Abstract
Although the beneficial effects of exercise training on conduit artery endothelial function are well-established in animals and humans with compromised basal function, whether exercise exerts favorable effects on a healthy endothelium is inconclusive. We sought to determine whether long-term exercise training enhances endothelial function in peripheral conduit arteries of healthy pigs. Using a retrospective analysis of data collected in our laboratory (n = 127), we compared in vitro brachial and femoral artery endothelium-dependent and -independent relaxation between a group of pigs that exercise-trained for 16–20 wk and a group that remained sedentary. No differences in vasomotor function were found between the 2 groups (P > 0.05). Additionally, in a subset of pigs (n = 16), expression levels of 18 proteins that are typically associated with the atherosclerotic process were measured by immunoblot analysis of endothelial cell scrapes obtained from the brachial and femoral arteries. We found no differences (P > 0.05) in endothelial gene expression between these exercise-trained and sedentary healthy pigs. These results indicate that pigs exhibiting the classic training-induced adaptations do not demonstrate enhanced endothelium-dependent dilation nor reveal a more atheroprotected endothelial cell phenotype in their brachial and femoral arteries than their sedentary but otherwise healthy counterparts.
Keywords: chronic exercise, peripheral conduit artery, endothelium
it is becoming increasingly accepted that exercise training exerts direct effects on the vascular wall, a concept recently referred to as vascular conditioning (9, 12, 29). The beneficial effects of exercise on conduit artery endothelial function are primarily notable in subject populations with preexisting cardiovascular risk factors or disease, that is, in individuals with compromised vascular function (11, 21, 23, 29). Conversely, in sedentary but otherwise healthy subjects, there is conflicting evidence regarding the influence of exercise training on endothelial function (11, 21, 23, 29). The disparity of results may be partially explained by the time point at which assessments are performed. In this regard, it is proposed in both healthy animals and humans (9, 11, 29) that the endothelial adaptive response in conduit arteries is training duration-dependent such that prolonging the exercise training leads to normalization of function, possibly as a result of an arterial remodeling (9, 11, 13, 14, 17, 19, 29). In the present investigation, we sought to determine whether, indeed, endothelial function of skeletal muscle arteries from pigs demonstrating the classic training-induced adaptations were similar to that of sedentary animals. With the goal of examining this question in a large data set (n = 127), we conducted a retrospective analysis of data collected in our laboratory during the past 2 decades. Specifically, we compared in vitro brachial and femoral artery endothelium-dependent and -independent relaxation between a group of healthy pigs that exercise-trained for 16–20 wk and a group that remained sedentary. No differences in vasomotor function were detected between the 2 groups in either vessel, thus supporting the concept that, in healthy animals, long-term exercise training does not enhance endothelium-dependent dilation in peripheral conduit arteries. Notwithstanding this outcome, it may be incorrect to presume that the absence of a training-induced adaptation in vasomotor function implies a lack of change toward an atheroprotective endothelial phenotype. To unravel this question, in a subset of pigs, expression of 18 proteins that are frequently altered in early atherosclerotic disease were measured from brachial and femoral artery endothelial cell scrapes. We hypothesized that trained animals would reveal a more antiatherogenic endothelial cell phenotype than their sedentary but otherwise healthy counterparts.
METHODS
Experimental animals.
