Abstract
Adriamycin (ADR) is an established, life-saving antineoplastic agent, the use of which is often limited by cardiotoxicity. ADR-induced cardiomyopathy is often accompanied by depressed myocardial high-energy phosphate (HEP) metabolism. Impaired HEP metabolism has been suggested as a potential mechanism of ADR cardiomyopathy, in which case the bioenergetic decline should precede left ventricular (LV) dysfunction. We tested the hypothesis that murine cardiac energetics decrease before LV dysfunction following ADR (5 mg/kg ip, weekly, 5 injections) in the mouse. As a result, the mean myocardial phosphocreatine-to-ATP ratio (PCr/ATP) by spatially localized 31P magnetic resonance spectroscopy decreased at 6 wk after first ADR injection (1.79 ± 0.18 vs. 1.39 ± 0.30, means ± SD, control vs. ADR, respectively, P < 0.05) when indices of systolic and diastolic function by magnetic resonance imaging were unchanged from control values. At 8 wk, lower PCr/ATP was accompanied by a reduction in ejection fraction (67.3 ± 3.9 vs. 55.9 ± 4.2%, control vs. ADR, respectively, P < 0.002) and peak filling rate (0.56 ± 0.12 vs. 0.30 ± 0.13 μl/ms, control vs. ADR, respectively, P < 0.01). PCr/ATP correlated with peak filling rate and ejection fraction, suggesting a relationship between cardiac energetics and both LV systolic and diastolic dysfunction. In conclusion, myocardial in vivo HEP metabolism is impaired following ADR administration, occurring before systolic or diastolic abnormalities and in proportion to the extent of eventual contractile abnormalities. These observations are consistent with the hypothesis that impaired HEP metabolism contributes to ADR-induced myocardial dysfunction.
Keywords: magnetic resonance imaging, 31P spectroscopy
adriamycin (ADR) represents one of the most potent and extensively used anticancer drugs (46); however, its antineoplastic use can be compromised in practice by cardiotoxic side effects (17, 35). Although ADR-induced cardiotoxicity is usually subclinical, symptoms of heart failure (HF) can develop acutely during therapy (18, 33) or chronically (25, 34). In general, HF with systolic and diastolic abnormalities develops in 18–36% of patients receiving a cumulative ADR dose of 250–601 mg/m2 (17, 35). A spectrum of cardiac metabolic (21, 39) and morphological (2, 6) abnormalities occurs following ADR treatment, but it is unclear which, if any of these, causes ADR cardiotoxicity. One metabolic abnormality that may contribute mechanistically to ADR cardiotoxicity is impaired creatine kinase (CK) energetics.
The CK reaction serves as the prime cardiac energy reservoir, quickly and reversibly converting adenosine diphosphate and phosphocreatine (PCr) to ATP and creatine (15, 44), where the PCr-to-ATP ratio (PCr/ATP) is commonly used to characterize a high-energy phosphate metabolism. An inhibition of CK impairs cardiac function or contractile reserve in normal hearts (13, 37), and an altered CK metabolism is observed in both experimental and human HF (14, 19, 23, 32, 47). In particular, an altered in vitro and in vivo CK energetics and a reduced cardiac PCr/ATP have been observed in ADR cardiotoxicity (8, 11, 24, 29). Whether the CK energetic decline contributes to ADR-induced cardiac dysfunction or is just another consequence of HF is still unresolved. This question has not been answered, in part, because there have been no serial studies performed in the same animals showing whether CK abnormalities precede or follow the development of contractile abnormalities in ADR cardiotoxicity.
