Abstract
We used a direct-contact endothelial cell-smooth muscle cell (EC-SMC) co-culture to examine whether quiescent SMCs regulate the EC inflammatory response to tumor necrosis factor (TNF)-α. ECs were cultured under static and physiological flow conditions. Compared with TNF-α-treated ECs in monoculture, TNF-α-treated ECs in co-culture had less NF-κB nuclear translocation; less intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin surface protein expression; no change in TNF receptor expression, but greater Kruppel-like factor 2 (KLF2) gene expression. After flow preconditioning for 24 h at 15 dyne/cm2, and exposure of ECs to flow and TNF-α for 4.5 h, ECs in co-culture had less ICAM-1, VCAM-1, and E-selectin surface protein expression. Exposure to flow greatly increased KLF2 gene expression levels in both EC cultures; as a result, ECs in co-culture and monoculture had similar levels of post-flow KLF2 gene expression. The reduced levels of TNF-α-induced adhesion molecule expression in co-culture required the presence of quiescent SMCs; adhesion to decellularized extracellular matrix (ECM) or co-culture with fibroblasts produced only a modest reduction in EC adhesion molecule expression. Furthermore, co-culture of quiescent SMCs and ECs on the opposite side of a 10-μm-thick porous membrane did not alter the TNF-α-mediated ICAM-1 surface protein expression. Although the ECM produced by SMCs plays some role in reducing TNF-α-mediated inflammation, these results suggest that the direct contact between ECs and quiescent SMCs is required to inhibit TNF-α-mediated activation.
Keywords: vascular inflammation, extracellular matrix, inflammatory cell adhesion molecules, tissue engineering, shear stress
atherosclerosis is a chronic inflammatory disease induced by an activated endothelium (23). An activated endothelium expresses vascular adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, which aid in monocyte rolling, firm adhesion, and transendothelial migration (as reviewed in Refs. 12 and 13). Endothelial cells (ECs) become activated when exposed to inflammatory cytokines, such as tumor necrosis factor (TNF)-α. The EC response to TNF-α may be regulated by its substrate and surroundings. After TNF-α treatment, different levels of adhesion molecule expression (E-selectin) were found between arterial and venous ECs in organ culture as well as ECs in organ and cell culture (14). Relative to ECs cultured alone, ECs cultured with proliferating smooth muscle cells (SMCs) on the opposite side of a porous membrane had increased EC adhesion molecule expression (4, 5) and TNF-α-mediated leukocyte adhesion (21, 22). These results suggest that proliferating SMCs, as found within diseased vessels, prime the endothelium to a more activated state. While flow waveforms present at sites susceptible to atherosclerosis promoted the inflammatory response of human umbilical vein endothelial cells co-cultured with quiescent SMCs (7), the role of quiescent SMCs upon the inflammatory response of ECs in the presence of cytokines that arise during atherosclerosis is not known.
We previously developed a direct-contact co-culture of ECs adhered to quiescent SMCs to act as a model of a healthy vessel wall (30). We found that quiescent SMCs produce fibrillar fibronectin (FN), which induces fibrillar adhesion formation and blocks focal adhesion formation within ECs in co-culture (31). Cell adhesion to fibrillar FN has a number of effects on cellular behavior including decreasing cell migration, increasing cell adhesiveness, and decreasing the shear-induced NF-κB nuclear translocation (18, 20). Therefore, EC interactions with extracellular matrix (ECM) produced by quiescent SMCs may regulate the response of ECs to TNF-α.
Since the inflammatory state of ECs influences early events in atherosclerosis, we tested the following two hypotheses. First, ECs in direct-contact co-culture with quiescent SMCs have a reduced inflammatory response to TNF-α compared with ECs cultured alone, which is due, in part, to the EC interactions with the SMC-produced ECM. Second, direct-contact co-culture increases the expression of the EC-specific transcription factor Kruppel-like factor 2 (KLF2), which promotes an anti-oxidant and anti-inflammatory EC phenotype. We compared the TNF-α-mediated EC inflammatory response of ECs cultured alone with ECs co-cultured in direct contact with quiescent SMCs. These responses were examined under both static and flow conditions. We found that quiescent SMCs reduce the TNF-α-mediated EC inflammatory response; thereby, suggesting that SMCs within healthy vessels play a role in preventing the initiation of atherosclerosis.
MATERIALS AND METHODS
Cell culture.
All media were supplemented with 1× antibiotic-antimycotic (GIBCO, Carlsbad, CA). To prepare co-cultures, human aortic SMCs (passages 7–11; Clonetics, Palo Alto, CA) were plated at 85,000 cells/cm2 on tissue culture plastic dishes (Corning, Corning, NY) or polystyrene Slideflasks (NUNC, Rochester, NY) that were incubated with 3.3 μg/ml human plasma FN (Sigma, St. Louis, MO) for over 1 h. Proliferative (actively dividing) SMCs were grown to confluence in smooth muscle basal media (Clonetics) supplemented with SmGM-2 singleQuots (Clonetics) forming one or two layers. One day after SMCs were seeded, the medium was changed to a serum-free quiescent medium composed of DMEM/F-12 (GIBCO) supplemented with 1× insulin-transferrin-selenium (GIBCO). NIH/3T3 fibroblasts (ATCC, Rockville, MD) were cultured under the same conditions as SMCs. Two days after addition of the quiescent medium, human aortic ECs (passages 7–10; Clonetics) were seeded at a confluent density directly on top of the quiescent (nondividing) SMCs in endothelial basal media-2 (EBM-2; Clonetics) supplemented with EGM-2 SingleQuots (Clonetics). After ∼24 h, the medium was changed to co-culture media composed of EBM-2 supplemented with 3.3% heat-inactivated FBS (GIBCO) and 1× insulin-transferrin-selenium (GIBCO). All cell cultures were maintained in a tissue culture incubator at 37°C and 5% CO2, and the medium was exchanged every other day. To assess the role of the SMC-produced ECM, the quiescent SMCs were treated with 0.02 N NH4OH for 5 min at 37°C and then rinsed three times with DPBS to remove all cellular content. Cell culture reagents and techniques, including co-culture formation, have also been described previously (31).
Immunofluorescence.