Between 1992 and 2010, 669 pigs entered our research program, which is devoted to the study of the cardiovascular effects of exercise. All pigs were used with protocols approved by the Animal Care and Use Committee at the University of Missouri. Pigs were housed in rooms maintained at 20–23°C with a 12:12-h light-dark cycle. With the purpose to examine the influence of long-term exercise training on peripheral artery endothelial function in healthy animals, we conducted a retrospective analysis of published and unpublished data from our swine database. Specifically, we selected data from pigs: 1) that were miniature Yucatan; 2) that were 12–14 mo old at time of death; 3) that were provided a standard diet (Purina Lab Mini-Pig Chow; 8% of daily caloric intake derived from fat); 4) that participated in a 16- to 20-wk exercise training program (EX) or remained sedentary (SED); and 5) in which in vitro endothelium-dependent and -independent relaxation of brachial and/or femoral arteries was assessed. In the present study, data were obtained from 127 pigs from which brachial (n = 113) and/or femoral arteries (n = 89) were harvested. Out of the 113 brachial arteries, 59 were derived from EX pigs (33 males, 26 females), and 54 were derived from SED pigs (34 males, 20 females). Out of the 89 femoral arteries, 47 were derived from EX pigs (30 males, 17 females), and 42 were derived from SED pigs (29 males, 13 females). It should be noted that some data included here have been reported in previous studies (37, 38, 40) in which the pigs were used as controls against high-fat-fed pigs. In a subset of 16 male pigs (8 EX, 8 SED), we measured protein expression in endothelial cell scrapes from brachial and femoral arteries. Furthermore, we used this subgroup of pigs to confirm our previous findings (34) that 1-yr-old Yucatan pigs that are fed a standard diet do not exhibit signs of atherosclerotic disease. All brachial and femoral arteries from this subset revealed 0% Sudan IV staining, a marker of atherosclerotic lesions (data not shown). This observation is vital because the objective of the present study was to examine the effects of exercise training on endothelial function and phenotype in healthy (disease-free) peripheral conduit arteries.
Training program.
All pigs were familiarized with running on a motorized treadmill and then randomly assigned to either an exercise or sedentary group. The exercise group completed a 16- to 20-wk endurance training program as described previously (24, 30, 40). Briefly, intensity and duration of exercise bouts increased steadily so that by week 10 of training the pigs ran 85 min/day, 5 days/wk. The 85-min training bouts consisted of a 5-min warm-up, a 15-min sprint run at 6–8 mile/h (mph), a 60-min endurance run at 4–6 mph, and a 5-min cool-down. Pigs assigned to the sedentary group were restricted to their enclosures (2 × 4-m pens) and did not exercise. At the conclusion of the intervention, both groups of pigs performed a graded intensity treadmill exercise test to exhaustion (20). In our laboratory, the efficacy of the training program is evaluated from measurements of endurance time on the treadmill, heart-to-body weight ratio, and citrate synthase activity assay as index of skeletal muscle oxidative capacity (31).
In vitro assessment of endothelium-dependent and -independent relaxation.
Procedures used to assess vasoactive responses of arterial rings have been published previously in detail (39, 40). Pigs from the exercise training group were killed 16–24 h following the last bout of exercise. Immediately following death, brachial and femoral arteries were harvested, trimmed of fat and connective tissue, and sectioned into 2- to 3-mm rings in cold Krebs bicarbonate buffer solution. Vasomotor reactivity was examined with the rings stretched to the length that produced maximal active tension. Before dose-response curves were initiated, arterial rings were preconstricted with PGF2α (30 μM). Endothelium-dependent relaxation was assessed by using bradykinin (10−11 to 10−6 M), whereas endothelium-independent relaxation was assessed with sodium nitroprusside (SNP; 10−10 to 10−4 M). After each dose-response protocol, the bicarbonate buffer solution was replaced to wash out the drug, and arterial segments were allowed 1 h to stabilize before initiation of the next protocol.
Solutions and drugs for vasomotor experiments.
Krebs bicarbonate buffer solution contained 131.5 mM NaCl, 5.0 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.5 mM CaCl2, 11.2 mM glucose, 20.8 mM NaHCO3, 0.003 mM propranolol, and 0.025 mM EDTA. Solutions were aerated with 95% O2-5% CO2 (pH 7.4) and maintained at 37°C. All drugs and chemicals were purchased from Sigma.
Endothelial cell phenotype: immunoblot analysis.
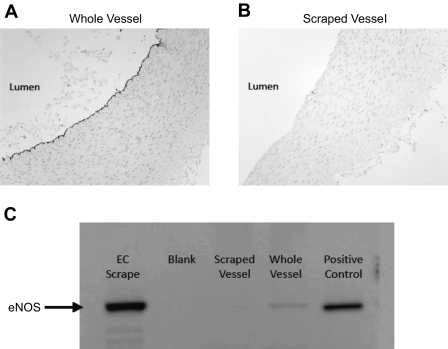
In a subset of male pigs (n = 16), expression levels of 18 proteins that are frequently altered in association with early atherosclerotic changes were measured from brachial and femoral artery endothelial cell scrapes. We selected markers of endothelium-derived dilators, antioxidant pathways, prooxidant pathways, and inflammation. Immediately following death, arteries were opened longitudinally and pinned. Laemmli buffer was applied on the endothelial surface, and, using a blade, the endothelial cell layer was gently scraped as described previously (4, 25). This method of scraping the luminal surface yields an endothelial enriched sample, as demonstrated in Fig. 1 and previously by others (6, 16, 27).
