Abstract
Impaired myocardial contractile function is a hallmark of heart failure (HF), which may present under resting conditions and/or during physiological stress. Previous studies have reported that high fat feeding in mild to moderate HF/left ventricular (LV) dysfunction is associated with improved contractile function at baseline. The goal of this study was to determine whether myocardial function is compromised in response to physiological stress and to evaluate the global gene expression profile of rats fed high dietary fat after infarction. Male Wistar rats underwent ligation or sham surgery and were fed normal chow (NC; 10% kcal fat; Sham + NC and HF + NC groups) or high-fat chow (SAT; 60% kcal saturated fat; Sham + SAT and HF + SAT groups) for 8 wk. Myocardial contractile function was assessed using a Millar pressure-volume conductance catheter at baseline and during inferior vena caval occlusions and dobutamine stress. Steady-state indexes of systolic function, LV +dP/dtmax, stroke work, and maximal power were increased in the HF + SAT group versus the HF + NC group and reduced in the HF + NC group versus the Sham + NC group. Preload recruitable measures of contractility were decreased in HF + NC group but not in the HF + SAT group. β-Adrenergic responsiveness [change in LV +dP/dtmax and change in cardiac output with dobutamine (0–10 μg·kg−1·min−1)] was reduced in HF, but high fat feeding did not further impact the contractile reserve in HF. The contractile reserve was reduced by the high-fat diet in the Sham + SAT group. Microarray gene expression analysis revealed that the majority of significantly altered pathways identified contained multiple gene targets correspond to cell signaling pathways and energy metabolism. These findings suggest that high saturated fat improves myocardial function at rest and during physiological stress in infarcted hearts but may negatively impact the contractile reserve under nonpathological conditions. Furthermore, high fat feeding-induced alterations in gene expression related to energy metabolism and specific signaling pathways revealed promising targets through which high saturated fat potentially mediates cardioprotection in mild to moderate HF/LV dysfunction.
Keywords: contractile function, infarction, high-fat diet, gene array analysis
chronic diseases and conditions including obesity, insulin resistance, and diabetes are linked to elevations in plasma free fatty acids (FFA) leading to enhanced lipid accumulation in nonadipose tissue (24). Enhanced myocardial lipid accumulation has been associated with decreases in myocardial contractile function, resulting in the progression of heart failure (HF). Nutritional guidelines from the American Heart Association (23) have suggested that increasing dietary fat intake negatively impacts myocardial structure/function through elevations in FFA. However, an epidemiology study (17) has reported no cardiovascular benefit to reducing dietary fat; in fact, low-carbohydrate/high-fat diets are associated with improvements in metabolic status and heart disease risk (19). In addition, overweight or obese patients demonstrate improved survival associated with cardiovascular disorders compared with normal weight or cachectic patients (20). Given that optimal pharmacotherapies targeted to inotropic support and/or neurohormonal activation have proven unsuccessful at decreasing morbidity/mortality in HF, the impact of high dietary fat on contractile function and its role as an adjunctive therapeutic tool require further investigation.
There is supporting evidence for a cardioprotective benefit of high dietary fat subsequent to cardiac injury. Our laboratory reported that the progression of infarction-induced HF is associated with improved contractile function after acute (39, 40) and chronic high fat feeding (38). Other studies (8, 12, 28, 44) have shown cardioprotection in a chronic pressure-overload model where a high-fat diet improved ventricular remodeling and the progression of contractile dysfunction. However, myocardial function was evaluated strictly under resting conditions. In chronic HF, exercise intolerance is a hallmark of disease severity, and exercise is a prognostic tool to unmask dysfunction not apparent under resting conditions (10, 18). Thus, to establish the impact of dietary interventions in HF, it is essential that contractile function be assessed under conditions of stress. A comprehensive evaluation of ventricular performance should include an evaluation of baseline performance, load-insensitive measures of systolic pump function, and an assessment of the contractile reserve using inotropic stimulation (5, 32).
In addition to changes in contractile function during the progression of failure, the heart also exhibits alterations at the level of transcription. Transcriptional changes accompanying myocardial infarction predominantly involve the expression of genes related to cytoskeletal architecture, excitation-contraction coupling, Ca2+ handling, contractility, and energy metabolism that likely impact ventricular remodeling processes and progression of dysfunction (46). As cardiac energy metabolism is critical to sustaining function, our previous work has focused on energy metabolism as a primary target altered with lipids in HF (39). Work done by others (2, 37, 41) has shown numerous metabolic genes regulated by the peroxisome proliferator-activated receptor (PPAR)-α-mediated pathway are downregulated in HF. Conversely, elevations in FFA achieved with high fat feeding or as seen in obesity and diabetes result in the activation of PPAR-α within the heart (8). Taken together, lipids have the potential to improve fatty acid oxidation in the face of cardiac injury by upregulating the PPAR transcriptional program. However, our previous work (39) did not reveal a robust alteration in the expression of PPAR-α-targeted genes despite increases in the activity of mitochondrial enzymes related to energy metabolism. Whether the extent of cardiac injury is influenced by the presence of metabolic substrates such as FFA remains a matter of debate in HF, and the cellular pathways that mediate the response are not fully understood. The impact of high fat on gene expression patterns during HF is unclear, and a genome-wide assessment of such would offer greater insight into the molecular mechanisms underlying the cardioprotective effects of high fat subsequent to infarction.
The present study was designed to evaluate the contractile improvement seen with high fat feeding subsequent to myocardial infarction under normal conditions and during physiological stress. In addition, a global gene expression profile was generated to identify potential molecular mechanisms underlying the physiology of improved contractility in HF with high fat. Taken together, these results contribute to our understanding of the improved cardiac function and cellular processes regulating the observed cardioprotective effects of high fat in the context of HF.
MATERIALS AND METHODS
Experimental Design: Animals, Dietary Manipulation, and Myocardial Infarction
This study was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996) and was approved by the Institutional Animal Care and Use Committee of Case Western Reserve University. Male Wistar rats (300–350 g) were maintained on a 12:12-h light/sleep-dark/awake cycle (lights on at 6 PM), and all procedures were done 3–6 h into the dark/awake cycle (13).
