Abstract
The atria play an important role in cardiac performance. We evaluated their function and the atrioventricular interaction in operated patients with tetralogy of Fallot (TOF). Twenty patients who had undergone surgical repair of TOF and seven controls were investigated. Patients had residual pulmonary but no major tricuspid valve insufficiency. Atrial and ventricular strain rates were obtained by echocardiographic speckle tracking. Cine MRI-derived volumetric analysis provided atrial and ventricular time volume and time volume change curves yielding emptying and filling parameters. In addition, at the atrial level, reservoir, conduit and pump function, and cyclic volume change were calculated. At the atrioventricular valve level, tricuspid and mitral annular plane systolic excursion (TAPSE and MAPSE, respectively) were measured by two-dimensional echocardiography. In the patients compared with controls, right ventricular end-diastolic volumes were increased and biventricular ejection fraction was decreased (all P < 0.05). Biventricular measures of early diastolic ventricular filling were at control levels, but in late diastole, right ventricular filling parameters and strain rates were decreased (P < 0.001). The maximal right atrial size was slightly but not significantly diminished, but cyclic volume change was significantly reduced (P < 0.0001). Pump and reservoir function were decreased (P < 0.05), and conduit function was elevated (P < 0.001). The left atrium showed reduced reservoir function and cyclic volume change (P < 0.05). TAPSE and MAPSE were also decreased (P < 0.05). There were statistically significant interdependencies between RV ejection fraction, TAPSE, and right atrial filling and emptying parameters (all P < 0.05). In TOF patients, moderate systolic and diastolic right ventricular dysfunction is associated with clearly impaired right atrial function. The left atrium is affected to a lesser extent.
Keywords: congenital heart disease, atrial function, magnetic resonance imaging
progressive right ventricular (RV) enlargement and dysfunction due to pulmonary regurgitation or obstruction across the reconstructed outflow tract dominate the long-term outcome in patients with surgically corrected tetralogy of Fallot (TOF) (8, 9). Recent studies (5, 22, 27) that have investigated the complex adaptive response of the RV to chronic overload in TOF have provided evidence that the RV cannot be seen as one entity. In addition, the impact of RV dysfunction on left ventricular (LV) and atrial function must be considered (1, 12, 15, 26). In 1974, Suga (25) highlighted the role of the atria in cardiac performance by transforming the continuous venous return into the intermittent ventricular filling flow.
The influence of different conditions on left atrial (LA) function, such as aging (24), LV dysfunction (2, 14, 19, 23), and aortic or mitral valve disease (6, 7), have been widely assessed. However, data about the interaction of the RV with the right atrium (RA) are sparse. Studies (10, 21, 28) have been conducted that assessed components of RA function in animals and humans with pulmonary arterial banding or pulmonary hypertension. A study by Hui and coworkers (15) investigated TOF patients with a focus on late atrial active emptying, the so-called atrial kick. However, a more systematic assessment of RA function that accounts for both filling and emptying parameters has not yet been studied in TOF patients with RV dysfunction. With this in mind, we designed the present study to use an integrated approach to investigate right and left atrial and ventricular function in TOF patients by MRI and two-dimensional echocardiographic speckle tracking imaging techniques.
METHODS
Study Population
Twenty patients with previously repaired TOF without additional cardiac malformations as well as seven controls without cardiac medical history were included in the study after random selection from the database of the Competence Network for Congenital Heart Defects (www.kompetenznetz-ahf.de) from one center. Exclusion criteria were as follows: 1) the existence of mitral or aortic valve insufficiency and tricuspid valve insufficiency greater than grade I in echocardiographic studies and 2) severe stenosis (>65 mmHg) of the pulmonary outflow and arteries, as assessed morphologically and reflected by elevated RV peak systolic pressure as calculated by echocardiography via tricuspid regurgitation. Sinus rhythm in the electrocardiogram was mandatory. This study was approved by the institutional research ethics committee, and written informed consent was obtained from the participants or their guardians.
The diagnostic procedures (see below) were all done on the same day in 11 subjects (8 patients and 3 controls). Seven subjects (3 patients and 4 controls) were studied within 1 wk; the remaining nine patients were assessed within 1 mo.
MRI
MRI was performed using a 1.5-T scanner (Release 2.6, Philips Medical Systems, Best, The Netherlands) with a cardiac surface array coil. ECG-triggered multislice multiphase steady-state free precession magnetic resonance images were acquired at end expiration in axial orientation, covering the whole heart (4). The sequence parameters were as follows: 25 phases/cardiac cycle, repetition time (TR) and echo time (TE) = shortest, flip angle = 60°, slice thickness = 6 mm, gap = 0 mm, field of view = 280–400 × 220–320 mm2, and matrix = 140–212 × 160–246, resulting in a plane resolution of 1.4 × 1.4 × 6 mm3.
Blood flow was measured in the main pulmonary artery with through-plane velocity-encoded cine MRI. The sequence parameters were as follows: TR/TE = 5.1/3.0 ms, flip angle = 15°, and encoding velocity = 200–400 cm/s (4).
Image analysis.