Fig. 1.
A: photomicrograph showing immunoreactive staining for endothelial nitric oxide synthase (eNOS) along the endothelial lining of an intact femoral artery. B: photomicrograph demonstrating loss of immunoreactivity to eNOS after scraping the luminal surface of the artery to remove endothelium. C: Western blot demonstrating enrichment of endothelial protein content with mechanical scraping of the luminal surface (femoral artery). Note the increase in eNOS immunoreactivity between scraped vessel, whole vessel, and endothelial scrape. EC, endothelial cell.
Total protein contents of arterial scrapes were measured using the NanoOrange protein assay. Protein samples from endothelial scrapes (10 μg/lane) were loaded on a 12-lane 5–12% NuPAGE Bis-Tris gradient gels, electrophoresed at 200 V for 50 min, and transferred at 34 V for 60 min to a polyvinylidene difluoride membrane (Hybond-ECL; Amersham). Brachial and femoral artery samples from the exercise and sedentary groups were loaded on the same gel in an alternating pattern. The membranes were blocked in 5% nonfat milk and TBST (20 mM Tris·HCl, 137 mM NaCl, and 0.1% Tween 20) at room temperature for 1 h. Overnight, the membrane was incubated with the primary antibody against endothelial nitric oxide synthase (eNOS; 1:1,000; BD Transduction), p47phox (1:500; BD Transduction), SOD-1 (1:5,000; Stressgen), SOD-2 (1:2,000; Stressgen), SOD-3 (1:4,000; Stressgen), catalase (1:5,000; Sigma), phosphor-eNOS (1:250; BD Transduction), Akt (1:500; Cell Signaling), phosphor-Akt (1:250; Cell Signaling), arginase 1 (1:1,000; BD Transduction), cyclooxygenase-1 (COX-1; 1:1,000; Santa Cruz Biotechnology), angiotensin AT1 (1:150; Santa Cruz Biotechnology), AT2 (1:1,000; Santa Cruz Biotechnology), VCAM-1 (1:250; Santa Cruz Biotechnology), p67phox (1:500; BD Transduction), Rac1 (1:1,000; Cytoskeleton), heat shock protein HSP90 (1:1,000; BD Transduction), caveolin-1 (1:250; BD Transduction), and MDA (1:1,000; Abcam). This was followed by 1-h incubation with a horseradish peroxidase-conjugated secondary antibody (1:2,500). Positive controls were included in all gels. Analysis of protein was performed with chemiluminescence and quantified by densitometry through the use of Kodak 4000R Imager and Molecular Imagery Software.
Statistical analysis.
All values are means ± SE. Dose-response curves were analyzed by two-way ANOVA with repeated measures on one factor (dose). Between-group differences in plasma cholesterol, body and heart weights, treadmill performance, skeletal muscle citrate synthase activity, brachial and femoral artery ring characteristics, and protein expression were determined by using an independent t-test. For all statistical tests, significance was set at 0.05. Statistical analyses were performed with SPSS 17.0. (SPSS, Chicago, IL).
RESULTS
Following the 16–20 wk intervention, total plasma cholesterol (EX = 64.5, SED = 67.3 mg/dl), low-density lipoprotein cholesterol (EX = 25.9, SED = 29.1 mg/dl), high-density lipoprotein cholesterol (EX = 34.0, SED = 32.4 mg/dl), and triglycerides (EX = 40.1, SED = 44.6 mg/dl) were not different between exercise and sedentary groups (P > 0.05). Although body weights were not different between groups (EX = 36.6, SED = 37.2 kg; P > 0.05) at termination of the study, heart weight (EX = 201.2, SED = 170.8 g) and heart-to-body weight ratio (EX = 5.5, SED = 4.6) were greater (P < 0.0001) in the exercise group compared with the sedentary group. Furthermore, following the intervention, exercise pigs increased endurance time on the treadmill by 43% (P < 0.0001) and revealed lower heart rates during rest and submaximal exercise (P < 0.0001). In addition, exercise training increased citrate synthase activity of the deltoid muscle, and the medial, lateral, long, and accessory heads of the triceps brachii muscle by approximately 15–40% (P < 0.001). These data confirm that pigs in the exercise group exhibited the classic training-induced adaptations.