31P magnetic resonance (MR) spectroscopy (MRS) and MR imaging (MRI) methods uniquely allow the serial noninvasive quantification of in vivo myocardial CK metabolites, ventricular anatomy, morphology, and function under physiological conditions (14, 19, 23, 32, 47). In vivo MRS/MRI techniques have been extended to the small dimensions and high heart rates of the mouse, enabling the quantification of murine cardiac CK energetics, anatomy, and function (7). The mouse heart exhibits similar in vivo PCr/ATP and measures of global function to those of the human heart (7), and the mouse has been frequently used to study ADR cardiotoxicity (5, 9, 10, 41). Recent experiments showed that the decline in cardiac PCr/ATP in pressure overload-induced murine HF precedes functional abnormalities (19). A causal role for impaired CK in ADR cardiotoxicity would be suggested if the energetic decline occurs before or at the same time as cardiac dysfunction, whereas a consequential role if decreased PCr/ATP occurs after cardiac dysfunction. Therefore, the aim of this study was to test the hypothesis that impaired CK energetics, indexed by a decreased cardiac PCr/ATP, precedes the development of ADR-induced systolic and/or diastolic dysfunction.
MATERIALS AND METHODS
Experimental animals.
All studies were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University School of Medicine. Adult male C57BL6 mice (weight, 30–40 g) were used and received intraperitoneal injection of ADR (5 mg/kg) weekly for a total of five injections. This ADR dose/scheme was chosen after experimental screening of doses/schemes previously reported (5, 9, 10, 41) with some modifications. Control mice received normal saline instead of ADR.
MRI/MRS study.
In vivo MRI/MRS studies were performed 6, 8, and 10 wk after the initiation of ADR administration. Mice were anesthetized with 1% isoflurane in a 50:50 oxygen and ambient air mixture and positioned prone on a Plexiglas platform with temperature control (37 ± 1°C). ECG leads, respiratory pad, and thermo couple (SA Instruments) were used to monitor basic vital parameters. 1H MRI and image-guided, spatially localized 31P MRS were used to study global left ventricular (LV) function and energetics, respectively, during the same examination, as previously reported (7). The probe set included 22-mm 1H MRI and 11-mm 31P MRS coils. Data were acquired on a Bruker Biospec MRS/MRI spectrometer equipped with a 4.7-T/40-cm Oxford magnet and a 12-cm (inner diameter) actively shielded BGA-12 gradient set capable of developing gradient strength of up to 400 mT/m. ECG and respiratory-gated multislice FLASH cine MRI (15 frames, echo time = 1.8 ms, repetition time = 7 ms, and slice thickness = 1 mm, flip angle = 30°) was used to acquire LV morphological and functional data. LV morphology and systolic function were quantified from LV short-axis slices and diastolic function from long-axis slices. Spatially localized 31P MRS was carried out using a one-dimensional chemical shift imaging sequence with a 16-mm field of view, 16 phase encoding steps in the direction perpendicular to the plane of the coil, 128 averages per phase encoding step, a modified BIR4 adiabatic excitation pulse of 60° flip angle, and an interpulse delay of 1 s. All mice awoke within ∼1 min after the study.
MRI/MRS data processing.
1H MR images were analyzed with ImageJ software and 31P MR spectra with in-house custom software (1). The largest and smallest LV volumes during the cardiac cycle were visually identified as end-diastolic and end-systolic volumes, respectively. LV mass was the sum of the LV areas of all end-diastolic short-axis slices multiplied by the slice thickness and by 1.05 (cardiac specific gravity). The LV ejection fraction was calculated from the relative difference in end-diastolic and end-systolic volumes. Diastolic function was assessed by the magnitude of the peak filling rate. The values for diastolic filling rates were calculated as the difference in LV volumes of the sequential frames in cine acquisitions from a long-axis slice divided by the duration of the frame interval. Cardiac PCr/ATP was quantified from the integrated peak areas of PCr and [β-P]ATP resonances from voxels intersecting the anterior LV wall and apart from chest skeletal muscle, as identified with 1H MR images as previously described (7). Voxel shifting was performed as necessary to avoid chest muscle contamination (3).
Statistical analysis.
Data are expressed as means ± SD. One-way ANOVA tests with Bonferroni correction for multiple comparisons were used. The relationship between metabolic and functional parameters was evaluated with Pearson product-moment correlational analysis. A value of P < 0.05 was considered statistically significant (GraphPad Prism, version 4.06).