Cell cultures were fixed with 3.7% paraformaldehyde for 15 min at 37°C. For NF-κB p65 immunostaining, the cells were permeabilized with 0.2% Triton X-100 (Sigma) at room temperature for 5 min. The cells were rinsed with DBPS and then incubated with 10% goat serum for 30 min at 37°C to block nonspecific binding. A mouse anti-human p65 antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) or a mouse anti-human ICAM-1 antibody (1:200; BD Biosciences Pharmingen, San Diego, CA) was incubated with the cells for 1 h at 37°C in 10% goat serum. Cells were rinsed multiple times and then incubated at 37°C for 45 min in 10% goat serum with Alexa Fluor 488 goat anti-mouse secondary antibody (1:500; Invitrogen, Carlsbad, CA).
NF-κB translocation.
Two days after the ECs were plated, cell cultures were treated with 5, 50, or 100 U/ml human TNF-α (100 U/ml ≈ 1 ng/ml; Sigma) for 4.5 h and then NF-κB p65 was immunolabeled as described above. For each experiment, 10 random images of the labeled NF-κB p65 were captured using a confocal laser-scanning microscope (LSM 510, Carl Zeiss, Thornwood, NY). The number of ECs per unit area that had positive nuclear staining of NF-κB p65 was counted along with the total number of ECs to calculate the percentage of ECs with NF-κB nuclear translocation.
Flow cytometry.
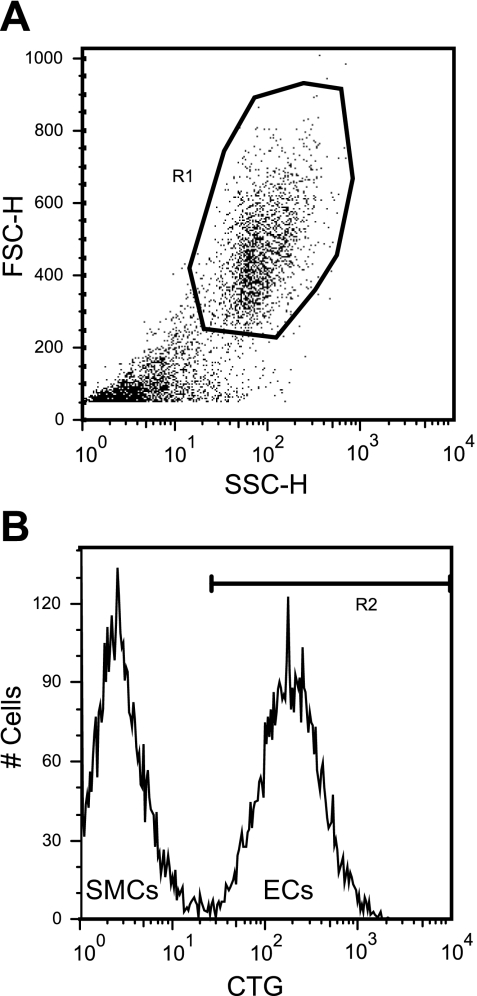
Before being plated, ECs were stained with 2 μM CellTracker Green (Invitrogen) for 15 min at 37°C. Two days after the ECs were plated, TNF-α (Sigma) was added to the culture media for 4.5 h before typsinizing with 0.5% trypsin/EDTA (GIBCO) for 5 min at 37°C. After trypsin-neutralizing solution (Clonetics) was added, the cell suspension was pipetted multiple times to break any remaining bonds between the cells. The cells were pelleted and resuspended in 10% goat serum containing mouse anti-human ICAM-1, VCAM-1, or E-selectin (BD Biosciences) at 1:200 dilution with a total volume of 300 μl. The cell solution was rotated for 45 min at 37°C, and then 500 μl of DBPS were added to the cell solution before the cells were pelleted. The cells were resuspended in 250 μl of 10% goat serum containing anti-mouse IgG-R-Phycoerythrin (PE; 1:50; Sigma). The cell solution was rotated at 37°C for 30 min, and then 600 μl of DBPS were added to the cell solution before the cells were pelleted. The cells were resuspended in 3.7% paraformaldhyde and analyzed on a FACSCalibur (Becton Dickinson, Lincoln Park, NJ) flow cytometer. ECs were positively gated by light scattering and CellTracker Green intensity as illustrated in Fig. 1. The mean immunofluorescence intensity of the adhesion molecule surface protein expression among ECs was determined for each experiment.
Fig. 1.
Typical flow cytometry gate setup to distinguish endothelial cells (ECs) from smooth muscle cells (SMCs). The flow cytometer gated the cells based on light scattering (A) and CellTracker Green (CTG) fluorescent intensity (B). ECs are separately gated from the SMCs due to the CTG labeling.
Separation of ECs from SMCs for total RNA isolation.
Co-cultures were washed with DPBS without Ca2+ and Mg2+ to remove media proteins. Trypsin/EDTA (0.5%; GIBCO) at 37°C was then added to the cells for 5 min. The trypsin was neutralized with trypsin-neutralizing solution, and the cell suspension was pipetted multiple times to break any remaining bonds between the cells. The cells were pelleted and resuspended with 1 ml of 0.1% BSA (Sigma) in DPBS. The ECs and SMCs were then separated using magnetic beads.
A volume of 26 μl of CD31 endothelial cell dynabeads (Invitrogen) was mixed with the cell sample for 20 min at room temperature. The bead-cell solution was placed in the Dynal MPC Magnet (Invitrogen) where the EC-bead complex was pulled to the side of the magnet, and the supernatant, containing SMCs in suspension, was removed. The EC-bead complexes were resuspended in 0.1% BSA in DPBS. The solution was placed in the magnet and the supernatant was discarded. ECs were then washed three more times to increase EC purity. EC purity was previously measured to be 98.7 ± 0.4% using this separation technique (31). Total EC RNA was isolated from the EC-bead complex using a commercially available RNA isolation kit (High-Pure Total RNA Isolation Kit, Roche Applied Science, Indianapolis, IN). Cell separation and EC total RNA isolation were also described previously (31).
Quantitative real-time RT-PCR.