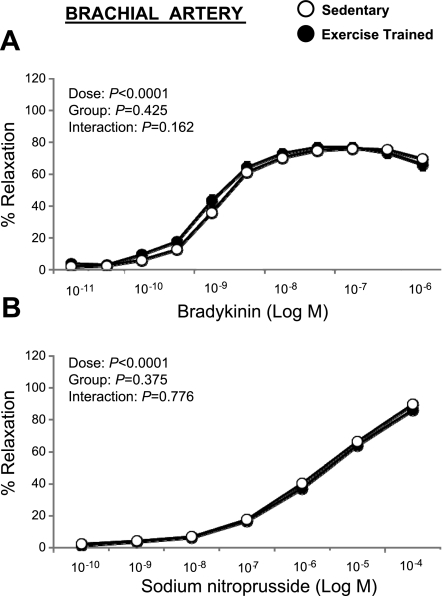
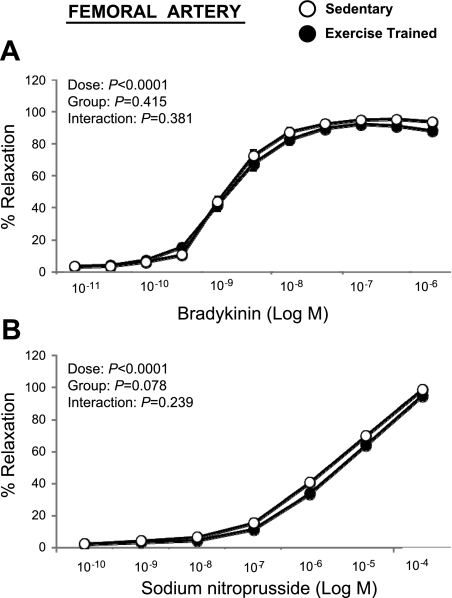
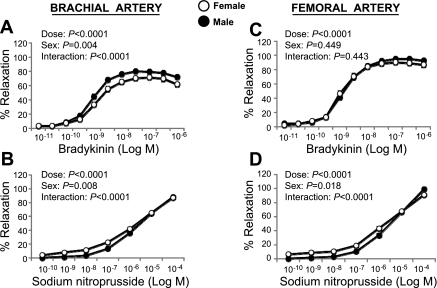
Bradykinin elicited a concentration-dependent relaxation of the brachial (Fig. 2A) and femoral (Fig. 3A) artery rings that was not different between the exercise and sedentary groups (P > 0.05). Similarly, SNP-induced relaxation of brachial (Fig. 2B) and femoral (Fig. 3B) artery rings did not differ between groups. Because the lack of differences between exercise-trained and sedentary groups occurred in both male and female pigs (P > 0.05), data were pooled across sex. Together, these data indicate that exercise training does not increase endothelium-dependent and -independent relaxation in healthy pigs. Although there was an absence of a group-by-sex interaction, we detected a main effect of sex on bradykinin-induced relaxation of the brachial (P < 0.05), but not femoral (P > 0.05), artery when pooling across groups (Fig. 4, A and C). Specifically, brachial arteries from male pigs exhibited greater endothelium-dependent relaxation compared with female arteries. Conversely, SNP-induced relaxation was greater (P < 0.05) in brachial and femoral arteries from female pigs compared with arteries from males (Fig. 4, B and D).
Fig. 2.
Bradykinin (A) and sodium nitroprusside (B) induced relaxation of brachial artery rings in exercise-trained (n = 59) vs. sedentary (n = 54) pigs (means ± SE). SE are not apparent because they are smaller than the symbols.
Fig. 3.