Analysis of the cine MRI was performed using View Forum software (release 5.1V1L2 SP3, Philips Medical Systems). For volumetric analysis, RA, RV, LA, and LV endocardial contours were manually traced in every slice and phase of the stack of axial cine MR images. The atrial appendages were included in the measurement of atrial volumes. The superior and inferior caval vein, coronary sinus, and pulmonary veins were excluded at their junction to the atrium, and ventricular trabeculations and papillary muscles were also excluded. To minimize interobserver variability, all tracing was done by one experienced observer, and traces were reviewed by a second observer.
RV and LV function.
The following standard parameters were determined for the RV and LV: end-diastolic volume (EDV), end-systolic volume (ESV), ejection fraction, and stroke volume. All volumes were indexed by dividing them by the body surface area.
RA and LA function.
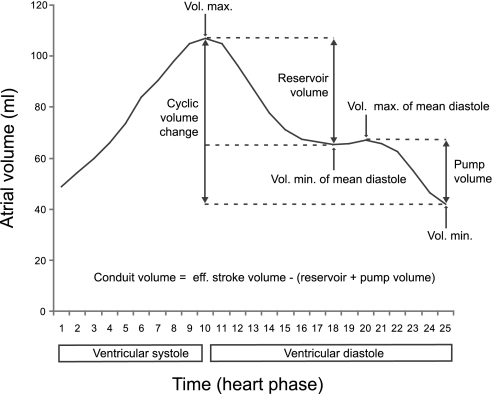
The following atrial function parameters were studied: reservoir, conduit and pump volumes and function, the cyclic volume change, and emptying fraction. The reservoir volume was calculated by subtracting the minimal atrial volume at middiastole from the maximal atrial volume (Fig. 1) (16, 20). The pump volume was calculated by subtracting the minimal atrial volume from the maximal atrial volume at middiastole. Conduit volume resulted after subtraction of the sum of reservoir and pump volume from effective stroke volume of the LV. The terms “systole” and “diastole” always refer to ventricular systole and ventricular diastole.
Fig. 1.
Atrial function. Shown is an exemplary atrial time volume curve of a control subject with assessment of atrial performance and function. Whereas reservoir volume, pump volume, and cyclic volume change are calculated from atrial volumes at special time points (see methods), the conduit volume, which is passing the atrium without causing atrial volume change, is calculated by the subtraction of the sum of reservoir and pump volume from the effective stroke volume of the left ventricle (LV).
Reservoir, conduit, and pump function were expressed as a percentage of LV effective stroke volume. The cyclic volume change was defined as the difference between maximal and minimal atrial volume (16, 20). Dividing the cyclic volume change by the maximal atrial volume multiplied by 100 resulted in the total atrial filling fraction. Again, all volumes were indexed by dividing them by the body surface area.
Atrial and ventricular time volume curves.
For the four heart chambers, time volume curves were generated by summation of the volumes of every slice for each phase. Time volume change curves, which were adapted from the work of Helbing et al. (13), were generated with assessment of the following parameters.
RV AND LV FUNCTION.
The following parameters were assessed: 1) early peak filling rate (in ml/s) as the maximal ventricular volume change in early diastole; 2) early filling fraction as the ventricular volume increase during the first third of diastole, normalized to ventricular stroke volume; 3) late peak filling rate (in ml/s) as the maximal ventricular volume change in late diastole; and 4) late filling fraction as the ventricular volume increase after the onset of atrial contraction, normalized to ventricular stroke volume.
RA AND LA FUNCTION.
The following parameters were assessed: 1) early peak emptying rate (in ml/s) as the maximal atrial volume change in early diastole; 2) early emptying fraction as the atrial volume decrease during the first third of diastole, normalized to the cyclic volume change; 3) late peak emptying rate (in ml/s) as the maximal atrial volume change in late diastole; and 4) late emptying fraction as the atrial volume decrease after the onset of atrial contraction, normalized to the cyclic volume change.
The conduit volume was calculated for each diastolic heart phase (t) according to the following formulas:
where LV(t) and RV(t) indicate the LV and RV volumes at a particular heart phase t, LVESV and RVESV are the LV and RV end-systolic volumes, LA Volmax and RA Volmax are the maximal volumes of the LA and RA, LA(t) and RA(t) are the LA and RA volumes at a particular heart phase t, and PR(t) is the pulmonary regurgitation volume during a particular heart phase t.
Heart phase t always refers to the same diastolic heart phase, and, for each subject, the time points of maximal and minimal volumes of the heart chambers were assessed individually.
Echocardiography
Transthoracic two-dimensional speckle tracking imaging was done at the ventricular level (apex, midsegment, and base) and atrial level (roof, midsegment, and base) to attain the parameters of myocardial deformation. Strain and strain rate were obtained in early diastole, late diastole, and systole on the basis of three cardiac cycles in the apical four chamber view (15, 17, 18). Peak strain was measured independently of aortic and pulmonary valve closure, and a mean value was calculated from the three measuring points. Images were acquired using a 2.5/3.5 transducer interfaced with a Vingmed System VII (GE Vingmed, Horten, Norway). Analysis was performed offline (EchoPAC 6.1.0, GE Vingmed). To evaluate the movement of the atrioventricular plane, maximal tricuspid and mitral annular plane systolic excursions (TAPSE and MAPSE, respectively) were measured (3).