Rats were randomly assigned to sham operation or coronary ligation to induce HF as previously described (26). Rats were anesthetized with isoflurane, intubated, and ventilated during surgical procedures. After surgery, rats were fed (8 wk) either normal chow (NC; 10% kcal fat) or a high saturated fat chow (SAT) with 60% kcal from fat (25% palmitic acid, 33% stearic acid, and 33% oleic acid, Research Diets). Thus, rats were divided into the following four groups: Sham + NC, Sham + SAT, HF + NC, and HF + SAT.
Echocardiography
Left ventricular (LV) function was evaluated via echocardiography 7 wk after ligation using the Sequoia C512 system (Siemens Medical) as described elsewhere (26). Briefly, anesthetized rats (1.5–2.0% isoflurane) were situated supine on a warming pad with ECG limb electrodes. Two-dimensional (2-D), 2-D-guided M-mode, and Doppler echocardiographic imaging of aortic and transmitral flows were performed via parasternal and foreshortened apical windows. End-diastolic and end-systolic dimensions were measured using ultrasonograph software. Fractional shortening (FS) was calculated as previously described (26). Results from the echocardiographic analysis were used to establish whether animals in the HF + NC and HF + SAT groups met the minimal criteria for HF/LV dysfunction: i.e., ejection fraction (EF) < 70% and FS < 0.40.
Hemodynamic Measurements with the Pressure-Volume Conductance Catheter
Hemodynamic assessments were made during the terminal surgery 8 wk after ligation surgery. During the terminal surgery, a 2.0-Fr pressure-volume (P-V) catheter (SPR-838, Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into the LV (31, 32), and the position was optimized by aiming for maximal stroke volume (SV). A 14-gauge angiocath was inserted into the right jugular vein for dobutamine infusions and saline boluses for calibration. A small balloon catheter was inserted through the right femoral vein into the inferior vena cava (IVC) to the level of the diaphragm for IVC occlusions. After 15 min of stabilization, steady-state parameters, including heart rate, maximal LV systolic pressure, and LV end-diastolic pressure, were recorded (n = 9–10 animals/group) using MPVS-400 signal conditioning hardware with integrated AD Instruments PowerLab DAQ technology (AD Instruments, Mountain View, CA). Maximal slopes of systolic and diastolic pressure (+dP/dt and −dP/dt), time constants of LV pressure decay, EF, SV, end-diastolic volume (EDV), cardiac output (CO), and stroke work (SW) were calculated using PVAN 3.2 software (Millar Instruments) (31, 32). Subsequently, LV P-V relationships were assessed using IVC occlusions by inflating the balloon to 8 mm over 5-s generating descending loops (n = 6–8 animals/group). LV end-systolic and end-diastolic P-V relationships (ESPVR and EDPVR, respectively) were obtained. Time-varying maximum elastance (Emax) was assessed from P-V regression curves at varying preloads, and preload recruitable SW (PRSW) was assessed by plotting SW against EDV. Thus, load-independent indexes of contractility were determined as measures of systolic function.
In a separate set of animals, dobutamine was used to assess the myocardial contractile reserve (35). Progressively increasing doses of dobutamine (0, 5, and 10 μg·kg−1·min−1), to simulate increasing workloads, were infused for 4 min each before P-V loops (n = 5 animals/group) were recorded. Absolute volumes were obtained via calibration as previously described (31). At the end of each experiment, 3–5 μl of 10% saline were injected intravenously, and from the shift of the P-V loops, parallel conductance volume was calculated to correct for cardiac mass volume (32).
Plasma and Tissue Metabolic Products
Nonfasted plasma glucose and FFA concentrations were measured using an enzymatic spectrophotometric kit (33). Triglyceride content was measured in homogenates of LV tissue samples using an enzyme-based spectrophotometric method (Wako Chemicals).
Microarray Analysis
Nonscar LV tissue from a separate set of animals (n = 6 animals/group) was quick frozen in liquid nitrogen. Tissue samples were processed at Baylor College of Medicine to perform RNA isolation and array hybridization. RNA was extracted from LV tissue using standard procedures as described elsewhere (9). Isolated RNA was converted to cDNA using ArrayScript reverse transcriptase and T7-(dT)24 primers followed by second-strand synthesis to generate double-stranded cDNA (Ambion). Purified cDNA was converted to biotin-labeled cRNA and hybridized to the Rat Ref-12 BeadChip Array (Illumina), which contains 22,517 probes for the rat genome. BeadChips were stained with strepavidin-Cy3 followed by chip image scanning and acquisition using BeadStation (Illumina). The resulting image files were evaluated for quality standards of hybridization, uniform staining, background, and housekeeping gene expression using BeadStudio software (version 3, Illumina). Detection scores were assigned for each gene probe by calculating probe mean fluorescence across replicates and determining the probability that the mean signal exceeded background. Probe signals were defined as significantly above background when the 75th percentile of each probe detection score met or exceeded 0.9 (detection P value = 0.2). The expression data were then normalized within centiles of the expression value distribution. The resulting list of detectable gene probes was analyzed to determine expression changes as described below.
Statistical Analysis
Experimental data.
All statistical analyses for metabolic and functional assessments were performed with SigmaStat software. Differences among the Sham + NC, Sham + SAT, HF + NC, and HF + SAT groups were determined using two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. LV P-V relations in response to dobutamine stimulation were assessed using three-way ANOVA followed by Bonferroni post hoc tests. Data are expressed as group means ± SE. Significance was established at P < 0.05.
Microarray analysis.