RESULTS
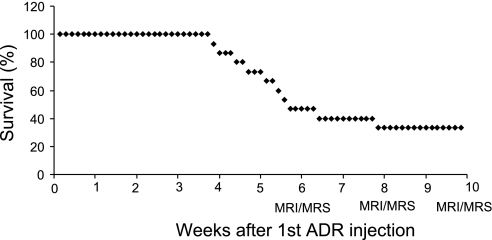
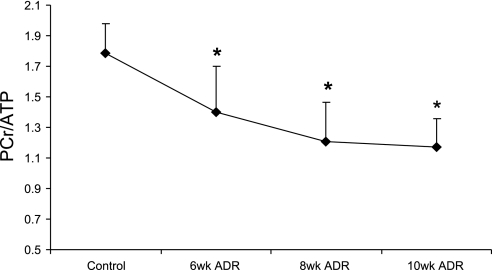
Fifteen animals received ADR, and the mortality was 53% at 6 wk and 67% at 10 wk (Fig. 1). Additional studies described in the supplemental material (posted with the online version of this article) suggest this early mortality is not due to structural cardiac damage or cell death and unrelated to typical ADR cardiotoxicity, consistent with prior reports. Thus five animals survived for all MRI/MRS studies between 6–10 wk. Representative MR images and cardiac 31P MR spectra are shown in Figs. 2 and 3. Six weeks of ADR treatment resulted in a cardiac energetic decline as evidenced by a reduction in mean cardiac PCr/ATP (1.79 ± 0.18 vs. 1.39 ± 0.30, control vs. ADR, respectively, P < 0.05, Fig. 4). At the same time, systolic and diastolic functional parameters in ADR mice remained similar to control values (Table 1).
Fig. 1.
Survival curve for Adriamycin (ADR)-treated mice. MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy.
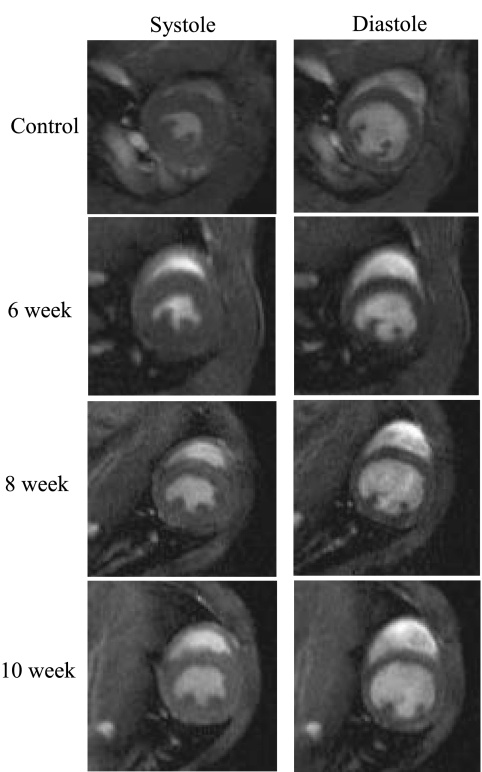
Fig. 2.
Typical transverse short-axis 1H magnetic resonance images of a mouse thorax through the mid-left ventricle at end systole and end diastole.
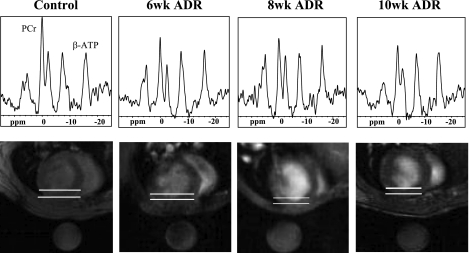
Fig. 3.
31P spectra (top) from the anterior myocardium, as indicated by the region between the white lines on the images (bottom), are shown with the prominent peaks of phosphocreatine (PCr) and β-phosphate of ATP (β-ATP). ppm, Parts/million. The round object below the animal in each image is a fiducial 31P standard contained within the probe. The cardiac PCr-to-ATP ratio (PCr/ATP) declines at 6 wk of ADR administration.