Exercise Testing
Maximal O2 uptake (in ml·min−1·kg body wt−1) was determined by bicycle ergometry with work load increase of 20 W/min. All subjects exercised until exhaustion.
Statistical Analysis
As the parameters of atrial and ventricular function showed an approximately Gaussian shape in a graphical check by boxplots, they were reported as means and SDs and compared between patients and controls using Student's t-test in the version for different variances. The association of variables was investigated with Pearson's correlation coefficient (r) including a test for the null hypothesis of independence. Computations were done with SPSS 17.0. P values of <0.05 were considered as significant.
RESULTS
Patient Characteristics
Twenty asymptomatic patients (New York Heart Association class I, mean age: 19.5 ± 8.9 yr, 8 men and 12 women, all Caucasian) with surgically repaired TOF as well as seven controls (mean age: 24.6 ± 8.7 yr, 4 men and 3 women, all Caucasian) were enrolled in the study (Table 1). All patients had volume load of the RV with a mean pulmonary regurgitation fraction of 31.8 ± 13.4% and mild pressure load with a mean RV peak pressure of 40.1 ± 14.8 mmHg. Patients were studied 14.6 ± 5.3 yr after TOF repair. The pulmonary regurgitation fraction differed between patients dependent on the type of surgical repair (24.5 ± 8.9% in n = 9 patients with homograft and 36.4 ± 16.9% in n = 11 patients without homograft). This difference just failed to reach statistical significance (P = 0.06). Nineteen patients had complete right bundle branch block, and one patient had incomplete right bundle branch block. Maximal O2 uptake was reduced in patients versus controls (P < 0.05).
Table 1.
Characteristics of the patient and control groups
| Patient Group | Control Group | |
|---|---|---|
| No. of patients | 20 | 7 |
| No. of men/no. of women | 8/12 | 4/3 |
| Age at study inclusion, yr | 19.5 ± 8.9 | 24.6 ± 8.7 |
| Body surface area, m2 | 1.57 ± 0.29 | 1.86 ± 0.28 |
| Mean heart rate, beats/min | 74.4 ± 12.1* | 63.4 ± 9.0 |
| QRS duration, ms | 141.1 ± 24.6* | 92.3 ± 9.5 |
| Pulmonary regurgitation fraction, % | 31.8 ± 13.4* | 2.06 ± 1.65 |
| RVSP, mmHg | 40.1 ± 14.8 | N/A |
| Maximal O2 consumption, ml·min−1·kg−1 | 26.8 ± 7.2* | 34.6 ± 6.7 |
| No. of previous palliations | 10 | |
| Time from repair, yr | 14.6 ± 5.3 | |
| No. of repeated surgeries | 4 | |
| No. of homografts/heterografts | 9 |
Values are means ± SD. RVSP, right ventricular (RV) systolic pressure; N/A, not applicable. Primary surgical correction had been performed with transannular patch (n = 9), without transannular patch (n = 2), and with valved conduit insertion (n = 9).
Significant difference compared with data in control subjects (P < 0.05).
RV
As expected, the patient group had significant RV enlargement (P < 0.005; Table 2) with reduced ejection fraction (P < 0.005), systolic strain rate (P < 0.05; Table 3), and global peak strain (P < 0.001).
Table 2.
Size and function of the RV and LV
| RV |
LV |
|||
|---|---|---|---|---|
| Control group | Patient group | Control group | Patient group | |
| Global parameters | ||||
| End-diastolic volume, ml/body surface area | 102.0 ± 17.4 | 135.2 ± 27.9* | 88.3 ± 5.6 | 87.1 ± 13.9 |
| End-systolic volume, ml/body surface area | 45.6 ± 11.1 | 70.7 ± 17.2* | 31.8 ± 5.8 | 37.6 ± 11.2 |
| Stroke volume, ml/body surface area | 56.8 ± 7.2 | 64.4 ± 12.5 | 56.5 ± 5.5 | 49.5 ± 8.4* |
| Regurgitation fraction, % | ||||
| Pulmonary valve | 2.06 ± 1.65 | 32.7 ± 14.7* | ||
| Aortic valve | None | 1.89 ± 1.24 | ||
| Ejection fraction, % | 55.7 ± 0.04 | 48.0 ± 4.1* | 64.1 ± 5.5 | 57.3 ± 8.1* |
| Cardiac index, l·min−1·body surface area−1 | 3.61 ± 0.71 | 4.78 ± 1.22* | 3.59 ± 0.68 | 3.66 ± 0.76 |
| Filling parameters | ||||
| EPFR, ml·s−1·body surface area−1 | 226.3 ± 121.3 | 237.8 ± 71.1 | 259.7 ± 108.5 | 266.6 ± 88.6 |
| Early filling fraction, % | 57.1 ± 17.5 | 41.2 ± 15.5 | 65.6 ± 14.3 | 62.7 ± 21.6 |
| LPFR, ml·s−1·body surface area−1 | 134.6 ± 66.4 | 74.6 ± 48.3* | 106.6 ± 40.7 | 73.2 ± 24.2 |
| Late filling fraction, % | 22.7 ± 6.8 | 11.0 ± 7.2* | 16.3 ± 4.9 | 10.1 ± 3.6* |
| EPFR/LPFR | 2.15 ± 1.50 | 10.70 ± 21.41 | 2.9 ± 1.5 | 4.1 ± 2.0 |
| Other parameters | ||||
| TAPSE, mm | 2.75 ± 0.14 | 1.64 ± 0.3* | ||
| MAPSE, mm | 1.59 ± 0.15 | 1.40 ± 0.21* | ||
Values are means ± SD. LV, left ventricle; EPFR, early peak filling rate; LPFR, late peak filling rate; TAPSE, tricuspid annular plane systolic excursion; MAPSE, mitral annular plane systolic excursion.