Normalized signal values meeting detection criteria were evaluated for statistical differences in expression between groups using two-way ANOVA. Models were run with and without an interaction term to examine main effects and potential interactions between experimental variables (HF and SAT). Probes meeting the criteria of P < 0.05 were compiled and evaluated for redundancy to generate a probe list representative of whole model expression changes. Gene probes with P < 0.05 for each variable (HF and SAT) were enumerated for the preliminary analysis of the impact of variables independently. To address the impact of SAT with underlying HF, a subset analysis was performed via a comparison of the HF groups for probes in which the overall model was significant (P < 0.05). Ontological terms were assigned for gene probes significantly altered in expression based on documentation from Illumina in addition to available annotative information in public databases (http://www.ncbi.nlm.nih.gov). Genes exhibiting significant differences were further analyzed using pathway and ontological classifications in GenMapp software (http://www.genmapp.org), a freely available program based on curated biochemical pathways in the Kyoto Encyclopedia of Genes and Genomes repository (11). Gene lists were also analyzed with the MappFinder component of GenMapp to statistically score pathways that possessed multiple altered targets. Normalized data have been submitted to GEO archive and are available at http://www/ncbi.nlm.nih.gov/geo/.
RESULTS
Body and Heart Weights
Body weights were not different with HF/LV dysfunction or high fat feeding 8 wk after ligation. LV-to-body weight ratios were significantly increased in both HF groups but not in either SAT group. Mean scar tissue weight did not differ between HF groups (Table 1).
Table 1.
Gravimetric data, plasma and tissue substrates, and echocardiographic data in the Sham + NC, Sham + SAT, HF + NC, and HF + SAT groups 8 wk after coronary artery ligation surgery
| Sham + NC Group | Sham + SAT Group | HF + NC Group | HF + SAT Group | |
|---|---|---|---|---|
| Gravimetric data | ||||
| Body mass, g | 512 ± 10 | 537 ± 9 | 532 ± 11 | 517 ± 12 |
| LV mass/body mass, mg/g | 1.91 ± 0.06 | 1.84 ± 0.05 | 2.03 ± 0.07† | 2.03 ± 0.06† |
| Scar, mg | 130 ± 9 | 144 ± 16 | ||
| Plasma and tissue substrates | ||||
| Glucose, mM | 5.44 ± 0.24 | 5.41 ± 0.25 | 5.68 ± 0.24 | 5.11 ± 0.20 |
| Fatty acids, μmol/ml | 0.45 ± 0.05 | 0.68 ± 0.08* | 0.44 ± 0.06 | 0.56 ± 0.4* |
| Tissue triglycerides, μmol/g wet wt | 1.59 ± 0.18 | 2.57 ± 0.37* | 1.65 ± 0.26 | 2.24 ± 0.33* |
| Echocardiographic data | ||||
| End-diastolic area, cm2 | 1.12 ± 0.03 | 1.10 ± 0.02 | 1.27 ± 0.5† | 1.24 ± 0.04† |
| End-systolic area, cm2 | 0.62 ± 0.04 | 0.56 ± 0.03 | 0.88 ± 0.05† | 0.86 ± 0.07† |
| Cardiac index, ml·min−1·mg−1 | 205 ± 17 | 189 ± 17 | 142 ± 22† | 149 ± 16† |
| Area of fractional shortening, cm2 | 0.45 ± 0.02 | 0.49 ± 0.02 | 0.31 ± 0.03† | 0.33 ± 0.03† |
Values are expressed as means ± SE; n = 9–10 animals/group. Animals were divided into the following four groups: sham operation (Sham) with a normal chow (NC) diet (Sham + NC), Sham with a high saturated fat (SAT) diet (Sham + SAT), heart failure (HF) with a NC diet (HF + NC), and HF with SAT diet (HF + SAT). LV, left ventricular.
P < 0.05, main effect for diet;
P < 0.05, main effect for treatment.
Metabolic Substrates
Plasma FFA and tissue triglycerides were increased with high fat in the Sham + SAT and HF + SAT groups. Plasma glucose was not altered by ligation surgery or the high-fat diet (Table 1).
Cardiac Function and Remodeling by Echocardiography
Contractile dysfunction and parameters of remodeling were assessed by echocardiography 7 wk after ligation to confirm dysfunction in the infarcted animals and normal function in the Sham groups. In this model, Sham rats typically exhibit an average EF of ∼89% and FS of ∼0.53. In contrast, HF groups average an EF of ∼59% and FS of ∼0.26. HF rats that did not meet this minimal criterion (see materials and methods) were considered nondysfunctional and were excluded from the study. Established markers of ventricular remodeling, end-diastolic areas, and end-systolic areas were increased and cardiac index and area of FS were decreased in HF versus Sham groups (Table 1). No evidence of systolic dysfunction or remodeling was found in the Sham + SAT group. Furthermore, LV dysfunction was not exacerbated in the HF + SAT group versus the HF + NC group.
Hemodynamics Under Baseline Steady-State Conditions
In vivo contractile function was assessed with a P-V catheter 8 wk after infarction. High fat feeding in the Sham group did not alter baseline parameters or any systolic or diastolic indexes (Table 2). LV end-systolic pressure was lower in the HF + NC group compared with the Sham + NC group but not relative to the HF + SAT group. LV end-systolic volume was increased in both HF groups, resulting in decreased SV and CO relative to dietary controls. It should be noted that EDV was not significantly different in HF groups (as seen with echocardiography) despite clear evidence of systolic dysfunction (increased end-systolic volume and decreased SV and CO). Infarction alone resulted in impaired LV systolic function in HF groups as defined by decreased EF, peak LV +dP/dt, SW, and maximal power. In contrast, diastolic dysfunction was evident with HF [peak LV −dP/dt was decreased and time constants of LV pressure decay (Weiss and Glantz) were increased] with no effect of diet (Table 2). However, high fat feeding improved peak LV +dP/dt, SW, and maximal power in the group HF + SAT group versus the HF + NC group.
Table 2.