RNA purity and quantity were measured using a NanoDrop Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Total RNA (50 ng) was reverse transcribed using the cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and a MyCycler (Bio-Rad) thermal cycler. One cycle of 5 min at 25°C, 30 min at 43°C, and 5 min at 85°C was performed. Primers, RNAse-free water, and IQ SYBR Green Supermix (Bio-Rad) were combined with the cDNA samples and placed in a MyIQ single color real-time PCR detection system (Bio-Rad). A two-step cycle configuration was performed with an initial denaturation for 3 min at 95°C and 50 cycles at 95°C for 15 s and 61°C for 1 min. All samples were performed in triplicate for all genes. The 2−ΔΔCT method was used to determine the relative gene expression (15). Primers were selected based on the gene sequence (PubMed) of interest and using the Primer 3 shared software (24) or purchased from BioChain Institute (for TNFR2). The primers (IDT Technologies, Coralville, IA) used to complete the studies were 1) β2-microglobulin: 5′-GGC TAT CCA GCG TAC TCC AAA G-3′ and 5′-CAA CTT CAA TGT CGG ATG GAT G-3′; 2) TNFR1: 5′-CTG CTG CCG CTG GTG CTC CTG-3′ and 5′-TGC CGG TAC TGG TTC TTC CTG-3′; 3) KLF2: 5′-GCA CGC ACA CAG GTG AGA AG-3′ and 5′-ACC AGT CAC AGT TTG GGA GGG-3′; and 4) eNOS: 5′-GTG ATG GCG AAG CGA GTG AAG-3′ and 5′-CCG AGC CCG AAC ACA CAG AAC-3′. Melting curves were plotted following each reaction to verify that only the target cDNA was amplified. Quantitative real-time RT-PCR techniques have also been described previously (31). Reference RNA, collected from a large batch of ECs, was used as a common reference for all samples tested except the TNFR1 and TNFR2 experiments (no reference used). The reference RNA was isolated, aliquoted, and stored at −80°C until needed.
Exposure of ECs to flow.
A pusatile pump (Cole Parmer, Vernon Hills, IL), pulse dampener (Cole Parmer), and silicon tubing (Cole Parmer) provided a recirculating, steady flow of co-culture media to a custom-made parallel-plate flow chamber. A silicon gasket was placed between the two plates to maintain the height (450 μm) and width (1.8 cm) of the fluid flow path. For a Newtonian fluid, the wall shear stress τw (dyne/cm2) is τw = 6μQ/wh2, where μ (0.97 cp) is the fluid viscosity, Q is the volumetric flow rate, w is the channel width, and h is the channel height.
One day after a confluent layer of ECs was plated on FN-coated plastic or quiescent SMCs, EC cell cultures were exposed to steady laminar fluid flow at a physiological shear stress of 15 dyne/cm2. After 24 h of flow, 100 U/ml of TNF-α were added to the media reservoir and the flow conditions continued for 4.5 h. Static cell cultures were also exposed to the TNF-α treatments in parallel with the flow samples.
Statistical analysis.
An ANOVA was performed when making multiple comparisons and a Tukey-Kramer analysis was used as a post hoc test. The Student's t-test was performed when comparing only two groups of data. A value of P < 0.05 was considered statistically significant. Data were expressed as means ± SE.
RESULTS
Effect of co-culturing ECs with quiescent SMCs on the TNF-α-mediated EC inflammatory response.
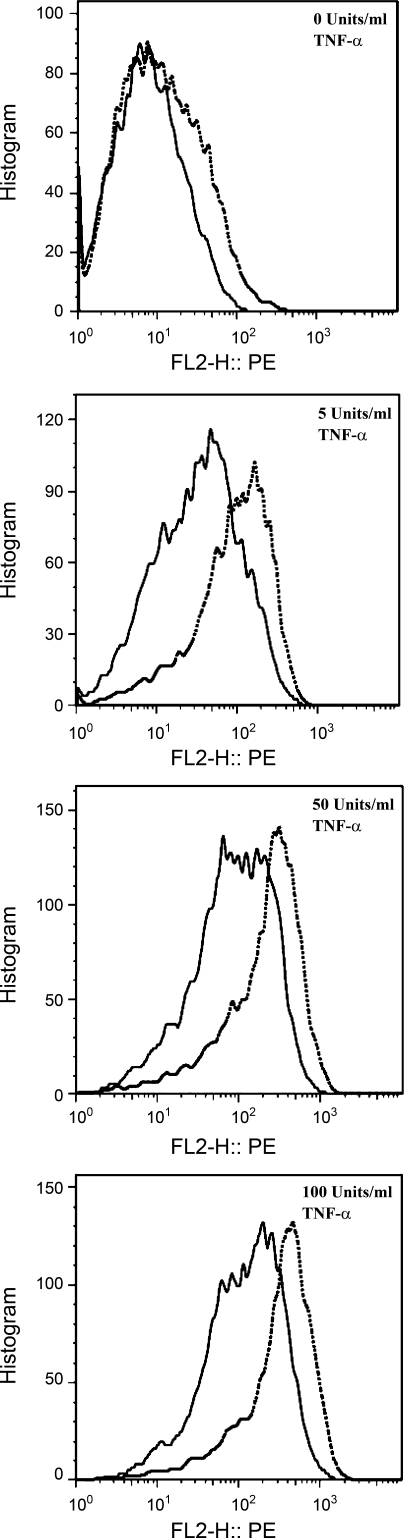
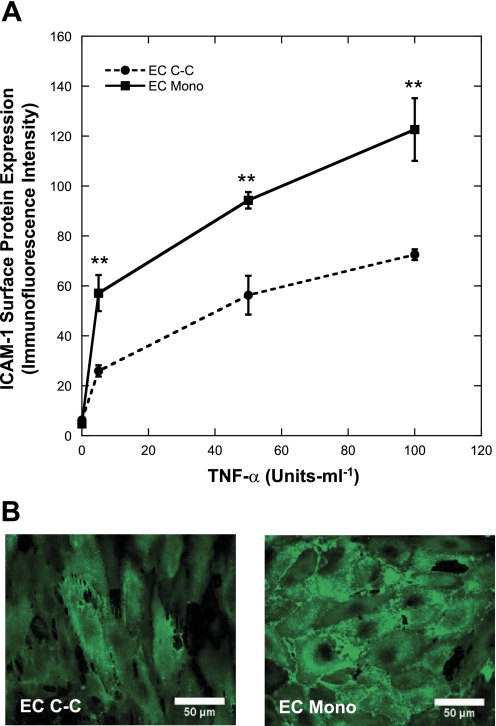
EC monocultures and co-cultures were treated with 0, 5, 50, or 100 U/ml TNF-α, and flow cytometry was used to measure the EC surface protein expression of ICAM-1, VCAM-1, and E-selectin. As shown with representative raw flow cytometry data after stimulation with various concentrations of TNF-α (Fig. 2), ICAM-1 surface protein expression on EC monocultures was greater than ECs co-cultured with quiescent SMCs. The mean intensity values were used in subsequent comparisons. Relative to TNF-α-treated ECs in monoculture, TNF-α treatment of ECs in co-culture produced significantly less ICAM-1 surface protein expression (Fig. 3A; P < 0.02, n = 3). VCAM-1 and E-selectin surface protein expression exhibited similar trends (not shown). Consistent with the flow cytometry results, the ICAM-1 immunofluorescence intensity is higher on ECs in monoculture compared with co-culture after treatment with 100 U/ml TNF-α (Fig. 3B).