Significant difference compared with data in control subjects (P < 0.05).
Table 3.
Strain rates and strain by two-dimensional echocardiographic speckle tracking imaging in the RV and LV
| RV |
LV |
|||
|---|---|---|---|---|
| Control group | Patient group | Control group | Patient group | |
| Ventricular strain rate, s−1 | ||||
| Early diastolic strain rate | ||||
| Ventricular apex | 2.00 ± 0.84 | 2.06 ± 0.72 | 2.28 ± 0.87 | 2.08 ± 0.70 |
| Midsegment | 2.10 ± 0.59 | 1.71 ± 0.59 | 1.80 ± 0.47 | 1.99 ± 0.68 |
| Ventricular base | 2.39 ± 0.77 | 2.12 ± 0.81 | 1.64 ± 0.38 | 2.31 ± 0.81 |
| Late diastolic strain rate | ||||
| Ventricular apex | 1.00 ± 0.33 | 0.51 ± 0.17* | 0.65 ± 0.22 | 0.66 ± 0.41 |
| Midsegment | 1.05 ± 0.37 | 0.57 ± 0.24* | 0.60 ± 0.17 | 0.66 ± 0.47 |
| Ventricular base | 1.27 ± 0.41 | 0.91 ± 0.42 | 0.81 ± 0.25 | 1.00 ± 0.70 |
| Systolic strain rate | ||||
| Ventricular apex | −1.69 ± 0.41 | −1.26 ± 0.25* | −1.35 ± 0.49 | −1.36 ± 0.51 |
| Midsegment | −1.57 ± 0.46 | −1.27 ± 0.26* | −1.10 ± 0.18 | −1.10 ± 0.29 |
| Ventricular base | −1.85 ± 0.92 | −1.58 ± 0.36 | −1.49 ± 0.50 | −1.47 ± 0.51 |
| Peak strain, % | ||||
| Mean | −26.3 ± 3.2 | −21.6 ± 2.8 | −19.5 ± 2.4 | −19.7 ± 3.7 |
Values are means ± SD. The early diasolic strain rate was the strain rate during the E wave in early ventricular diastole, the late diastolic strain rate was the strain rate during the A wave in late ventricular diastole, and the systolic strain rate was the strain rate during ventricular systole.
Significant difference compared with data in control subjects (P < 0.05).
In early diastole, covering the first third of diastole, there were no significant changes of ventricular filling parameters and strain rates. However, at late diastole, starting with the onset of atrial contraction, peak filling rate and filling fraction were significantly diminished (P < 0.05 and P < 0.005; Table 2 and Fig. 2B). At the same time, the late diastolic strain rate was reduced (P < 0.001; Table 3). TAPSE was significantly lower in the patient group (P < 0.0001; Table 2) and correlated with RV ejection fraction (r = 0.555, P < 0.005).
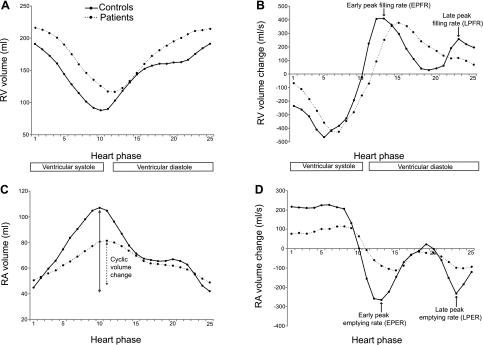
Fig. 2.
Right atrial (RA) and right ventricular (RV) time volume (A and C) and time volume change curves (B and D) of the patient and control groups. Mean values are shown.
LV
The patient group had a lower ejection fraction and EDV (P < 0.05 and P = 0.09; Table 2). The effective stroke volume was reduced (P < 0.05), but the cardiac index was at control levels (P = 0.83) due to a slightly elevated heart rate (P < 0.05; Table 1). In early diastole, there were no significant changes of ventricular filling parameters (Table 2 and Fig. 3B), but the late filling fraction was significantly reduced in the patients (P < 0.05). MAPSE was lower (P < 0.05), whereas all other echocardiographic measurements were at control levels (Table 3). There was no correlation between MAPSE and RV ejection fraction (r = 0.293).
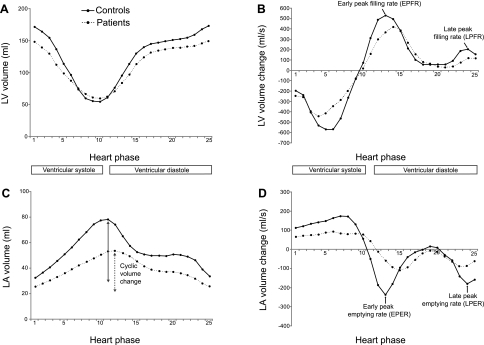
Fig. 3.