Steady-state hemodynamic properties in the Sham + NC, Sham + SAT, HF + NC, and HF + SAT groups 8 wk after coronary artery ligation surgery
| Sham + NC Group | Sham + SAT Group | HF + NC Group | HF + SAT Group | |
|---|---|---|---|---|
| Heart rate, beats/min | 313 ± 12 | 305 ± 10 | 297 ± 11 | 305 ± 9 |
| LV end-systolic pressure, mmHg | 117 ± 4 | 105 ± 4 | 101 ± 5‡ | 109 ± 3 |
| LV end-diastolic pressure, mmHg | 7.3 ± 0.6 | 7.3 ± 0.7 | 11.9 ± 2.5 | 9.1 ± 0.8 |
| LV end-diastolic volume, μl | 215 ± 16 | 195 ± 11 | 181 ± 17 | 219 ± 9 |
| LV end-systolic volume, μl | 66 ± 8 | 53 ± 9 | 102 ± 17* | 121 ± 13* |
| Stroke volume, μl | 166 ± 11 | 167 ± 9 | 93 ± 9*‡ | 122 ± 9*‡ |
| Cardiac output, ml/min | 51.9 ± 3.9 | 51.0 ± 2.9 | 26.0 ± 2.2*‡ | 36.8 ± 2.6*‡ |
| Systolic Indexes | ||||
| Ejection fraction, % | 76 ± 2 | 80 ± 3 | 50 ± 4*‡ | 54 ± 4*‡ |
| LV +dP/dtmax, mmHg·s−1·103 | 9.19 ± 0.47 | 8.37 ± 0.42 | 5.53 ± 0.24*‡ | 7.30 ± 0.22*§ |
| Stroke work, mmHg·μl−1·103 | 15.8 ± 1.2 | 14.2 ± 1.1 | 6.0 ± 0.8*‡ | 9.5 ± 0.8*‡§ |
| Maximal power, mW | 91 ± 8 | 104 ± 12 | 37.6 ± 4.6*‡ | 73.0 ± 7.4*‡§ |
| Diastolic Indexes | ||||
| LV −dP/dtmax, mmHg·s−1·103 | 8.59 ± 0.57 | 7.22 ± 0.33 | 4.45 ± 0.30*‡ | 5.26 ± 0.24*‡ |
| Weiss time constant, ms | 12.5 ± 0.6 | 12.7 ± 0.8 | 17.4 ± 1.0*‡ | 15.2 ± 0.8* |
| Glantz time constant, ms | 13.1 ± 0.7 | 12.7 ± 0.4 | 18.5 ± 0.6*‡ | 18.8 ± 0.9* |
Values are expressed as means ± SE; n = 9–10 animals/group.
P < 0.05, main effect for treatment (Sham vs. HF); †P < 0.05, main effect for diet (NC vs. SAT);
P < 0.05, HF vs. Sham groups within diet;
P < 0.05, HF + NC group vs. HF + SAT group.
Hemodynamics During IVC Occlusions
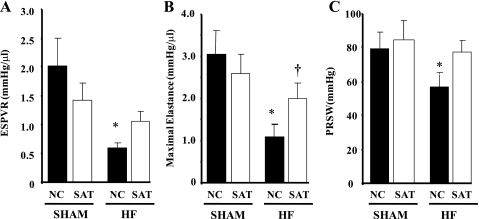
IVC occlusions allow for the determination of systolic and diastolic function with varying preloads and thus assesses load-independent contractility through evaluations of ESPVR, Emax, and PRSW. ESPVR and PRSW were decreased in the HF + NC group but not in the HF + SAT group. Interestingly, ESPVR was reduced in the Sham + SAT group versus the Sham + NC group (P = 0.070). Emax was reduced in the HF + NC group but was significantly increased in the HF + SAT group versus the HF + NC group (Fig. 1B). Thus, the high-fat diet improved systolic performance in the HF + SAT group relative to the HF + NC group (Fig. 1, A and C). EDPVR as an index of diastolic function was not different (data not shown).
Fig. 1.
Inferior vena caval occlusion parameters: A: slope of the end-systolic pressure-volume relationship (ESPVR). B: maximal elastance, which was obtained from a series of pressure-volume regression curves at varying preloads. C: preload recruitable stroke work (PRSW), which was generated with varying left ventricular (LV) end-diastolic volumes. Animals were divided into the following four groups: sham operation (Sham) with a normal chow (NC) diet (Sham + NC), Sham with a high saturated fat (SAT) diet (Sham + SAT), heart failure (HF) with a NC diet (HF + NC), and HF with SAT diet (HF + SAT). n = 6–8 animals/group. *P < 0.05 Sham vs. HF within diet; † P < 0.05, diet within treatment.
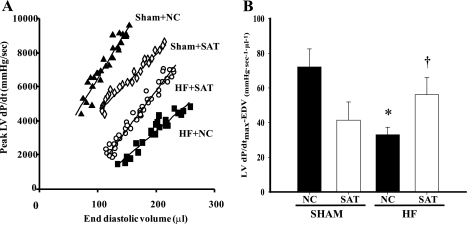
The dP/dtmax-EDV relation also serves as a load-independent measure of contractility (Fig. 2A). The slope of the dP/dtmax-EDV relationship was reduced in the HF + NC group versus the Sham + NC group but was significantly increased in the HF + SAT group versus the HF + NC group (Fig. 2B). The dP/dtmax-EDV relationship was reduced in the Sham + SAT group versus the Sham + NC group (similar to ESPVR), suggesting a negative impact of high fat in Sham animals. Overall, the results demonstrate that load-independent measures of contractility are improved by high fat feeding in HF.
Fig. 2.
A: preload adjusted peak +dP/dtmax (in mmHg/s)-end-diastolic volume (EDV; in μl) in representative Sham + NC, Sham + SAT, HF + NC, and HF + SAT animals. B: slope of the peak LV +dP/dtmax-EDV relationship. n = 6–8 animals/group. *P < 0.05, Sham vs. HF within diet; †P < 0.05, diet within treatment.