Fig. 2.
Representative raw flow cytometry data of intercellular adhesion molecule-1 (ICAM-1) surface protein expression after tumor necrosis factor (TNF)-α treatment. The surface protein expression of ICAM-1 on ECs in monoculture (dotted line) and co-culture (solid line) after TNF-α stimulation was determined by flow cytometry techniques.
Fig. 3.
Effect of co-culture on ICAM-1 surface protein expression after TNF-α treatment. The surface protein expression of ICAM-1 (n = 3; A) in monoculture (EC Mono) and co-culture (EC C-C) after TNF-α stimulation was determined by flow cytometry techniques (A). Immunofluorescent images of ICAM-1 on the surface of EC monocultures and co-cultures were captured to verify flow cytometry results after treatment with 100 U/ml TNF-α (B). **P < 0.02 compared with EC C-C.
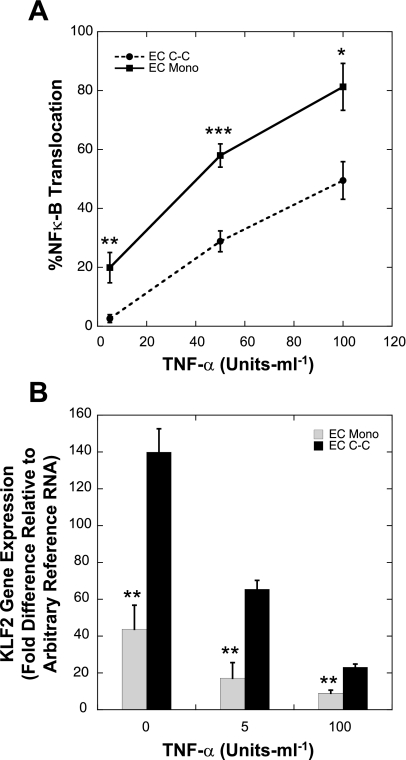
To investigate the possible mechanism responsible for the reduction of the TNF-α-mediated EC adhesion molecule expression induced by co-culture, we measured the gene expression of the TNF-α receptors along with the response of two key EC transcription factors, NF-κB (2) and KLF2 (26), known to play a role in regulating the EC inflammatory response. Relative to ECs in monoculture, ECs in co-culture did not have significantly different expression levels of TNFR1 (1.06 ± 0.26) or TNFR2 (1.17 ± 0.53), as measured by real-time quantitative RT-PCR (n = 3). After stimulation with increasing concentrations of TNF-α, the percentage of cells exhibiting nuclear translocation of NF-κB p65 increased in both EC monocultures and co-cultures (n = 4; Fig. 4A). Relative to ECs in monoculture, ECs in co-culture had significantly less TNF-α-mediated NF-κB p65 nuclear translocation at each of the TNF-α treatments tested (5 U/ml, P < 0.02; 50 U/ml, P < 0.002; 100 U/ml, P < 0.05). As expected, TNF-α reduced the expression of KLF2 in ECs in co-culture and monoculture (Fig. 4B; n = 4). More importantly, relative to EC monocultures, ECs in co-culture had significantly higher KLF2 gene expression levels (P < 0.02 for all TNF-α concentrations tested). The endothelial nitric oxide synthase (eNOS) gene expression level in static untreated EC monocultures was found to be 1.01 ± 0.13, while the level in EC co-cultures was 1.51 ± 0.07 compared with a reference RNA. These results suggest that the reduced NF-κB p65 nuclear translocation and the increased KLF2 and eNOS gene expression within ECs in co-culture may lead to the reduced expression of the EC adhesion molecules.
Fig. 4.
Effect of co-culture on nuclear translocation of NF-κB and Kruppel-like factor 2 (KLF2) gene expression after TNF-α treatment. NF-κB nuclear translocation within ECs in monoculture (EC Mono) or co-culture (EC C-C) after TNF-α stimulation was determined by measuring the percent of ECs with positive nuclear NF-κB p65 immunofluorescent staining (n = 4; A). KLF2 gene expression of ECs in monoculture or co-culture after TNF-α stimulation was determined by quantitative real-time RT-PCR (n = 4; B). *P < 0.05, **P < 0.02, ***P < 0.002 compared with EC C-C.
Effect of quiescent SMC co-culture with ECs on the TNF-α-mediated response during exposure to steady laminar shear stress.
To investigate whether the reduced inflammatory response found in static EC co-cultures persisted after exposure to steady laminar flow at a physiological shear stress, we exposed the cell cultures to a steady shear stress of 15 dyne/cm2 for 24 h, added 100 U/ml TNF-α, continued the flow for an additional 4.5 h, and then measured the EC adhesion molecule expression. Static controls were not exposed to flow at any time during the experiment. ICAM-1, VCAM-1, and E-selectin expression on ECs in co-culture was still significantly different from adhesion molecule expression on ECs in monoculture when the cell cultures were exposed to steady physiological shear stress (P < 0.02, n = 4; Fig. 5A); even though, exposure to flow reduced the ratio of the amount of cell adhesion molecules between ECs in monoculture and co-culture (P < 0.02; Fig. 5B). From these results, we hypothesized that shear stress increased the KLF2 within ECs in monoculture and ECs in co-culture to similar expression levels, which yielded the lower ratio of adhesion molecule expression of ECs in monoculture relative to ECs in co-culture.