Left atrial (LA) and LV time volume (A and C) and time volume change curves (B and D) of the patient and control groups. Mean values are shown.
RA
Reservoir function was lower in the patient group than in the control group (P < 0.001; Table 5), conduit function was elevated (P < 0.001), and pump function was diminished (P < 0.05). There was strong negative correlation between reservoir and conduit function (r = −0.916, P < 0.001). The maximal size of the atrium was slightly diminished in the patient group (P = 0.23), whereas the minimal size of the atrium was significantly larger (P < 0.05). This resulted in a significant decrease of the cyclic volume change (P < 0.001; Fig. 2C) and atrial filling fraction (P < 0.001; Table 5). The latter was significantly correlated to RV ejection fraction (r = 0.675, P < 0.001) and TAPSE (r = 0.698, P < 0.001). In addition, during atrial filling, strain and strain rates were reduced in every segment of the RA (all P < 0.001; Table 4). Atrial emptying was reduced in early and late diastole (P < 0.001 and P < 0.05, Table 5 and Fig. 2D). The strain rate, measured close to the tricuspid valve, was significantly lower in early and late diastole (both P < 0.05; Table 4). The values of the other atrial segments were at control levels.
Table 5.
Size and function of the RA and LA
| RA |
LA |
|||
|---|---|---|---|---|
| Control group | Patient group | Control group | Patient group | |
| Global parameters | ||||
| Maximum volume, ml/body surface area | 57.3 ± 9.3 | 50.8 ± 17.1 | 43.8 ± 10.2 | 34.6 ± 9.6 |
| Minimum volume, ml/body surface area | 22.5 ± 4.9 | 29.8 ± 11.7* | 18.1 ± 5.0 | 15.5 ± 6.2 |
| Cyclic volume change, ml/body surface area | 34.9 ± 5.4 | 21.1 ± 6.5* | 25.6 ± 6.0 | 19.2 ± 5.0* |
| Filling fraction, % | 61.0 ± 4.2 | 42.3 ± 8.2* | 58.4 ± 4.5 | 56.3 ± 8.8 |
| Reservoir volume, ml/body surface area | 23.0 ± 1.4 | 12.6 ± 4.3* | 17.7 ± 3.7 | 12.4 ± 2.7* |
| Conduit volume, ml/body surface area | 19.8 ± 3.2 | 28.1 ± 8.8* | 28.5 ± 4.9 | 29.9 ± 7.8 |
| Pump volume, ml/body surface area | 13.7 ± 4.5 | 8.7 ± 2.7* | 9.9 ± 3.4 | 7.2 ± 3.0 |
| Reservoir function, % | 41.0 ± 3.4 | 26.1 ± 9.2* | 31.2 ± 5.4 | 25.5 ± 6.4* |
| Conduit function, % | 35.2 ± 5.5 | 56.2 ± 12.6* | 50.6 ± 9.6 | 60.0 ± 10.5 |
| Pump function (atrial kick), % | 23.9 ± 6.3 | 17.8 ± 6.0* | 17.3 ± 4.4 | 14.8 ± 6.2 |
| Emptying parameters | ||||
| Atrial emptying in early diastole, ml/body surface area | 20.1 ± 2.4 | 9.8 ± 4.7* | 14.2 ± 4.1 | 6.1 ± 3.8 |
| EPER, ml·s−1·body surface area−1 | −265.1 ± 73.6 | −115.1 ± 71.3* | −238.4 ± 101.8 | −110.4 ± 75.0* |
| Early emptying fraction, % | 58.6 ± 10.1 | 48.0 ± 19.9 | 58.2 ± 20.1 | 32.5 ± 18.4* |
| Atrial emptying in late diastole, ml/body surface area | 11.03 ± 4.43 | 6.30 ± 3.15* | 8.4 ± 3.4 | 5.5 ± 2.3 |
| LPER, ml·s−1·body surface area−1 | −233.3 ± 131.4 | −102.8 ± 65.9* | −179.8 ± 73.5 | −92.4 ± 61.1* |
| Late emptying fraction, % | 31.0 ± 8.6 | 31.3 ± 13.1 | 32.1 ± 6.6 | 28.7 ± 12.0 |
| Other parameters | ||||
| Atrioventricular E-to-A ratio | 2.00 ± 0.17 | 1.66 ± 0.51 | 1.90 ± 0.39 | 2.33 ± 0.70 |
Values are means ± SD. EPER, early peak emptying rate; LPER, late peak emptying rate; E-to-A ratio, ratio of the early ventricular filling wave to late atrial contraction filling wave.
Significant difference compared with data in control subjects (P < 0.05).
Table 4.