Hemodynamics During Dobutamine Stimulation
Baseline heart rates were not different between any of the groups but increased significantly from 0 to 10 μg·kg−1·min−1 dobutamine in all groups (Table 3). SV and CO were both decreased in the HF + NC group but not in the HF + SAT group versus Sham groups. However, SV and CO were both greater in the HF + SAT group versus the HF + NC group at each dobutamine dose. EF was significantly lower at baseline in the HF + NC group only, but both HF groups were lower compared with Sham group at 5 and 10 μg·kg−1·min−1 dobutamine (Table 3). EF increased with dobutamine in the Sham + NC group only, suggesting decreased β-adrenergic responsiveness in both HF groups and in the Sham + SAT group. There were no increases in SW with dobutamine in any group; however, SW was significantly depressed in the HF + NC group only at all dobutamine doses. Both Sham groups showed increases in LV +dP/dtmax with dobutamine, but there was a blunted dobutamine response in both HF groups. LV +dP/dtmax at baseline was lower in the HF + NC group versus both Sham groups and the HF + SAT group (Table 3).
Table 3.
Hemodynamics properties at baseline and during dobutamine infusion in the Sham + NC, Sham + SAT, HF + NC, and HF + SAT groups 8 wk after coronary artery ligation surgery
| Sham + NC Group | Sham + SAT Group | HF + NC Group | HF + SAT Group | |
|---|---|---|---|---|
| Heart rate, beats/min | ||||
| Baseline | 313 ± 24f,h | 310 ± 5h | 306 ± 11h | 289 ± 7h |
| Dobutamine | ||||
| 5 μg·kg−1·min−1 | 369 ± 36 | 340 ± 23 | 333 ± 17 | 293 ± 15 |
| 10 μg·kg−1·min−1 | 411 ± 9 | 377 ± 10b,c | 363 ± 12a,e | 343 ± 11a,b,e |
| Stroke volume, μl | ||||
| Baseline | 177 ± 20 | 175 ± 30 | 95 ± 18a,e | 163 ± 21a |
| Dobutamine | ||||
| 5 μg·kg−1·min−1 | 189 ± 11 | 187 ± 25 | 98 ± 25a,e | 154 ± 14a |
| 10 μg·kg−1·min−1 | 212 ± 27 | 199 ± 20 | 99 ± 19a,e | 158 ± 17a |
| Cardiac output, ml/min | ||||
| Baseline | 55.7 ± 8.6 | 54.1 ± 8.8 | 27.5 ± 2.6a,e | 45.8 ± 6.1a |
| Dobutamine | ||||
| 5 μg·kg−1·min−1 | 69.4 ± 6.9 | 63.9 ± 10.5 | 31.6 ± 6.7a,e | 45.3 ± 4.8a |
| 10 μg·kg−1·min−1 | 87.3 ± 11.6 | 75.0 ± 7.3 | 37.8 ± 4.7a,e | 56.1 ± 7.5a |
| Ejection fraction, % | ||||
| Baseline | 74 ± 2h | 77 ± 8 | 49 ± 5a,e | 60 ± 4a |
| Dobutamine | ||||
| 5 μg·kg−1·min−1 | 87 ± 6 | 84 ± 7 | 53 ± 9a,e | 64 ± 7a |
| 10 μg·kg−1·min−1 | 91 ± 2 | 82 ± 9 | 60 ± 9a,e | 73 ± 8a |
| Stroke work, mmHg·μl−1·103 | ||||
| Baseline | 16.6 ± 2.5 | 15.8 ± 2.9 | 6.6 ± 0.8a,e | 13.5 ± 2.4a |
| Dobutamine | ||||
| 5 μg·kg−1·min−1 | 17.4 ± 1.6 | 19.1 ± 3.1 | 7.9 ± 2.5a,e | 12.7 ± 1.6a |
| 10 μg·kg−1·min−1 | 16.6 ± 2.5 | 17.7 ± 3.5 | 7.9 ± 0.9a,e | 12.8 ± 1.7a |
| LV +dP/dt, mmHg·s−1·103 | ||||
| Baseline | 8.61 ± 0.54f,h | 8.55 ± 0.20h | 5.48 ± 0.23a,e | 7.27 ± 0.46a,d |
| Dobutamine | ||||
| 5 μg·kg−1·min−1 | 13.6 ± 2.44g | 11.4 ± 1.19 | 7.21 ± 0.62a,e | 9.11 ± 1.20a |
| 10 μg·kg−1·min−1 | 17.1 ± 0.91 | 13.6 ± 1.69 | 8.43 ± 1.08a,e | 10.3 ± 1.90a |
Values are expressed as means ± SE; n = 5 animals/group.
aP < 0.05, main effect for treatment (Sham vs. HF);
bP < 0.05, main effect for diet (NC vs. SAT);
cP < 0.05, diet effect within Sham groups;
dP < 0.05, diet effect within HF groups;
eP < 0.05, HF vs. Sham groups within diet;
fP < 0.05, 0 vs. 5 μg·kg−1·min−1 dobutamine;
gP < 0.05, 5 vs. 10 μg·kg−1·min−1 dobutamine;
hP < 0.05, 0 vs. 10 μg·kg−1·min−1 dobutamine.
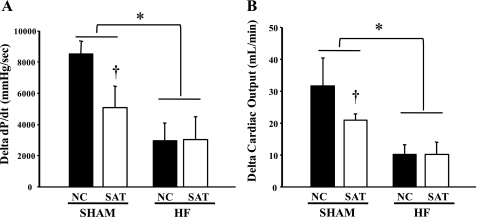
Interestingly, CO and LV +dP/dtmax showed differing responses to β-adrenergic stimulation in HF versus Sham groups. There were no differences in the overall response to dobutamine between the two HF groups (i.e., change in CO and LV +dP/dtmax from 0 to 10 μg·kg−1·min−1 dobutamine); however, the improvement in contractile function at baseline was maintained through all dobutamine doses, resulting in greater function at each workload in the HF + SAT group compared with the HF + NC group. In contrast, the Sham + SAT group showed a blunted response to dobutamine compared with the Sham + NC group at all doses (Fig. 3, A and B). Thus, the absolute change in both LV +dP/dtmax and CO from 0 to 10 μg·kg−1·min−1 dobutamine was not affected by high fat feeding in HF, yet high fat had a significant effect on the β-adrenergic responses in the Sham + SAT group.
Fig. 3.