Fig. 5.
Effect of co-culture on ICAM-1, vascular cell adhesion molecule-1 (VCAM-1), and E-selectin surface protein expression after flow and TNF-α treatment. EC Mono and EC C-C were exposed to a shear stress of 15 dyne/cm2 for 24 h before the addition of 100 U/ml of TNF-α. The cultures were then simultaneously exposed to the TNF-α and shear stress for 4.5 h. The surface protein expression of inflammatory cell adhesion molecules was determined by flow cytometry techniques after exposure to flow (n = 4; A). To illustrate the effect of flow on the adhesion molecule expression, the results are presented as the ratio of the surface protein expression of ECs in monoculture relative to ECs in co-culture (n = 4; B). **P < 0.02 compared with EC Mono (A) or equivalent static (B).
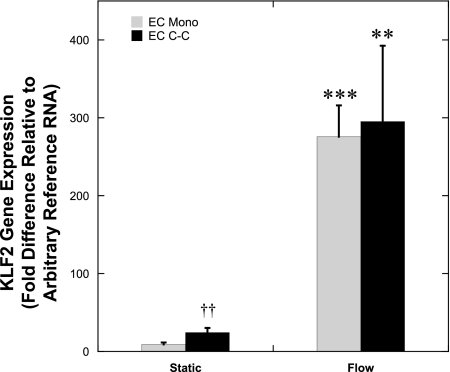
We measured the KLF2 gene expression within ECs in co-culture and monoculture after flow preconditioning and treatment with 100 U/ml of TNF-α during flow (n = 5–6; Fig. 6). Exposure to flow significantly increased KLF2 gene expression in both EC monocultures (P < 0.002) and EC co-cultures (P < 0.02). There was no significant effect of co-culture on KLF2 gene expression when the cells were exposed to flow. These results suggest that under flow conditions, there are additional factors, other than KLF2, responsible for the lower TNF-α-mediated EC adhesion molecule expression found within ECs in co-culture.
Fig. 6.
Effect of co-culture on KLF2 gene expression after flow and TNF-α treatment. EC Mono and EC C-C were exposed to a shear stress of 15 dyne/cm2 for 24 h before the addition of 100 U/ml of TNF-α. The cultures were then simultaneously exposed to the TNF-α and shear stress for 4.5 h. EC KLF2 gene expression after flow and TNF-α stimulation was determined by quantitative real-time RT-PCR (n = 5–6). **P < 0.02, ***P < 0.002 compared with equivalent static, and ††P < 0.002 compared with static EC Mono.
Effect of the direct contact between co-cultured ECs and SMCs on the TNF-α-mediated EC adhesion molecule expression.
We examined whether the reduced EC inflammatory response in co-culture was influenced by the ECM secreted by the SMCs and/or specific interactions unique to the underlying SMCs. To determine whether the ECM produced by the quiescent SMCs aided in the reduction of the EC adhesion molecule expression, the quiescent SMCs were decellularized, the ECs were plated on the remaining ECM, and the TNF-α (100 U/ml)-mediated EC adhesion molecule expression was measured by flow cytometry. EC-fibroblast co-cultures were formed to determine whether the inhibition of the TNF-α-mediated ICAM-1 surface expression is unique to EC-SMC interactions. No adverse effects on the number of ECs attached or the confluent cell density were observed when ECs were adhered to the decellularized matrix or quiescent fibroblasts.
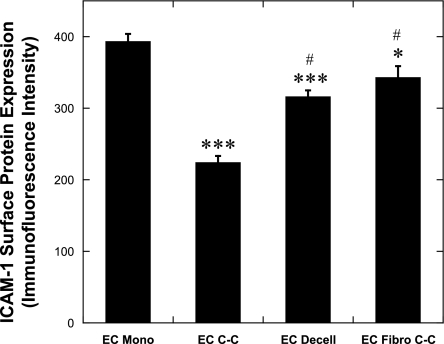
Relative to ECs adhered to FN-coated plastic (EC monoculture), ECs adhered to the decellularized ECM produced by quiescent SMCs had significantly less ICAM-1 (P < 0.002, n = 12; Fig. 7). VCAM-1 and E-selectin expression was regulated similarly to ICAM-1 (not shown). ECs adhered to quiescent fibroblasts had significantly less ICAM-1 surface protein expression compared with ECs in monoculture (P < 0.02, n = 4–12; Fig. 7); however, the reduction was not as large as the results obtained when ECs were cultured on quiescent SMCs. Immunofluorescence labeling of FN (not shown) illustrated that the surface of quiescent fibroblasts was covered with fibrillar FN as we observed previously on quiescent SMCs (31). Therefore, the fibrillar FN and other ECM proteins within the decellularized matrix and on the surface of the quiescent fibroblasts may reduce the TNF-α-mediated adhesion molecule expression on the EC surface.
Fig. 7.
Effect of substrate on TNF-α-mediated ICAM-1 surface protein expression. ECs adhered to fibronectin (FN)-coated plastic (EC Mono, n = 12), quiescent SMCs (EC C-C, n = 12), decellularized ECM produced by quiescent SMCs (EC Decell, n = 12), or quiescent fibroblasts (EC Fibro C-C, n = 4) were stimulated with 100 U/ml TNF-α before ICAM-1 surface protein expression was measured by flow cytometry techniques. *P < 0.02, ***P < 0.002 compared with EC Mono and #P < 0.002 compared with EC C-C.
ECs in co-culture with quiescent SMCs had significantly less ICAM-1 surface protein expression compared with ECs in monoculture, ECs on the decellularized ECM, or ECs co-cultured with quiescent fibroblasts. VCAM-1 and E-selectin surface protein expression for ECs on decellularized matrix exhibited a similar trend as found with ICAM-1 (not shown). These results suggest that the underlying SMCs contribute to the reduction of EC adhesion molecule expression, and this is specific to EC-SMC interactions.
