Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail 8: 132–140, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Spadari-Bratfisch RC, dos Santos IN. Adrenoceptors and adaptive mechanisms in the heart during stress. Ann N Y Acad Sci 1148: 377–383, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Sucharov CC. Beta-adrenergic pathways in human heart failure. Expert Rev Cardiovasc Ther 5: 119–124, 2007 [DOI] [PubMed] [Google Scholar]
- 39.van der Heyden MA, Wijnhoven TJ, Opthof T. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res 65: 28–39, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res 95: 798–806, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Warnholtz A, Munzel T. The failing human heart: another battlefield for the NAD(P)H oxidase? J Am Coll Cardiol 41: 2172–2174, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science 300: 1530–1532, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol 279: H429–H436, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest 111: 617–625, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A 98: 1607–1612, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]