Change in LV +dP/dtmax (A) and change in cardiac output (B) during dobutamine stress (change from 0 to 10 μg·kg−1·min−1 dobutamine). n = 5 animals/group. *P < 0.05, main effect of Sham vs. HF; †P < 0.05, Sham + NC group vs. Sham + SAT group.
Gene expression Profiling of the Infarcted Heart Exposed to High Fat
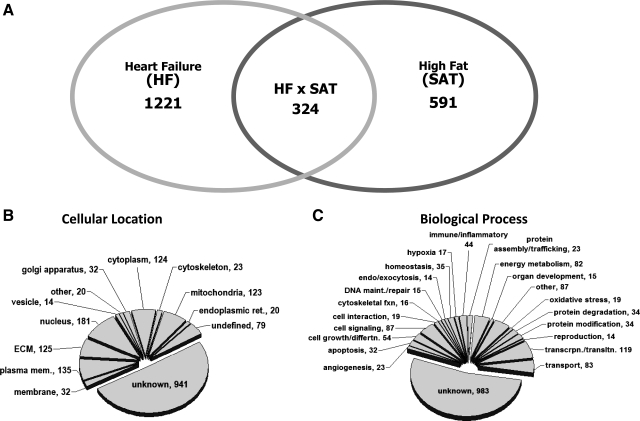
Microarray analysis was performed to generate a gene expression profile for the model as a whole and subsequently for the impact of dietary high fat with underlying HF. Of the 22,517 gene probes present on the microarray, 11,013 gene probes were detectable above background. Statistical analysis revealed that 1,848 gene probes were differentially expressed in the whole model analysis (see the Supplemental Table in the Supplemental Material).1 Within this whole model data set, a total of 1,221 gene probes were altered with respect to HF alone, and 591 gene probes were affected by the high-fat diet (Fig. 4A). Ontological terms were assigned for each of the 1,848 gene probes altered in the whole model analysis to define both their cellular localization and biological processes. The categorical breakdown of this data is shown as pie charts in Fig. 4, B and C. A number of subcellular locations were identified in the gene probe list, primarily the cytoplasm, extracellular matrix, nucleus, and mitochondrion (Fig. 4). Several biological processes were represented in the whole model analysis, including the category of transcription and translation along with cell signaling and energy metabolism (Fig. 4).
Fig. 4.
Venn diagram and ontological assignments for gene probes with altered expression in rats subjected to infarction followed by high fat feeding. A: Venn diagram of gene probes altered across model variables of HF or SAT from the 1,848 whole model analysis probe list (P < 0.05). Gene probes altered by HF and SAT were determined in the subset analysis (P < 0.05 by t-test). B: ontology terms associated with the cellular location of the 1,848 gene probes found to be statistically altered in the model. ECM, extracellular matrix. C: ontological breakdown of the gene probes by biological process.
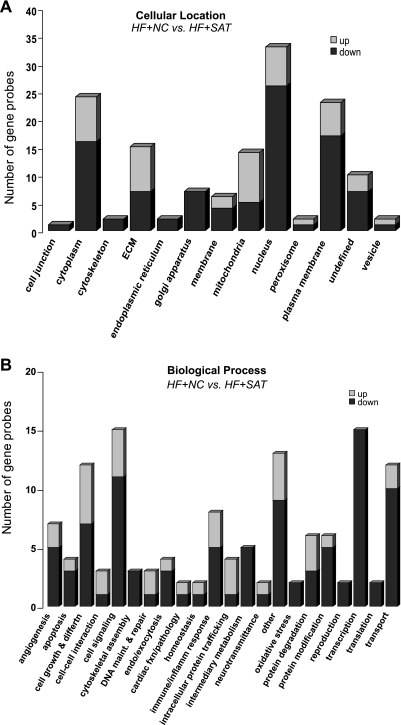
As the primary interest of the gene expression analysis for this study was the impact of high fat on HF, an additional statistical analysis was performed on the 1,848 gene probes to determine the subset of genes differentially expressed between the HF groups (HF + SAT vs, HF + NC groups). This analysis generated a list of 324 gene probes with significantly altered expression with respect to high fat in rats with cardiac dysfunction (P < 0.05). The ontological breakdown for genes in this subset analysis is shown as bar graphs in Fig. 5. Figure 5A shows the distribution of genes according to their cellular compartmentalization. Interestingly, the large majority of the gene probes with decreased expression in the HF + SAT group were associated with the nucleus, hinting at genes associated with the control of transcription. Figure 5B provides additional insight on the biological function of genes altered in HF by high dietary fat; several categories were populated with >10 gene probes, including cell growth and differentiation, cell signaling, transcription/translation, and transport.
Fig. 5.
Ontological assignments of the genes altered between the HF + NC and HF + SAT groups (324 gene probes). A: ontology terms associated with the cellular location of the gene probes found to be statistically significant (P < 0.05) when the HF + SAT and HF + NC groups were compared within the whole model list (1,848 gene probes). B: ontological breakdown of the gene probes by biological process (P < 0.05). The “undefined” designation was used for probes with multiple localization terms. “Other” was used for ontology categories containing fewer than two gene probes. “Unknown” designates gene probes with no annotative information available.
To fully explore the biological relevance of each gene and potential mechanisms pertaining to the cardioprotective effect of a high-fat diet, gene probes were analyzed in the context of biochemical pathways. MappFinder (GenMapp) was used to elucidate functional correlates in terms of classic cellular pathways and ontology using an objective scoring system within the software to define enrichment of gene representation within each biological pathway. As GenMapp relies on classic, well-defined pathways, a retrospective review of the gene probe list was performed to assure all genes relevant to identified pathways were included. This compilation produced 53 gene probes representing 10 pathways and 2 ontological categories, as shown in Table 4 (several genes are listed in more than one pathway/category). The pathways identified include several signaling pathways and energy metabolism. Cell signaling pathways altered with high fat during cardiac dysfunction include the androgen receptor, transforming growth factor (TGF)-β, and TNF-α cascade signaling. With the exception of energy metabolism, most of the genes associated with cell signaling pathways were downregulated with respect to high fat in HF. Genes categorized as related to energy metabolism are primarily proteins that break down fatty acids, and their expression was upregulated in HF with high fat feeding. The exception was pyruvate dehydrogenase kinase 4 (Pdk4), the regulatory enzyme controlling pyruvate oxidation by pyruvate dehydrogenase. Two categories based more on ontological classification are shown in Table 4 as they are relevant to energy metabolism as well as overall cardiac function and include genes localized to the mitochondrion. The majority of these genes were upregulated, and their function overlaps with energy metabolism.