Bradykinin (A) and sodium nitroprusside (B) induced relaxation of femoral artery rings in exercise-trained (n = 47) vs. sedentary (n = 42) pigs (means ± SE). SE are not apparent because they are smaller than the symbols.
Fig. 4.
Bradykinin and sodium nitroprusside induced relaxation of brachial (A and B) and femoral artery (C and D) in male vs. female pigs (means ± SE). SE are not apparent because they are smaller than the symbols.
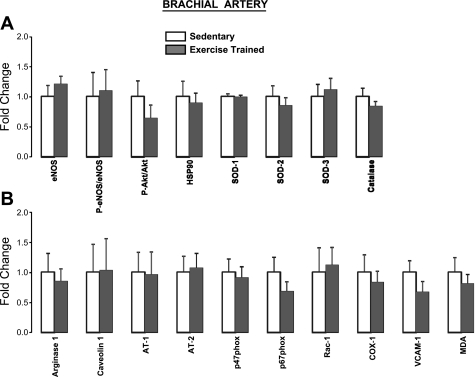
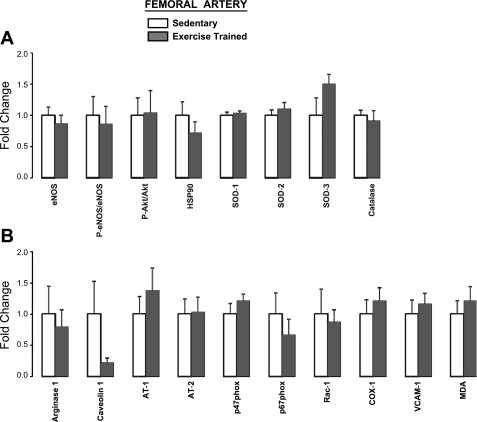
Furthermore, in the subset of pigs, expression of proteins of interest in brachial (Fig. 5) and femoral (Fig. 6) artery endothelial scrapes was not different (P > 0.05) between exercise (n = 8) and sedentary (n = 8) groups. Although it may appear as if trends for a group difference existed for certain proteins, it should be noted that the lowest P value was 0.138 (SOD-3 in femoral artery; Fig. 6A). Therefore, these data suggest that exercise training in healthy pigs does not markedly alter endothelial expression of the selected 18 genes. Importantly, this subset of pigs were representative of the entire study sample in that no differences in vasomotor function were found between the exercise and sedentary groups (P > 0.05). Moreover, the classic training-induced adaptations, as reported above, were also confirmed in this subgroup of pigs (P < 0.05).
Fig. 5.
Brachial artery endothelial expression of proteins that are considered antiatherogenic (A) and proatherogenic (B) in exercise-trained (n = 8) vs. sedentary (n = 8) pigs (means ± SE). All P > 0.05. P-, phosphorylated; HSP90, heat shock protein 90; AT, angiotensin; COX-1, cyclooxygenase-1; MDA, malondialdehyde.
Fig. 6.
Femoral artery endothelial expression of proteins that are considered antiatherogenic (A) and proatherogenic (B) in exercise-trained (n = 8) vs. sedentary (n = 8) pigs (means ± SE). All P > 0.05.
DISCUSSION
Using a large data set, the present study confirms that, in healthy adult male and female swine, long-term exercise training does not increase endothelium-dependent dilation of peripheral conduit arteries. Contrary to our hypothesis, we demonstrated that exercise-trained pigs do not reveal a more antiatherogenic endothelial cell phenotype compared with sedentary but otherwise healthy pigs. Furthermore, we also report that male pigs exhibit enhanced endothelium-dependent relaxation in the brachial but not femoral artery compared with female pigs.