Strain rates and strain by two-dimensional echocardiographic speckle tracking imaging in the RA and LA
| RA |
LA |
|||
|---|---|---|---|---|
| Control group | Patient group | Control group | Patient group | |
| Atrial strain rate, s−1 | ||||
| Early emptying strain rate | ||||
| Atrial base | −3.52 ± 1.02 | −2.36 ± 1.29* | −3.37 ± 0.73 | −2.81 ± 1.08 |
| Midsegment | −2.62 ± 0.87 | −2.38 ± 1.13 | −3.25 ± 0.91 | −2.66 ± 0.88 |
| Atrial roof | −2.34 ± 1.06 | −2.59 ± 1.17 | −3.19 ± 1.14 | −2.61 ± 0.69 |
| Late emptying strain rate | ||||
| Atrial base | −3.82 ± 0.92 | −2.76 ± 1.00* | −1.43 ± 0.48 | −2.36 ± 1.47 |
| Midsegment | −2.50 ± 0.44 | −2.59 ± 1.01 | −1.80 ± 0.57 | −2.20 ± 1.11 |
| Atrial roof | −2.11 ± 0.91 | −2.50 ± 0.44 | −2.13 ± 0.80 | −2.22 ± 1.15 |
| Filling strain rate | ||||
| Atrial base | 4.61 ± 0.97 | 2.78 ± 0.89* | 2.43 ± 0.84 | 2.41 ± 0.64 |
| Midsegment | 4.05 ± 0.64 | 2.64 ± 0.83* | 2.26 ± 0.87 | 2.25 ± 0.82 |
| Atrial roof | 3.87 ± 0.96 | 2.80 ± 0.94* | 2.48 ± 0.69 | 2.26 ± 0.94 |
| Peak strain, % | ||||
| Mean | 96.2 ± 15.8 | 42.4 ± 10.7* | 52.9 ± 10.3 | 46.3 ± 12.7 |
Values are means ± SD. RA, right atrium; LA, left atrium. The early emptying strain rate was the strain rate during the E wave in early ventricular diastole, the late emptying strain rate was the strain rate during the A wave in late ventricular diastole, and the filling strain rate was the strain rate during ventricular systole.
Significant difference compared with data in control subjects (P < 0.05).
LA
Reservoir function was diminished (P < 0.05; Table 5), but conduit and pump function were at control levels. The maximal and minimal size of the atrium did not differ significantly between patients and controls, but cyclic volume change was decreased (P < 0.05; Table 5 and Fig. 3C). Atrial emptying was lower in early diastole (P < 0.001; Fig. 3D) and unchanged in late diastole. Speckle tracking imaging revealed no significant differences between patients and controls (Table 4).
Grouping the patients according to functional aspects or the preceding surgical procedure revealed no major differences in the analysis of RA and LA function parameters. In patients with combined pressure-volume load of the RV (n = 9, mean pulmonary regurgitation fraction of 24.1 ± 8.0%, mean RV peak pressure of 54.1 ± 10.0 mmHg, existing homograft in n = 8 patients) compared with those with predominant volume load (n = 11, mean pulmonary regurgitation fraction of 36.7 ± 17.1%, mean RV peak pressure of 28.6 ± 3.5 mmHg), the only difference was a decreased RA reservoir function (P < 0.05).
DISCUSSION
In patients with surgically corrected TOF, residual pulmonary insufficiency and/or stenosis of the RV outflow tract is an important disease that requires close follow-up (9). RV enlargement and dysfunction are typically progressive, and the time of transition from compensated into irreversible right heart failure remains difficult to predict. There is evidence that alterations in LA function have an important impact on cardiac performance (19); however, there are little data about the interaction of the RA and RV in patients with TOF. In the present study, we systematically assessed right and left atrial and ventricular function in a group of patients with pulmonary regurgitation as the leading pathophysiological characteristic. We found clearly abnormal RA function even though RV function was only moderately impaired. In addition, our data provide new points of view on the mechanisms of atrioventricular interactions that allow us to propose cause-and-effect chains. In the following sections, we discuss our observations in a point-by-point manner.
Interaction of the RA and RV
Ventricular systole and atrial filling.
In the patient group, the RA cyclic volume change and filling fraction were below control values. Cyclic volume change is thought to reflect atrial distensibility. Previous investigators have concluded from studies (11, 25) that were performed for the LA or RA with normal RV systolic function that it has a positive impact on global cardiac output. In our noninvasive study, atrial pressure was not measured, and thus the compliance of the atria could not be determined. However, the clearly diminished strain and strain rates of the RA during atrial filling are indicative for reduced atrial compliance (17). In addition, Barbier and colleagues (2) showed interdependencies among LA filling, mitral valve plane displacement, LV ejection fraction, and atrial filling being a strong predictor of ventricular performance. Bazaz et al. (3) reported that the coupling of ventricular ejection fraction and valvular plane displacement is even more pronounced for the right side compared with the left side of the heart, but they did not evaluate the effect on atrial function. Our study, which was performed in a homogeneous group of TOF patients with moderate RV dysfunction, shows a strong coupling between RA filling, TAPSE, and RV ejection fraction. Additional research is warranted to evaluate these interdependencies in a group of TOF patients with different degrees of RV dysfunction.
Ventricular filling and atrial emptying in the early and middiastolic phases.