Fig. 4.
Time course of in vivo cardiac energetic decline in ADR-treated mice. *P ≤ 0.05 compared with control.
Table 1.
LV morphology and function in ADR-treated mice
| n | HR, beats/min | EDV, μl | ESV, μl | SV, μl | EF, % | CO, ml/min | LV Mass, mg | PFR, μl/ms | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 13 | 519 ± 26 | 49.7 ± 8.7 | 16.3 ± 3.5 | 33.4 ± 5.9 | 67.3 ± 3.9 | 17.4 ± 3.4 | 94.1 ± 16.4 | 0.56 ± 0.13 |
| ADR (6 wk) | 5 | 519 ± 29 | 45.5 ± 3.5 | 16.1 ± 3.5 | 29.4 ± 3.2 | 64.7 ± 6.1 | 15.3 ± 2.6 | 79.1 ± 12.3 | 0.51 ± 0.06 |
| ADR (8 wk) | 5 | 508 ± 80 | 55.8 ± 11.2 | 24.4 ± 4.4 | 31.4 ± 7.6 | 55.9 ± 4.2*† | 15.5 ± 2.4 | 78.0 ± 8.4 | 0.34 ± 0.12* |
| ADR (10 wk) | 5 | 525 ± 46 | 48.8 ± 9.8 | 23.0 ± 6.3 | 25.8 ± 4.7 | 53.2 ± 4.7*† | 13.4 ± 1.9* | 73.8 ± 10.0* | 0.27 ± 0.08*† |
Values are means ± SD; n, number of mice. HR, heart rate; EDV, end-diastolic volume; ESV, end-systolic volume; SV, systolic volume; EF, ejection fraction; CO, cardiac output, LV, left ventricular; PFR, peak filling rate.
P ≤ 0.05 compared with control;
P ≤ 0.05 compared to 6 wk after 1st Adriamycin (ADR) injection.
Over the ensuing 2 wk, i.e., 8 wk after the first ADR exposure, a further energetic decline (Fig. 4) was accompanied by LV systolic and diastolic dysfunction. Specifically, the mean ejection fraction decreased to 55.9% (P < 0.002) and the mean peak diastolic filling rate decreased by ∼40% from that of control mice (P < 0.01, Table 1).
After an additional 2 wk, i.e., 10 wk after the first ADR injection, mean cardiac PCr/ATP was 35% lower than that of control mice (1.79 ± 0.18 vs. 1.17 ± 0.18, control vs. ADR, respectively, P < 0.003, Fig. 4). LV ejection fraction was significantly lower (53.2 ± 4.7%) than that of control mice (67.3 ± 3.9%, P < 0.05), whereas end-systolic volumes trended higher and stroke volumes lower (Table 1). LV mass decreased by 22% and mean resting cardiac output by 23% (P < 0.05) (Table 1).
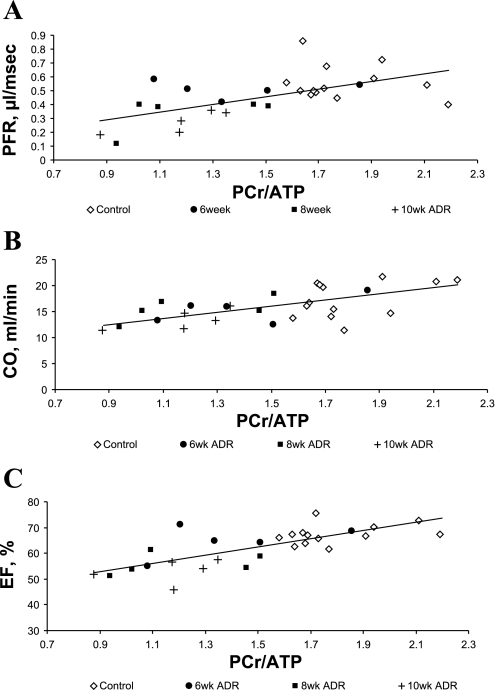
ADR administration was associated with an early significant decline in cardiac PCr/ATP and later significant decreases in mean LV filling rate, ejection fraction, and cardiac output. To determine whether the LV energetics correlated with the extent of contractile and morphological abnormalities, cardiac PCr/ATP was compared with the functional and morphological parameters. There was a significant correlation of in vivo cardiac PCr/ATP with peak filling rate, cardiac output, and ejection fraction (Fig. 5).