A plethora of research indicates that habitual exercise improves endothelial function in peripheral conduit arteries of patients with heart failure, coronary artery disease, hypertension, hypercholesterolemia, obesity, diabetes, and aging (11, 21, 23, 29). These beneficial effects of exercise training on the peripheral circulation have also been demonstrated in animal models of cardiovascular risk or disease (14). For example, studies in hypercholesterolemic pigs reveal that exercise training markedly preserves or restores endothelium-dependent dilation of skeletal muscle arteries (37, 38, 40). At the opposite end of the spectrum, studies in healthy animals and humans indicate that exercise training does not consistently cause increases in endothelial function (11, 14, 21, 23, 29). The discrepancies in these findings may be attributed to the time point at which measurements of endothelial function are performed. In fact, although it may appear counterintuitive, the animal literature has long supported the proposal that short- but not long-term exercise training improves conduit artery endothelial function (14, 17). The actual profile of the time course of vascular adaptations during training has also been recently described in healthy humans (32, 33). In a series of longitudinal studies involving multiple repeated measurements, Tinken and colleagues (32, 33) established that endothelial function at the brachial and popliteal arteries improves rapidly (2 wk) from the start of exercise training and returns to baseline levels (at 8 wk) as dilatory capacity increases. As previously proposed for coronary arteries (17), these data from Tinken and coworkers (32, 33) support the notion that remodeling of conduit arteries may partly displace the need for acutely responsive vasodilatory mechanisms (9, 11, 14, 17, 19, 29). It is currently believed that during long-term exercise training, as the conduit arteries remodel and enlarge, shear stress during exercise bouts may become normalized, thus resulting in the return of endothelial function to basal levels (9, 11, 13, 14, 17, 19, 29). However, further research is necessary to establish to what extent arterial remodeling induced by training can alter wall shear stress at rest and during exercise. Taken together, it is proposed that, in sedentary but otherwise healthy animals and humans, the endothelial adaptive responses in conduit arteries occur very early in the training process, and as the exercise program continues these functional changes tend to disappear. Indeed, the present retrospective analysis of data from our swine database supports the latter portion of this contention; that is, long-term exercise training does not alter endothelium-dependent dilation in healthy peripheral conduit arteries. A previous study from our laboratory (22) using the same porcine model showed that 1 wk of exercise training does increase brachial artery sensitivity to bradykinin. Interestingly, this effect was not detected in the femoral artery. Given that blood flows to muscles served by the brachial artery are more uniformly elevated than those supplied by the femoral artery, it is possible that this differential vascular adaptation to short-term training is due to a greater brachial than femoral artery exposure to shear stress during the exercise bouts (22).
These findings in healthy pigs and those in humans do not appear to parallel observations made in healthy rats in which endothelium-dependent dilation of the aorta increases as the duration of the exercise training program progresses (7, 8). The effects of body size on cardiovascular hemodynamics should be considered when interpreting these contrasting data. Differences in vessel sizes between species, relative to differences in arterial blood flows, largely impact vascular shear stress such that resting and exercise-induced shear in rat arteries is ∼4-fold greater than shears observed in large mammals and humans (5). These inherent differences between species may contribute to the divergence in vascular adaptations to training.
The measurement of endothelium-dependent dilation is established as a surrogate marker for overall endothelial health and, in the human literature, has been referred to as the barometer for cardiovascular risk (35). Given that regulation of vascular tone is only one of the many functions of the endothelium (2), it is essential to recognize that endothelial alterations may not always be manifested with changes in endothelium-dependent dilation. With this in mind, we believe that it may be presumptuous to assume that the absence of a training-induced adaptation in vasomotor function implies a lack of change toward an atheroprotective endothelial phenotype. To address this issue, in a subset of exercise-trained and sedentary healthy pigs, expression levels of 18 proteins that are frequently associated with early atherosclerotic disease were measured from brachial and femoral artery endothelial enriched samples. Contrary to our premise, there were no differences in the magnitude of expression for any of the measured proteins. Furthermore, in the same subset of pigs, none of the histological markers that were assessed in the vascular wall (immunohistochemical staining for macrophage scavenger receptor A, nitrotyrosine, and MDA as well as intima-media thickness) revealed differences between exercise-trained and sedentary animals (data not shown). Together, these data suggest that, in healthy pigs, endurance training does not modulate vascular gene expression.
The current finding that exercise training has no effect on endothelial function/phenotype in healthy animals, contrasted with previous observations from diseased animals and humans in which exercise exerts a beneficial impact (11, 14, 21, 23, 29), supports the concept that there may be an interaction between endothelial health and exercise (14). That is, it is plausible that the endothelium is not amendable with long-term exercise unless impairment or disease is present. In other words, when endothelial function/phenotype is near optimal levels, as in healthy arteries, improvements may not occur as a result of a “ceiling effect.”