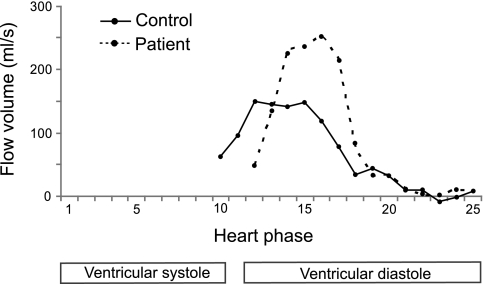
In the patient group, in early diastole, which relates to the first third of diastole, we noted normal RV filling despite abnormal RA emptying. RV filling was delayed but otherwise unsuspicious, as indicated by early peak filling and strain rates that were at control levels. In contrast, RA reservoir function was substantially reduced, and atrial emptying was also slowed and delayed. One could speculate that pulmonary regurgitation, which is highest in early diastole, contributes to maintained early RV filling but “competes” hydrodynamically with atrial emptying. Then, in middiastolic phases, where pulmonary regurgitation abates, we noted in the patient group an atrial conduit function that was delayed, and its magnitude was clearly above control levels (Fig. 4).
Fig. 4.
Conduit flow for one typical patient and one typical control subject. In the patient, the conduit flow is delayed but higher in its magnitude.
The transformation of the continuous venous return into the intermittent ventricular filling flow is one major task of the atrium (25). This implies that conduit volumes should be low in relation to reservoir volumes. This goes along with the observations of Gaynor et al. (11), who reported that a low conduit-to-reservoir ratio favors high cardiac output. In our study of TOF patients, we noted augmented conduit but decreased reservoir volumes being associated with RV dysfunction. Again, future research is warranted to evaluate the impact of this finding in subgroups of TOF patients with different degrees of RV dysfunction.
Ventricular filling and atrial emptying in the late diastolic phases.
After the onset of atrial contraction, RV filling fraction and ventricular strain rates were decreased, implying abnormal RV filling in late diastole. These findings were associated with simultaneously diminished RA pump function (atrial kick), as evidenced by decreased late diastolic RA emptying fraction and strain rate. This observation is in line with several studies (7, 15) that reported a reduced active atrial pump function in volume-loaded ventricles. Other authors (7, 10, 28) have reported increased active atrial performance in the presence of severe, isolated pressure loaded LVs or RVs, for example, in the setting of pulmonary hypertension. In our selected group of patients with only moderate pressure load of the RV in addition to volume load, we did not see this effect of increased atrial pump function. Although the relation between active atrial pump function and ventricular load reported by other investigators is striking, it is not possible to explain the underlying mechanisms on the basis of the studies performed so far. One might speculate that atrial filling, which correlated in our study with atrial pump function, has an impact on atrial activation. However, systematic future research is necessary to further evaluate this matter.
Limitations
To keep variability low, this study was conducted in preselected TOF patients who had a homogeneous moderate degree of RV enlargement and dysfunction. In addition, we excluded patients with major tricuspid insufficiency, as this will cause atrial distension and thus induce other pathophysiological mechanisms of atrial function. Therefore, the results of our study are not representative for all TOF patients. The values for natriuretic peptides are lacking, as these were not measured in our patients. Whether the observed abnormalities of RA function are from intrinsic causes or express the functional response to abnormal RV function cannot be fully determined at present. A possible intrinsic cause might be the scar in the RA after open-heart surgery. Furthermore, after surgery, the pericardium was left open, whereas the intact pericardium seems to play a role in regular atrial function (11).
Conclusions
In the TOF patients studied, moderate systolic and diastolic RV dysfunction is already associated with impaired RA function. There is a strong coupling of RV ejection fraction, tricuspid annular plane displacement, and RA filling. Future studies need to systematically evaluate if RA dysfunction is a predictor of RV failure.
GRANTS
This work was supported by Federal Ministry of Education and Research Grant 01EV0704 and Competence Network for Congenital Heart Defects Grant FKZ 01G10210.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
The authors thank Anne Gale for editorial assistance.
REFERENCES
- 1.Abd El Rahman MY, Hui W, Timme J, Ewert P, Berger F, Dsebissowa F, Hetzer R, Lange PE, Abdul-Khaliq H. Analysis of atrial and ventricular performance by tissue Doppler imaging in patients with atrial septal defects before and after surgical and catheter closure. Echocardiography 22: 579–585, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation 100: 427–436, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Bazaz R, Edelman K, Gulyasy B, Lopez-Candales A. Evidence of robust coupling of atrioventricular mechanical function of the right side of the heart: insights from M-mode analysis of annular motion. Echocardiography 25: 557–561, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Beerbaum P, Barth P, Kropf S, Sarikouch S, Kelter-Kloepping A, Franke D, Gutberlet M, Kuehne T. Cardiac function by MRI in congenital heart disease: impact of consensus training on interinstitutional variance. J Magn Reson Imaging 30: 956–966, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bodhey NK, Beerbaum P, Sarikouch S, Kropf S, Lange P, Berger F, Anderson RH, Kuehne T. Functional analysis of the components of the right ventricle in the setting of tetralogy of Fallot. Circ Cardiovasc Imaging 1: 141–147, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Borg AN, Pearce KA, Williams SG, Ray SG. Left atrial function and deformation in chronic primary mitral