Fig. 5.
A: relationship between in vivo cardiac PCr/ATP and peak filling rate (PFR) [maximal rate of volume change (−dV/dtmax)]: y = 0.27x + 0.05, R2 = 0.35, R = 0.59, P < 0.001. B: relationship between in vivo cardiac PCr/ATP and cardiac output (CO): y = 5.5x + 7.7, R2 = 0.38, R = 0.62, P < 0.001. C: relationship between in vivo cardiac PCr/ATP and ejection fraction (EF): y = 15.4x + 39.1, R2 = 0.54, R = 0.73, P < 0.0001.
DISCUSSION
There are several novel observations from this in vivo murine study of serial changes in cardiac energetics and function following ADR administration. First, we observe that CK energetics decrease, as shown by lower cardiac PCr/ATP, early after ADR administration. Second, abnormalities of LV systolic and diastolic function appear weeks after the lower cardiac PCr/ATP, indicating that the impaired CK energetics is an early phenomenon that may contribute to subsequent LV dysfunction. Third, the systolic (LV ejection fraction and cardiac output) and diastolic (peak filling rate) dysfunction is significantly associated with the energetic abnormality (cardiac PCr/ATP).
Although ADR is an effective anticancer drug, cardiotoxicity occurs and limits the amount administered (17, 35). Energy metabolism, a requisite for normal cardiac function, is a likely target for ADR (38). CK functions as the major myocardial ATP reserve and temporal buffer (26, 43) and is impaired in experimental and human HF (14, 19, 23, 32, 47). Even though the existence of a relationship between impaired CK energetics and ADR cardiotoxicity was suggested years ago (8, 11, 38, 39), the chronological sequence of events necessary for establishing possible causality has not been defined in the same animals.
What are the possible mechanisms responsible for the energetic decline after ADR administration, and how can they interfere with cardiac function? The mechanisms of action of the anthracyclines against tumor cells remain controversial, although the generation of reactive oxygen species through the interaction with mitochondrial enzymes via their quinone ring is thought to be one likely contributing factor (36). ADR-generated reactive oxygen species may oxidize important enzymes containing sulfhydryl groups and may also interfere with DNA replication (36). ADR is known to oxidize sulfhydryl groups of CK and attenuate CK activity (22). Prior studies from cultured cardiomyocytes and in vivo hearts demonstrate reductions in the absolute and relative cardiac CK metabolite pool sizes following ADR exposure (11, 29). In addition, ADR alters the structure and function of purified mitochondrial CK (CKMt), leading to a dissociation of octamers into dimers and an inhibition of CKMt binding to the mitochondrial membrane (38). CKMt also mediates the formation of important multienzyme complexes, including the adenine nucleotide translocator and voltage-dependent anion channel-facilitating chemical energy transfer (4, 28). The disruption of the functional coupling of CKMt and adenine nucleotide translocator after ADR exposure, in part, may explain the reduction of the rate and efficiency of energy transfer (40). On the other hand, multiple abnormalities in myofibrillar CK (CKM) can contribute to the impaired overall inefficiency of the CK system as well. For instance, ADR inhibits CKM gene expression (16) and causes an inhibition of the CKM isoform in vitro and in vivo (20, 44). Another reported response of the CK system to ADR is an increase in the B-isoform of CK (CKB), which occurs in some other forms of HF (30, 48). Although the antitumor effect of ADR may be related to interference with DNA synthesis (12, 31), the augmentation of CKB suggests that ADR does not interfere with all DNA processes. The effect of ADR may be also mediated by the downregulation of CKMt and/or CKM mRNA and the upregulation of CKB mRNA. The prior reports of ADR interference with CK metabolism are consistent with our current data showing a reduced in vivo cardiac PCr/ATP.