When interpreting the present results, we acknowledge that the beneficial effects of exercise might be apparent in the expression of genes that have yet to be identified as crucial and therefore were not included in our analysis. In this regard, we are currently directing efforts toward globally examining the impact of training on endothelial cell phenotype with the use of genome-wide microarray analysis of gene expression. It is also possible that the magnitude of variability in protein expression among pigs, as evidenced by the large error bars (Figs. 5 and 6), impeded the potential for detecting the effects of exercise. Accordingly, we believe that further research should evaluate the effectiveness of exercise on maintaining a healthy endothelial phenotype in a longitudinal design. Such an approach would require the involvement of cutting-edge techniques capable of in vivo endothelial cell sampling (28). Last, we recognize that the lack of adaptations in large peripheral arteries does not imply an absence of vascular changes throughout the entire arterial tree. Endothelial phenotypic heterogeneity between beds (18) (e.g., conduit vs. resistant vessels) may dictate the adaptability of vascular cells to a given stimulus (26). In this regard, data from Green et al. (10) and Tinken et al. (33) suggest that, in healthy humans, the time course of endothelial adaptations to repeated episodes of shear stress is variable throughout the vasculature. Specifically, it appears that in conduit arteries (e.g., brachial), endothelium-dependent dilation is initially enhanced followed by normalization to baseline levels (33), whereas in the microvasculature (e.g., forearm skin), improvements in vasomotor function are sustained without indication of succeeding normalization (10, 36).
The present data demonstrate that brachial artery bradykinin-induced relaxation is slightly but significantly greater in male vs. female pigs, a difference that was not detected in the femoral artery. Previous studies conducted in humans have indicated that sex differences in endothelial function are age-dependent, with older women exhibiting a more pronounced attenuation in brachial artery function (15). Our data indicate that, in pigs, sex-related differences may appear earlier in life. More interestingly, for the first time, our study suggests that the sex effect on endothelium-dependent dilation may be vessel-specific, an observation that will warrant further investigation. Furthermore, similar to studies in humans (3), we found that endothelium-independent relaxation is augmented in females compared with males, thus suggesting that females exhibit greater smooth muscle sensitivity than males. The present sex differences should be, however, interpreted with caution given that the death of female pigs was not standardized according to the menstrual cycle. Although no studies in swine have systemically examined the impact of the menstrual cycle on vasomotor function, data in humans strongly suggest that vascular function is altered throughout the menstrual cycle with the peak function occurring during the late follicular phase before ovulation (1).
In summary, the present investigation indicates that pigs exhibiting the classic training-induced adaptations do not demonstrate enhanced endothelium-dependent dilation nor reveal a more atheroprotected endothelial cell phenotype than their sedentary, but otherwise healthy, counterparts. This is the first study comprehensively characterizing brachial and femoral artery vasomotor function and endothelial phenotype in exercise-trained and sedentary pigs. Furthermore, we report for the first time that the sex effect on endothelium-dependent dilation is vessel-specific, with brachial but not femoral arteries from male pigs exhibiting enhanced function compared with females pigs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-52490 and HL-36088 (to M. H. Laughlin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of Pam Thorne, Jennifer Casati, Ann Melloh, and David Harah.