To determine whether the proximity of the ECs from the quiescent SMCs affected EC adhesion molecule expression, we plated ECs and quiescent SMCs on opposite sides of a 10-μm-thick porous membrane and measured ICAM-1 surface protein expression after exposure to TNF-α. Quiescent SMCs on the opposite side of a porous membrane did not reduce the TNF-α-mediated ICAM-1 surface protein expression compared with ECs in monoculture (n = 3–12; Fig. 8). Thus, the direct contact between ECs and SMCs in co-culture is required to inhibit the TNF-α-mediated ICAM-1 surface protein expression.
Fig. 8.
Effect of the direct contact between ECs and SMCs in co-culture on TNF-α-Mediated ICAM-1 surface protein expression. EC Mono (n = 12), EC C-C (n = 12), ECs in monoculture on a porous membrane (EC Mono PM, n = 3), and ECs on opposite side of porous membrane as quiescent SMCs (EC C-C PM, n = 3) were treated with 100 U/ml TNF-α and ICAM-1 surface protein expression was measured with flow cytometry techniques. ***P < 0.002 compared with EC Mono.
DISCUSSION
Previous studies indicate that when ECs and proliferating SMCs are cultured on opposite sides of a porous membrane, the proliferating SMCs produce an increase in the EC inflammatory response, even in the absence of cytokines (3–5, 21, 22). While the two cell types may form connections through the narrow pores (9), the use of the porous membrane limits the interactions between the ECs and SMCs, increasing the diffusion distance and reducing the frequency of myoendothelial gap junction formation and EC interaction with the ECM produced by SMCs. The porous membrane also introduces a synthetic and stiffer surface between the ECs and SMCs than occurs in vivo. In addition, many of the previous co-culture studies on porous membranes utilized venous ECs, rather than arterial ECs, and proliferative SMCs, which model the state of a diseased vessel rather than quiescent SMCs that are found within healthy vessels. To address these limitations, we utilized our novel direct-contact co-culture system to investigate the effect of culturing arterial ECs in direct contact with quiescent arterial SMCs on the TNF-α-mediated EC inflammatory response.
Our direct-contact co-culture system replicates the growth state and architecture of tissue-engineered blood vessels and represents a better model of healthy native arteries compared with EC cultures grown on rigid, synthetic surfaces. Our novel co-culture system enables investigations of the effect of EC adhesion to a compliant cellular substrate, EC interactions with quiescent SMCs, and EC adhesion on SMC-produced ECM (30, 31). In the current study, we found that, under static and physiological flow conditions, quiescent SMCs reduced the TNF-α-mediated EC inflammatory response. This response was dependent on the direct contact of ECs with SMCs and their apical ECM.
Prior studies report that proliferating SMCs induced an inflammatory state within ECs by increasing EC NF-κB p65 and p50 mRNA expression; NF-κB-DNA binding activity (3); EC ICAM-1, VCAM-1, and E-selectin mRNA expression (3, 5); E-selectin protein expression (4); and leukocyte adhesion to ECs with (21) or without the presence of TNF-α (3). In contrast, we found that quiescent SMCs did not affect adhesion molecule expression and NF-κB nuclear translocation in the absence of cytokines and reduced the EC inflammatory response to TNF-α.
There are two key differences between the co-culture systems that may explain the contrasting results: the separation of cells with a porous membrane and the SMC phenotype. The results within the literature were obtained using a membrane co-culture system where ECs and proliferative SMCs were grown on opposite sides of a porous membrane. Our co-culture system is a direct-contact model consisting of an EC monolayer adhered to the surface of quiescent SMCs and their apical ECM. Structurally, with the ECs adhered to ECM produced by and in close proximity to quiescent SMCs, our direct-contact co-culture system represents a model of a healthy blood vessel. The membrane co-culture system used in many previous studies mimicked a diseased vessel, since the SMCs were maintained in a proliferative phenotype. Therefore, these results suggest that SMCs within a diseased vessel may aid in the activation of ECs, whereas SMCs within a healthy vessel may aid to reduce the activation of ECs.
Different experimental procedures cannot explain the difference between our results and previously published results. In the current studies, a high concentration (0.25–0.5%) of trypsin/EDTA was needed to detach ECs from the surface SMCs for flow cytometry. Since VCAM-1 may be hydrolyzed to a certain extent due to trypsinization (6), EC co-cultures and monocultures were always treated in parallel with the same procedures, thus any hydrolysis of the EC adhesion molecules would have occurred to the same extent for both conditions. During the flow cytometry analysis, the ECs were distinguished from the SMCs by positive staining of CellTracker Green. A gate was placed between the two distant peaks formed by the positive and negative CellTracker Green staining, so that only the EC adhesion molecule expression would be quantified in the samples (Fig. 1). Therefore, the difference in EC adhesion molecule expression between ECs in co-culture and monoculture is not due to the SMCs diluting the EC population in the co-culture samples.
While SMCs express receptors for TNF-α, several lines of evidence suggest that SMCs in the co-culture are not likely to act as a TNF-α sink, depleting the stimulating cytokine. One, Fig. 8 illustrates that ECs in co-culture with SMCs separated by a porous membrane do not have a reduced ICAM-1 expression as found with ECs in direct contact with SMCs. Two, Fig. 7 shows that ECs in a direct-contact co-culture with fibroblasts do not have a similar level of reduced ICAM-1 expression as our EC-SMC co-culture. If the fibroblasts in direct-contact co-culture and the SMCs in a porous membrane co-culture did bind significant numbers of TNF-α to deplete the level of soluble TNF-α, then we would expect similar responses of ECs to direct-contact co-culture and co-culture on opposite sides of porous membranes. Furthermore, TNF receptor levels on EC and SMCs are low (17, 34). The confluent EC layer likely acts as a barrier to the TNF-α, limiting exposure of the SMCs so binding of TNF-α to receptors on SMCs is not likely to reduce the concentration sufficiently to affect the amount of TNF-α bound to ECs.
While the SMC-produced ECM reduces the EC adhesion molecule expression relative to ECs in monoculture (Fig. 7), ECs in co-culture have a significantly lower EC adhesion molecule expression compared with ECs attached to the decellularized ECM. Thus, other factors aid in the reduction of the adhesion molecules in co-culture besides the SMC-produced ECM. Another possibility is that the SMC-produced ECM does not fully mimic the ECM on the surface of the SMCs in co-culture. The decellularization process did remove some of the ECM and may have damaged some of the ECM causing reduced EC interactions with the SMC-produced ECM compared with ECs in co-culture.