Even though these data were acquired in vivo under physiological conditions and provide new insights into ADR cardiotoxicity, there are distinct limitations of this study. First, the degree of systolic dysfunction, as measured by the LV ejection fraction, was modest and not to the degree at 10 wk that was likely to cause HF at that time. However, this degree of dysfunction is clinically important, since it would be sufficient to discontinue ADR therapy if observed in patients and it did allow testing of the proposed hypothesis. Second, although cardiac PCr/ATP is an important index of myocardial energetics, the rate of ATP flux through CK may be a more important factor underlying contractile dysfunction in HF (32, 47). However, at this time, in vivo measures of CK flux in the murine heart have not been reported. Thus future studies to measure ATP flux through CK, as well as other purported potential energetic contributors to dysfunction, are needed. Finally, the early mortality observed here in the mouse is higher than that observed clinically today. It is important to point out that comparable mortality rates have been reported in prior murine studies of ADR cardiotoxicity (26, 42) and the fundamental observation that cardiac dysfunction is observed well after ADR administration parallels the clinical observation that ADR cardiotoxicity is generally a chronic condition often occurring long after ADR exposure.
In conclusion, ADR cardiotoxicity in the mouse is associated with an early, significant decline of in vivo cardiac energetics that occurs before systolic and diastolic abnormalities are detected by serial MRI examinations. The extent of systolic and diastolic dysfunction correlates with reduced cardiac PCr/ATP following ADR exposure, consistent with the hypothesis that abnormalities in cardiac CK energetics underlie mechanical abnormalities in ADR cardiotoxicity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-63030, by the D. W. Reynolds Cardiovascular Center at Johns Hopkins, and by the Clarence Doodeman Endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of M. Y. Maslov: Caritas St.Elizabeth's Medical Ctr., Tufts Univ. School of Medicine, 736 Cambridge St., Boston, MA 02135 (e-mail: mikhail.maslov@caritaschristi.org).
REFERENCES
- 1.Barker PB, Sibisi S. Non-linear least squares analysis of in vivo 31P NMR data (Abstract). Soc Magn Res Med 9: 1089, 1990 [Google Scholar]
- 2.Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep 62: 865–872, 1978 [PubMed] [Google Scholar]
- 3.Bottomley PA, Hardy CJ, Roemer PB, Weiss RG. Problems and expediencies in human 31P spectroscopy. The definition of localized volumes, dealing with saturation and the technique-dependence of quantification. NMR Biomed 2: 284–289, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Brdiczka DG, Zorov DB, Sheu SS. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochim Biophys Acta 1762: 148–163, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bruynzeel AM, Vormer-Bonne S, Bast A, Niessen HW, van der Vijgh WJ. Long-term effects of 7-monohydroxyethylrutoside (monoHER) on DOX-induced cardiotoxicity in mice. Cancer Chemother Pharmacol 60: 509–514, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Buja LM, Ferrans VJ, Mayer RJ, Roberts WC, Henderson ES. Cardiac ultrastructural changes induced by daunorubicin therapy. Cancer 32: 771–788, 1973 [DOI] [PubMed] [Google Scholar]