Table 4.
Pathways affected by high fat feeding with underlying cardiac dysfunction
| Pathway and Gene Title | Gene | Accession Number | Fold Change |
|---|---|---|---|
| Androgen receptor signaling | |||
| Cyclin D1* | Ccnd1 | NM_171992.2 | −1.25 |
| v-Jun sarcoma virus 17 oncogene homolog* | Jun | NM_021835.2 | −1.22 |
| Filamin A | Flna | NM_001134599.1 | −1.15 |
| Apoptosis | |||
| v-Jun sarcoma virus 17 oncogene homolog | Jun | NM_021835.2 | −1.22 |
| MAPK kinase 4* | Map2k4 | NM_001030023.1 | −1.22 |
| Bcl2/adenovirus E1B 19-kDa interacting protein 1 | Bnip1 | NM_080897.1 | −1.20 |
| Interferon regulatory factor 1 | Irf1 | NM_012591.1 | −1.18 |
| Apoptosis antagonizing transcription factor | Aatf | NM_053720.1 | −1.18 |
| Caspase 8 | Casp8 | NM_022277.1 | −1.16 |
| Perforin 1 (pore-forming protein) | Prf1 | NM_017330.1 | −1.06 |
| Clusterin | Clu | NM_053021.2 | 1.36 |
| Insulin signaling | |||
| v-Jun sarcoma virus 17 oncogene homolog* | Jun | NM_021835.2 | −1.22 |
| MAPK kinase 4* | Map2k4 | NM_001030023.1 | −1.22 |
| PKC-θ (predicted) | PRKCQ | XM_341553.2 | −1.20 |
| PKC-α* | Prkca | NM_001105713 | −1.13 |
| Tuberous sclerosis 1 | Tsc1 | NM_021854.1 | 1.13 |
| Phosphatidylinositol 3-kinase, C2 domain, γ-polypeptide | Pik3c2g | NM_053923.1 | 2.61 |
| Energy metabolism | |||
| Coenzyme Q3 homolog methyltransferase* | Coq3 | NM_019187.1 | −1.19 |
| Phospholipase C-δ4 | Plcd4 | NM_080688.1 | −1.17 |
| Cytochrome c oxidase subunit IV isoform 1* | Cox4i1 | NM_017202.1 | 1.09 |
| Acetyl-CoA dehydrogenase, medium chain* | Acam | NM_016986.1 | 1.17 |
| 3-Hydroxy-3-methylglutaryl-CoA synthase 2* | Hmgcs2 | NM_173094.1 | 1.30 |
| Acetyl-CoA acyltransferase 2, mitochondrial* | Acaa2 | NM_130433.1 | 1.67 |
| Pyruvate dehydrogenase kinase isoenzyme 4* | Pdk4 | NM_053551.1 | 1.81 |
| Cytosolic acyl-CoA thioesterase 1* | Cte1 | NM_031315.1 | 2.32 |
| Transforming growth factor-β receptor signaling | |||
| Cyclin D1* | Ccnd1 | NM_171992.2 | −1.25 |
| v-Jun sarcoma virus 17 oncogene homolog* | Jun | NM_021835.2 | −1.22 |
| Syndecan 2 | Sdc2 | NM_013082.2 | −1.20 |
| Cullin 1* | Cul1 | NM_001108627 | −1.19 |
| Retinoblastoma-like 2 | Rbl2 | NM_031094.1 | −1.13 |
| Anaphase-promoting complex subunit 4 (predicted) | XM_223496.3 | −1.13 | |
| Activin A receptor type II-like 1 | Acvrl1 | NM_022441.1 | −1.11 |
| TNF-α signaling | |||
| Cullin 1* | Cul1 | NM_001108627 | −1.19 |
| Serine/threonine kinase* | Mark2 | NM_021699.1 | −1.17 |
| Similar to 2610301G19Rik protein (predicted) | LOC306007 | XM_224329.3 | −1.16 |
| Caspase 8* | Casp8 | NM_022277.1 | −1.16 |
| Filamin A* | Flna | NM_001134599.1 | −1.15 |
| Minichromosome maintenance deficient 5, cell division cycle 46 | Mcm5 | NM_001106170.1 | −1.12 |
| WNT signaling | |||
| Cullin 1* | Cul1 | NM_001108627 | −1.19 |
| Serine/threonine kinase | Mark2 | NM_021699.1 | −1.17 |
| MAPK signaling | |||
| MAPK kinase 4* | Map2k4 | NM_001030023.1 | −1.22 |
| PKC-α | Prkca | NM_001105713 | −1.13 |
| Growth arrest and DNA damage-inducible 45, γ isoform | XM_237999.3 | 1.30 | |
| G protein signaling | |||
| Olfactory receptor 1232 | Olr1232 | NM_001000445.1 | −1.15 |
| Prepro-neuropeptide W polypeptide | LOC259224 | NM_001003409 | −1.14 |
| Regulator of G protein signaling 9 | Rgs9 | NM_019224.1 | −1.07 |
| G protein-coupled receptor 116 | Gpr116 | NM_139110.1 | 1.21 |
| mRNA processing | |||
| Heterogeneous nuclear ribonucleoprotein A3 | Hnrpa3 | NM_198132.2 | −1.14 |
| PRP4 preprocessing factor 4 homolog (predicted) | Prpf4 | XM_233022.3 | −1.13 |
| Splicing factor arginine/serine-rich 5 | Sfrs5 | NM_019257.1 | −1.11 |
| RNA (guanine-7-)methyltransferase | Rnmt | NM_001008299.1 | −1.10 |
| Mitochondrial compartment | |||
| Coenzyme Q3 homolog methyltransferase* | Coq3 | NM_019187.1 | −1.19 |
| Solute carrier family 25 member 30 | Slc25a30 | NM_001013187 | −1.19 |
| DEAD (Asp-Glu-Ala-Asp) box polypeptide 24 | Ddx24 | NM_199119.1 | −1.13 |
| MRS2-like magnesium homeostasis factor | Mrs21 | NM_024001.1 | −1.13 |
| Transcription factor A mitochondrial | Tfam | NM_031326.1 | −1.09 |
| Cytochrome c oxidase subunit IV isoform 1* | Cox4i1 | NM_017202.1 | 1.09 |
| Vesicle-associated membrane protein 1 | Vamp1 | NM_013090.1 | 1.13 |
| Acetyl-CoA dehydrogenase, medium chain* | Acadm | NM_016986.1 | 1.17 |
| Thioredoxin 1 | Txn1 | NM_053800.2 | 1.17 |
| 3-Hydroxy-3-methylglutaryl-CoA synthase 2* | Hmgcs2 | NM_173094.1 | 1.30 |
| Uncoupling protein 3 | Ucp3 | NM_013167.2 | 1.49 |
| Acetyl-CoA acyltransferase 2, mitochondrial* | Acaa2 | NM_130433.1 | 1.67 |
| Pyruvate dehydrogenase kinase isoenzyme 4* | Pdk4 | NM_053551.1 | 1.81 |
| Cytosolic acyl-CoA thioesterase 1* | Cte1 | NM_031315.1 | 2.32 |
| Cardiac function | |||
| ATPase Na+-K+ transporting α2-polypeptide | Atp1a2 | NM_012505.1 | −1.22 |
| Cardiac ankyrin repeat kinase | Cark | NM_181769.1 | −1.14 |
| Leprecan 1 | Lpre1 | NM_053667.1 | −1.12 |
| Aldehyde dehydrogenase family 1 subfamily A2 | Aldh1a2 | NM_053896.1 | 1.22 |