The results presented in this study suggest that, under static or physiological flow conditions, quiescent SMCs reduce the TNF-α-mediated EC inflammatory response and that this response is dependent on the direct contact with SMCs and their apical ECM. Although there were not enough samples gathered to draw a valid conclusion (n = 1), Rainger and Nash (21) noted that contractile (and presumably quiescent) SMCs in their membrane co-culture system did not produce significant TNF-α-mediated leukocyte adhesion as found in their co-cultures using proliferative SMCs. Thus, the SMC phenotype seems to play a significant role in determining the inflammatory response of the endothelium.
Our results provide some insight into possible candidate pathways. Since ECs in monoculture and co-culture had similar expression levels of the TNF-α receptors, ECs in both conditions received the same degree of stimulation from the TNF-α. This suggests that the underlying mechanism causing the different EC inflammatory responses to the TNF-α in monoculture and co-culture is due to altered intercellular signaling mechanisms. TNF-α induces the phosphorylation of IκB, which increases its degradation. The reduction in IκB levels led to the nuclear translocation of the inflammatory transcription factor NF-κB (29) and subsequent increase in EC adhesion molecule expression (2). Conversely, NF-κB nuclear translocation is inhibited by NO production, which increases the amount of cytoplasmic IκBα (27). eNOS and KLF2 (Fig. 4B), an EC transcription factor that increases eNOS enzymatic activity (26), gene expression were found to be increased in static EC co-cultures compared with EC monocultures. The eNOS gene expression levels in EC co-cultures were 50% higher than the levels found in EC cultured alone. Therefore, ECs in static co-culture, relative to ECs in static monoculture, could have increased NO production due to higher KLF2 and eNOS gene expression levels, yielding more cytoplasmic IκBα to inhibit NF-κB nuclear translocation (Fig. 4A) and EC adhesion molecule expression (Fig. 3). The possible difference in NO production between static EC monocultures and co-cultures may be negated after exposure to flow due to similar levels of KLF2 gene expression (Fig. 6), which reduced the ratio of ICAM-1, VCAM-1, and E-selectin surface protein expression between ECs in monoculture and co-culture after flow (Fig. 5B). Even though flow reduced the EC adhesion molecule expression, significant differences remained between ECs in monoculture and co-culture (Fig. 5A). Thus, KLF2 could be responsible for the reduced TNF-α-mediated inflammatory response in static ECs in co-culture, but additional factors inhibit this inflammatory response in co-culture after exposure to flow.
The low time-averaged shear stress found in atheroprone shear waveforms induces an EC phenotype that may be similar to statically cultured ECs compared with ECs exposed to atheroprotective shear levels. For example, ECs cultured under static conditions or exposed to an atheroprone oscillatory shear waveform had very low levels of KLF2 gene expression relative to a pulsatile atheroprotective shear waveform (32). Thus, it is conceivable that EC-SMC interactions, yielding increased KLF2, could aid in the inhibition of the progression of atherosclerosis in flow-reduced areas within the vasculature.
We did find that the ECM produced by the SMCs contributed to the altered functional response of the ECs in co-culture, which could be due to the distinct mechanism by which ECs adhere to quiescent SMCs (31). In co-culture, quiescent SMCs present the endothelium an ECM-containing fibrillar FN, which induces tensin-rich fibrillar adhesion formation due to interactions with the fibrillar FN and EC α5β1 integrin complex. ECs in co-culture lacked focal adhesions and, relative to ECs in monoculture, had less RhoA mRNA expression (31). RhoA activation is suppressed by the clustering of β1 integrin molecules (19). RhoA is a key mediator of 1) the clustering of cell adhesion molecules on the surface of ECs (33) and 2) the TNF-α-mediated NF-κB nuclear translocation (8). Additional mediators, such as p38, regulate NF-κB nuclear translocation. Shear-induced NF-κB nuclear translocation is inhibited by p38 activation through EC interactions with the fibrillar form of FN (20), and overexpression of tensin increases p38 activation (10). Activation of p38 kinase is known to inhibit TNF-α-mediated IκBα phosphorylation and degradation leading to reduced NF-κB nuclear translocation (1, 25). Therefore, EC interactions with the ECM on the surface of the SMCs could influence the inhibition of TNF-α-mediated NF-κB-induced EC inflammatory response by a decreased amount of active RhoA and/or increased p38 activation resulting from fibrillar adhesion formation and the absence of focal adhesions.
Our results suggest that alterations in the healthy intimal ECM could adversely affect the function of ECs. The low shear stress environment found within atheroprone areas may play a significant role in altering the vessel wall ECM and inducing the activation of the endothelium. Reduced flow through the carotid artery induced the production of cyclophilin A (CyPA) from the vascular SMCs (24a). CyPA promotes the activation of matrix metalloproteinases (11), which degrade the ECM within the vascular wall. Therefore, alteration of the ECM within the vascular wall not only promotes SMC proliferation and migration, but it can also affect the EC interactions with intimal ECM proteins that aid in the functional response of the endothelium.
ECs communicate with SMCs through many paracrine mediators, such as endothelium-derived hyperpolarizing factor (28) and various shear stress-sensitive mediators of vessel wall remodeling, ECM synthesis, and SMC migration (16). These mediators might then induce SMCs to send signals back to the ECs altering their inflammatory response. Since the 10-μm porous membrane inhibited the reduced EC inflammatory response in co-culture, such paracrine signaling may only play a secondary role in decreasing the EC inflammation. The data support the hypothesis that EC inflammation reduction in co-culture occurs through the ECs interaction with the SMC-produced ECM and its unique formation of fibrillar adhesions and lack of focal adhesions and the molecule signaling associated with these complexes.
Utilizing our direct-contact co-culture system, we previously found that quiescent SMCs can induce the endothelium to present a less thrombotic surface due to reduced tissue factor expression compared with ECs cultured alone (30), and these current results suggest that quiescent SMCs and their apical ECM reduce the endothelium's inflammatory response to TNF-α. Thus, this model of a healthy vessel wall illustrates possible functions of the underlying quiescent SMCs on the thrombotic and inflammatory response of the endothelium. While the current study highlights the important role of shear stress in mediating the response to TNF-α in co-culture, subjecting ECs and EC-SMC co-cultures to different mechanical stimuli, such as strain and pulsatile shear waveforms, could alter the EC-SMC interactions causing varied EC inflammatory responses to TNF-α. Additional studies are needed to fully characterize the effects of various mechanical stimuli on ECs co-cultured with SMCs.