- 7.Chacko VP, Aresta F, Chacko SM, Weiss RG. MRI/MRS assessment of in vivo murine cardiac metabolism, morphology, and function at physiological heart rates. Am J Physiol Heart Circ Physiol 279: H2218–H2224, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Dekker T, van Echteld CJ, Kirkels JH, Ruigrok TJ, van Hoesel QG, de Jong WH, Schornagel JH. Chronic cardiotoxicity of adriamycin studied in a rat model by 31P NMR. NMR Biomed 4: 16–24, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Delgado RM, 3rd, Nawar MA, Zewail AM, Kar B, Vaughn WK, Wu KK, Aleksic N, Sivasubramanian N, McKay K, Mann DL, Willerson JT. Cyclooxygenase-2 inhibitor treatment improves left ventricular function and mortality in a murine model of doxorubicin-induced heart failure. Circulation 109: 1428–1433, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Doroshow JH, Locker GY, Ifrim I, Myers CE. Prevention of doxorubicin cardiac toxicity in the mouse by N-acetylcysteine. J Clin Invest 68: 1053–1064, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eidenschink AB, Schröter G, Müller-Weihrich S, Stern H. Myocardial high-energy phosphate metabolism is altered after treatment with anthracycline in childhood. Cardiol Young 10: 610–617, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Eliot H, Gianni L, Myers C. Oxidative destruction of DNA by the adriamycin-iron complex. Biochemistry 23: 928–936, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Hamman BL, Bittl JA, Jacobus WE, Allen PD, Spencer RS, Tian R, Ingwall JS. Inhibition of the creatine kinase reaction decreases the contractile reserve of isolated rat hearts. Am J Physiol Heart Circ Physiol 269: H1030–H1036, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J 122: 795–801, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Ingwall JS, Kramer MF, Fifer MA, Lorell BH, Shemin R, Grossman W, Allen PD. The creatine kinase system in normal and diseased human myocardium. N Engl J Med 313: 1050–1054, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Miller SC, Billingham ME, Akimoto H, Torti SV, Wade R, Gahlmann R, Lyons G, Kedes L, Torti FM. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc Natl Acad Sci USA 87: 4275–4279, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefrak EA, Pitha J, Rosenheim S, Gottleib JA. A clinicopathologic analysis of ADR cardiotoxicity. Cancer 32: 302–314, 1973 [DOI] [PubMed] [Google Scholar]
- 18.Lenaz L, Page JA. Cardiotoxicity of adriamycin and related anthracyclines. Cancer Treat Rev 3: 111–120, 1976 [DOI] [PubMed] [Google Scholar]
- 19.Maslov MY, Chacko VP, Stuber M, Moens AL, Kass DA, Champion HC, Weiss RG. Altered high-energy phosphate metabolism predicts contractile dysfunction and subsequent ventricular remodeling in pressure-overload hypertrophy mice. Am J Physiol Heart Circ Physiol 292: H387–H391, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Br J Pharmacol 135: 581–588, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mingyan E, Hongli L, Shufeng L, Bo Y. Effects of pyrrolidine dithiocarbamate on antioxidant enzymes in cardiomyopathy induced by adriamycin in rats. Cardiology 111: 119–125, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Muraoka S, Fujimoto Y. Inactivation of creatine kinase by Adriamycin during interaction with horseradish peroxidase. Biochem Pharmacol 60: 95–99, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86: 1810–1818, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Nicolay K, Aue WP, Seelig J, van Echteld CJ, Ruigrok TJ, de Kruijff B. Effects of the anti-cancer drug adriamycin on the energy metabolism of rat heart as measured by in vivo 31P-NMR and implications for adriamycin-induced cardiotoxicity. Biochim Biophys Acta 929: 5–13, 1987 [DOI] [PubMed] [Google Scholar]
- 25.Praga C, Beretta G, Vigo PL, Lenaz GR, Pollini C, Bonadonna G, Canetta R. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep 63: 827–834, 1979 [PubMed] [Google Scholar]
- 26.Sakaguchi H, Kodama A, Tomonari M, Ando Y, Tabuchi M, To H, Araki R, Kitahara T, Sasaki H, Ohdo S, Higuchi S. Pre-administration of docetaxel protects against adriamycin-induced cardiotoxicity. Breast Cancer Res Treat 109: 443–450, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Saks VA, Kuznetsov AV, Kupriyanov VV, Miceli MV, Jacobus WE. Creatine kinase of rat heart mitochondria. The demonstration of functional coupling to oxidative phosphorylation in an inner membrane-matrix preparation. J Biol Chem 260: 7757–7764, 1985 [PubMed] [Google Scholar]