regurgitation. Eur J Echocardiogr 10: 833–840, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cioffi G, Stefenelli C. Comparison of left ventricular geometry and left atrial size and function in patients with aortic stenosis versus those with pure aortic regurgitation. Am J Cardiol 90: 601–606, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Davlouros PA, Kilner PJ, Hornung TS, Li W, Francis JM, Moon JC, Smith GC, Tat T, Pennell DJ, Gatzoulis MA. Right ventricular function in adults with repaired tetralogy of Fallot assessed with cardiovascular magnetic resonance imaging: detrimental role of right ventricular outflow aneurysms or akinesia and adverse right-to-left ventricular interaction. J Am Coll Cardiol 40: 2044–2052, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 356: 975–981, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Gaynor SL, Maniar HS, Bloch JB, Steendijk P, Moon MR. Right atrial and ventricular adaptation to chronic right ventricular pressure overload. Circulation 112: I212–I218, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Gaynor SL, Maniar HS, Prasad SM, Steendijk P, Moon MR. Reservoir and conduit function of right atrium: impact on right ventricular filling and cardiac output. Am J Physiol Heart Circ Physiol 288: H2140–H2145, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Geva T, Sandweiss BM, Gauvreau K, Lock JE, Powell AJ. Factors associated with impaired clinical status in long-term survivors of tetralogy of Fallot repair evaluated by magnetic resonance imaging. J Am Coll Cardiol 43: 1068–1074, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Helbing WA, Niezen RA, Le Cessie S, van der Geest RJ, Ottenkamp J, de Roos A. Right ventricular diastolic function in children with pulmonary regurgitation after repair of tetralogy of Fallot: volumetric evaluation by magnetic resonance velocity mapping. J Am Coll Cardiol 28: 1827–1835, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Hoit BD, Gabel M. Influence of left ventricular dysfunction on the role of atrial contraction: an echocardiographic-hemodynamic study in dogs. J Am Coll Cardiol 36: 1713–1719, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Hui W, Abd El Rahman M. Y, Dsebissowa F, Rentzsch A, Gutberlet M, Alexi-Meskishvili V, Hetzer R, Lange PE, Abdul-Khaliq H. Quantitative analysis of right atrial performance after surgical repair of tetralogy of Fallot. Cardiol Young 14: 520–526, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jarvinen VM, Kupari MM, Hekali PE, Poutanen VP. Right atrial MR imaging studies of cadaveric atrial casts and comparison with right and left atrial volumes and function in healthy subjects. Radiology 191: 137–142, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Kim DG, Lee KJ, Lee S, Jeong SY, Lee YS, Choi YJ, Yoon HS, Kim JH, Jeong KT, Park SC, Park M. Feasibility of two-dimensional global longitudinal strain and strain rate imaging for the assessment of left atrial function: a study in subjects with a low probability of cardiovascular disease and normal exercise capacity. Echocardiography 26: 1179–1187, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Knirsch W, Dodge-Khatami A, Kadner A, Kretschmar O, Steiner J, Bottler P, Kececioglu D, Harpes P, Valsangiacomo Buechel ER. Assessment of myocardial function in pediatric patients with operated tetralogy of Fallot: preliminary results with 2D strain echocardiography. Pediatr Cardiol 29: 718–725, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kono T, Sabbah HN, Rosman H, Alam M, Stein PD, Goldstein S. Left atrial contribution to ventricular filling during the course of evolving heart failure. Circulation 86: 1317–1322, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Lauerma K, Harjula A, Jarvinen V, Kupari M, Keto P. Assessment of right and left atrial function in patients with transplanted hearts with the use of magnetic resonance imaging. J Heart Lung Transplant 15: 360–367, 1996 [PubMed] [Google Scholar]
- 21.Leeuwenburgh BP, Steendijk P, Helbing WA, Baan J. Indexes of diastolic RV function: load dependence and changes after chronic RV pressure overload in lambs. Am J Physiol Heart Circ Physiol 282: H1350–H1358, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Morcos P, Vick GW, 3rd, Sahn DJ, Jerosch-Herold M, Shurman A, Sheehan FH. Correlation of right ventricular ejection fraction and tricuspid annular plane systolic excursion in tetralogy of Fallot by magnetic resonance imaging. Int J Cardiovasc Imaging 25: 263–270, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol 82: 756–761, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Spencer KT, Mor-Avi V, Gorcsan J, 3rd, DeMaria AN, Kimball TR, Monaghan MJ, Perez JE, Weinert L, Bednarz J, Edelman K, Kwan OL, Glascock B, Hancock J, Baumann C, Lang RM. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart 85: 272–277, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suga H. Importance of atrial compliance in cardiac performance. Circ Res 35: 39–43, 1974 [DOI] [PubMed] [Google Scholar]
- 26.Tzemos N, Harris L, Carasso S, Subira LD, Greutmann M, Provost Y, Redington AN, Rakowski H, Siu SC, Silversides CK. Adverse left ventricular mechanics in adults with repaired tetralogy of Fallot. Am J Cardiol 103: 420–425, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Wald RM, Haber I, Wald R, Valente AM, Powell AJ, Geva T. Effects of regional dysfunction and late gadolinium enhancement on global right ventricular function and exercise capacity in patients with repaired tetralogy of Fallot. Circulation 119: 1370–1377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willens HJ, Fertel DP, Qin J, Labrador E, Lowery MH. Effects of age and pulmonary arterial hypertension on the different phases of right atrial function. Int J Cardiovasc Imaging 24: 703–710, 2008 [DOI] [PubMed] [Google Scholar]