REFERENCES
- 1.Adkisson EJ, Casey DP, Beck DT, Gurovich AN, Martin JS, Braith RW. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med 235: 111–118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Endothelial cell heterogeneity and atherosclerosis. Curr Atheroscler Rep 8: 69–75, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol 298: H1889–H1901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng C, Helderman F, Tempel D, Segers D, Hierck B, Poelmann R, van Tol A, Duncker DJ, Robbers-Visser D, Ursem NT, van Haperen R, Wentzel JJ, Gijsen F, van der Steen AF, de Crom R, Krams R. Large variations in absolute wall shear stress levels within one species and between species. Atherosclerosis 195: 225–235, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein reponse in arterial endothelium in regions of suceptibility to atherosclerosis. Circ Res 105: 453–461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delp MD, Laughlin MH. The time course of enhanced endothelium-mediated dilation in aorta of trained rats. Med Sci Sports Exerc 29: 1454–1461, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Delp MD, McAllister RM, Laughlin MH. Exercise training alters endothelium-dependent vaosreactivity of rat abdominal aorta. J Appl Physiol 75: 1354–1363, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev 37: 196–202, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green DJ, O'Driscoll G, Joyner MJ, Cable NT. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J Appl Physiol 105: 766–768, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green DJ, Swart A, Exterkate A, Naylor LH, Black MA, Cable NT, Thijssen DH. Impact of age, sex and exercise on brachial and popliteal artery remodelling in humans. Atherosclerosis 210: 525–530, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Jasperse JL, Laughlin MH. Endothelial function and exercise training: evidence from studies using animal models. Med Sci Sports Exerc 38: 445–454, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med 250: 29–36, 2001 [DOI] [PubMed] [Google Scholar]
- 16.LaMack JA, Himburg HA, Friedman MH. Distinct profiles of endothelial gene expression in hyperpermeable regions of the porcine aortic arch and thoracic aorta. Atherosclerosis 195: e35–e41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc 27: 1135–1144, 1995 [PubMed] [Google Scholar]
- 18.Laughlin MH. Joseph B. Wolfe Memorial Lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc 36: 352–362, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol 104: 588–600, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Maiorana A, O'Driscoll G, Taylor R, Green D. Exercise and the nitric oxide vasodilator system. Sports Med 33: 1013–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 22.McAllister RM, Laughlin MH. Short-term exercise training alters responses of porcine femoral and brachial arteries. J Appl Physiol 82: 1438–1444, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol Scand 180: 113–123, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Muller JM, Meyers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308–2314, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Newcomer SC, Taylor JC, Bowles DK, Laughlin MH. Endothelium-dependent and -independent relaxation in the forelimb and hindlimb vasculatures of swine. Comp Biochem Physiol 148: 292–300, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Newcomer SC, Taylor JC, McAllister RM, Laughlin MH. Effects of chronic nitric oxide synthase inhibition on endothelium-dependent and -independent relaxation in arteries that perfuse skeletal muscle of swine. Endothelium 15: 17–31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, Pritchard WF, Powell S, Chang GY, Stoeckert CJ, Jr, Davies PF. Coexisting proinflammatory and antioxidative endothleial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA 101: 2482–2487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silver AE, Christou DD, Donato AJ, Beske SD, Moreau KL, Magerko KA, Seals DR. Protein expression in vascular endothelial cells obtained from human peripheral arteries and veins. J Vasc Res 47: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108: 845–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas TR, Pellechia J, Rector RS, Sun GY, Sturek MS, Laughlin MH. Exercise training does not reduce hyperlipidemia in pigs fed a high fat diet. Metabolism 51: 1587–1595, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price EM, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Tinken TM, Thijssen DH, Black MA, Cable NT, Green DJ. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 586: 5003–5012, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinken TM, Thijssen DH, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension 55: 312–318, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol 86: 335–345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vita JA, Keaney JF. Endothelial function: a barometer for cardiovascular risk? Circulation 106: 640–642, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Wang J. Effects of exercise training and detraining on cutaneous microvascular function in man: the regulatory role of endothelium-dependent dilation in skin vasculature. Eur J Appl Physiol 93: 429–434, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Woodman CR, Ingram D, Bonagura J, Laughlin MH. Exercise training improves femoral artery blood flow responses to endothelium-dependent dilators in hypercholesterolemic pigs. Am J Physiol Heart Circ Physiol 290: H2362–H2368, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodman CR, Thompson MA, Turk JR, Laughlin MH. Endurance exercise training improves endothelium-dependent relaxation in brachial arteries from hypercholesterolemic male pigs. J Appl Physiol 99: 1412–1421, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Woodman CR, Turk JR, Rush JW, Laughlin MH. Exercise attenuates the effects of hypercholesterolemia on endothelium-dependent relaxation in coronary arteries from adult female pigs. J Appl Physiol 96: 1105–1113, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Woodman CR, Turk JR, Williams DP, Laughlin MH. Exercise training preserves endothelium-dependent relaxation in brachial arteries from hyperlipidemic pigs. J Appl Physiol 94: 2017–2026, 2003 [DOI] [PubMed] [Google Scholar]