While the current studies provide novel insights into the influence of quiescent SMCs on the inflammatory response of ECs, the work also has implications for the development of tissue-engineered blood vessels. We previously showed that our direct-contact EC-SMC co-culture could be used as an effective model of a tissue-engineered blood vessel (30). Therefore, we predict that the EC-SMC interactions within tissue-engineered blood vessels will help to protect the endothelium from the TNF-α-induced inflammation, which could reduce the initiation and progression of atherosclerosis within the tissue-engineered blood vessel.
GRANTS
The National Institutes of Health Grants RO1-HL-57446 and RO1-HL-88825 and National Institute of Biomedical Imaging and Bioengineering Grant predoctoral fellowship for C. S. Wallace F31EB006298 supported this project.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Special thanks go to A. Munnelly for assistance with the fibroblast experiments, as well as T. Dissanayake and M. Cook at the Flow Cytometry Shared Resource of the Comprehensive Cancer Center at Duke University Medical Center for analyzing the flow cytometry samples.
REFERENCES
- 1.Bowie AG, O'Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol 165: 7180–7188, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Chen CC, Manning AM. Transcriptional regulation of endothelial cell adhesion molecules: a dominant role for NF-kappa B. Agents Actions Suppl 47: 135–141, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Chiu JJ, Chen LJ, Chang SF, Lee PL, Lee CI, Tsai MC, Lee DY, Hsieh HP, Usami S, Chien S. Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: role of NF-kappaB. Arterioscler Thromb Vasc Biol 25: 963–969, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Chiu JJ, Chen LJ, Lee CI, Lee PL, Lee DY, Tsai MC, Lin CW, Usami S, Chien S. Mechanisms of induction of endothelial cell E-selectin expression by smooth muscle cells and its inhibition by shear stress. Blood 110: 519–528, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu JJ, Chen LJ, Lee PL, Lee CI, Lo LW, Usami S, Chien S. Shear stress inhibits adhesion molecule expression in vascular endothelial cells induced by coculture with smooth muscle cells. Blood 101: 2667–2674, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Grabner R, Till U, Heller R. Flow cytometric determination of E-selectin, vascular cell adhesion molecule-1, and intercellular cell adhesion molecule-1 in formaldehyde-fixed endothelial cell monolayers. Cytometry 40: 238–244, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes proinflammatory priming. Am J Physiol Cell Physiol 293: C1824–C1833, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Hippenstiel S, Schmeck B, Seybold J, Krull M, Eichel-Streiber C, Suttorp N. Reduction of tumor necrosis factor-alpha (TNF-alpha) related nuclear factor-kappaB (NF-kappaB) translocation but not inhibitor kappa-B (Ikappa-B)-degradation by Rho protein inhibition in human endothelial cells. Biochem Pharmacol 64: 971–977, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97: 44–51, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Katz BZ, Zohar M, Teramoto H, Matsumoto K, Gutkind JS, Lin DC, Lin S, Yamada KM. Tensin can induce JNK and p38 activation. Biochem Biophys Res Commun 272: 717–720, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Kim WJ, Jeon ST, Koh EM, Cha HS, Ahn KS, Lee WH. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol 116: 217–224, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med 8: 1235–1242, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Kluger MS, D'Alessio A, Garcia-Cardena G, Pober JS. Regulation of arterial-venous differences in tumor necrosis factor responsiveness of endothelial cells by anatomic context. Am J Pathol 172: 1088–1099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Mack PJ, Zhang Y, Chung S, Vickerman V, Kamm RD, Garcia-Cardena G. Biomechanical regulation of endothelium-dependent events critical for adaptive remodeling. J Biol Chem 284: 8412–8420, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med 177: 1277–1286, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature 367: 193–196, 1994 [DOI] [PubMed] [Google Scholar]
- 19.O'Connor KL, Nguyen BK, Mercurio AM. RhoA function in lamellae formation and migration is regulated by the alpha6beta4 integrin and cAMP metabolism. J Cell Biol 148: 253–258, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol 169: 191–202, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rainger GE, Nash GB. Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ Res 88: 615–622, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Rainger GE, Stone P, Morland CM, Nash GB. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J Immunol Methods 255: 73–82, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by Krawetz S, Misener S. Totowa, NJ: Humana, 2000, p. 365–386 [DOI] [PubMed] [Google Scholar]
- 24a.Satoh K, Matoba T, Suzuki J, O'Dell MR, Nigro P, Cui Z, Mohan A, Pan S, Li L, Jin Z, Yan C, Abe J, Berk BC. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation 117: 3088–3098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwenger P, Alpert D, Skolnik EY, Vilcek J. Activation of p38 mitogen-activated protein kinase by sodium salicylate leads to inhibition of tumor necrosis factor-induced IkappaB alpha phosphorylation and degradation. Mol Cell Biol 18: 78–84, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, Feinberg MW, Chen Z, Simon DI, Luscinskas FW, Michel TM, Gimbrone MA, Jr, Garcia-Cardena G, Jain MK. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med 199: 1305–1315, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J Biol Chem 272: 30969–30974, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Takaki A, Morikawa K, Tsutsui M, Murayama Y, Tekes E, Yamagishi H, Ohashi J, Yada T, Yanagihara N, Shimokawa H. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J Exp Med 205: 2053–2063, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanos D, Maniatis T. NF-kappa B: a lesson in family values. Cell 80: 529–532, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng 35: 375–386, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Wallace CS, Strike SA, Truskey GA. Smooth muscle cell rigidity and extracellular matrix organization influence endothelial cell spreading and adhesion formation in coculture. Am J Physiol Heart Circ Physiol 293: H1978–H1986, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern specific. Biochem Biophys Res Commun 341: 1244–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol 145: 1293–1307, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamawaki H, Lehoux S, Berk BC. Chronic physiological shear stress inhibits tumor necrosis factor-induced proinflammatory responses in rabbit aorta perfused ex vivo. Circulation 108: 1619–1625, 2003 [DOI] [PubMed] [Google Scholar]