- 28.Schlattner U, Wallimann T. Metabolite channeling: creatine kinase microcompartments. In: Encyclopedia of Biological Chemistry, ed. by Lennarz WJ. New York: Academic, 2004, p. 646–651 [Google Scholar]
- 29.Seraydarian MW, Artaza L, Goodman MF. Adriamycin: effect on mammalian cardiac cells in culture. I. Cell population and energy metabolism. J Mol Cell Cardiol 9: 375–382, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Shen W, Spindler M, Higgins MA, Jin N, Gill RM, Bloem LJ, Ryan TP, Ingwall JS. The fall in creatine levels and creatine kinase isozyme changes in the failing heart are reversible: complex post-transcriptional regulation of the components of the CK system. J Mol Cell Cardiol 39: 537–544, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Sinha K, Chignell CF. Binding mode of chemically activated semiquinone free radicals from quinone anticancer agents to DNA. Chem Biol Interact 28: 301–308, 1979 [DOI] [PubMed] [Google Scholar]
- 32.Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 114: 1151–1158, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinberg JS, Cohen AJ, Wasserman AG, Cohen P, Ross AM. Acute arrhythmogenicity of doxorubicin administration. Cancer 60: 1213–1238, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266: 1672–1677, 1991 [PubMed] [Google Scholar]
- 35.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97: 2869–2879, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49: 330–352, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Tian R, Ingwall JS. Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Physiol Heart Circ Physiol 270: H1207–H1216, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Tokarska-Schlattner M, Wallimann T, Schlattner U. Multiple interference of anthracyclines with mitochondrial creatine kinases: preferential damage of the cardiac isoenzyme and its implications for drug cardiotoxicity. Mol Pharmacol 61: 516–523, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T, Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J Mol Cell Cardiol 41: 389–405, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Tolekis AI, Kal'venas AA, Dzheia PP, Prashkiavichius AK, Iasatis AA. Functional changes in the mitochondrial site of adenylate kinase and creatine kinase systems of energy transport induced by myocardial ischemia and adriablastin. Biokhimiia 53: 649–654, 1988 [PubMed] [Google Scholar]
- 41.van Acker SA, Voest EE, Beems DB, Madhuizen HT, de Jong J, Bast A, van der Vijgh WJ. Cardioprotective properties of O-(beta-hydroxyethyl)-rutosides in doxorubicin-pretreated BALB/c mice. Cancer Res 53: 4603–4607, 1993 [PubMed] [Google Scholar]
- 42.Vedam K, Nishijima Y, Druhan LJ, Khan M, Moldovan NI, Zweier JL, Ilangovan G. Role of heat-shock factor-1 activation in the doxorubicin-induced heart failure in mice. Am J Physiol Heart Circ Physiol 298: H1832–H1841, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallimann T, Kuhn HJ, Pelloni G, Turner DC, Eppenberger HM. Localization of creatine kinase isoenzymes in myofibrils. II. Chicken heart muscle. J Cell Biol 75: 318–325, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallimann T. Bioenergetics. Dissecting the role of creatine kinase. Curr Biol 4: 42–46, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Weinstein DM, Mihm MJ, Bauer JA. Cardiac peroxynitrite formation and left ventricular dysfunction following doxorubicin treatment in mice. J Pharmacol Exp Ther 294: 396–401, 2000 [PubMed] [Google Scholar]
- 46.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol 19: 670–686, 1992 [PubMed] [Google Scholar]
- 47.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA 102: 808–813, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams RE, Kass DA, Kawagoe Y, Pak P, Tunin RS, Shah R, Hwang A, Feldman AM. Endomyocardial gene expression during development of pacing tachycardia-induced heart failure in the dog. Circ Res 75: 615–623, 1994 [DOI] [PubMed] [Google Scholar]