| Fast myosin alkali light chain | Mlc3 | NM_020104.1 | 1.26 |
Shown are pathway analysis hits in the HF + SAT group compared with the HF + NC group. The list of genes found to be statistically significant for the model (1,848 gene probes) was then used to perform a t-test to define genes altered by high fat in the context of HF (P < 0.05). This list was then analyzed to determine what biochemical pathways were represented within the subset of data. Accession number refers to the unique gene identifier within the RefSeQ database.
Gene probes with more than one pathway assignment within the table.
DISCUSSION
The major findings of the present study reveal that high saturated fat feeding improves myocardial function at rest and during physiological stress in rats with LV dysfunction after myocardial infarction. Steady-state indexes of systolic function and preload recruitable measures of contractility were decreased after infarction but not in HF animals fed high saturated fat. Furthermore, reductions in the myocardial contractile reserve was evident in all HF animals, but high fat did not exacerbate the response to a β-adrenergic challenge. In contrast, the contractile reserve was reduced by high saturated fat feeding in normal animals. Thus, in mild to moderate HF/LV dysfunction, high fat feeding improves contractile function at rest and during physiological stress but may negatively impact the contractile reserve under nonpathological conditions.
Pathological conditions such as diabetes (3) and obesity (1, 50) are associated with enhanced myocardial lipid accumulation and contractile dysfunction, a condition referred to as lipotoxicity (42). Interestingly, the obesity paradox counters this assertion in that obese patients have a better prognosis once cardiovascular pathology has been established. Recent studies (12, 28, 38, 39, 44) have provided evidence for this alternative hypothesis, one in which exposure of the myocardium to elevated lipids serves in a protective rather than pathological capacity. The results of the present study expand on our previous findings in that contractile function at baseline was improved in HF animals fed high fat, i.e., load-dependent measures, LV peak +dP/dtmax , SW, and maximal power were all significantly increased. However, our previous studies focused solely on the effects on function of elevated lipids under resting or baseline conditions and did not evaluate the myocardial response to preload reductions or physiological stress. This study reports that during IVC occlusions and load-independent measures of contractility (ESPVR, Emax, PRSW, and peak +dP/dtmax-EDV) were increased with high fat feeding in HF/LV dysfunction. Trifunovic et al. (47) also reported no deleterious effects of high-fat diets (both saturated and unsaturated) on myocardial function under different loading conditions. In contrast, in isolated working hearts, a 55% high saturated fat diet was associated with decreased external cardiac work and efficiency of cardiac oxygen used during reductions in preload; however, the impaired coronary flow reserve may have accounted for this maladaptive response to increased metabolic requirements (34). Thus, our data suggest that despite an increased exposure to dietary lipids, the contractile responses to alterations in preload are improved in HF animals fed a high saturated fat diet and thus contradicts the notion of lipotoxicity. An increase in myocardial energy supply through increased fatty acid availability might account for this improvement in function, a concept that would be consistent with the protective effect espoused by the obesity paradox (22).
Impaired myocardial contractile function is a hallmark of HF, which may present under resting conditions and/or when the heart is challenged by increasing cardiac work. During a β-adrenergic challenge to evaluate the myocardial contractile reserve, we observed a diminished cardiac response with increasing dobutamine doses in the HF + NC group that was not evident in the HF + SAT group. Furthermore, β-adrenergic responsiveness was reduced in the infarcted groups, although high fat feeding did not further impact the myocardial contractile reserve. A diminished β-adrenergic response in HF is consistent with our understanding that β-adrenergic receptors are downregulated and/or desensitized during HF (25). The fact that high saturated fat feeding did not further compromise the response to a physiological stress argues against lipotoxicity in this model. However, studies in models with elevated plasma and myocardial lipids (such as in diabetes and obesity) have reported alterations in the responsiveness of both isolated hearts and papillary muscles in high-fat diet-induced obese rabbits. Carroll et al.(6) reported a negative effect of obesity on the β-adrenergic responsiveness of both isolated hearts and papillary muscle preparations in high-fat diet-induced obese rabbits. Diabetic patients manifesting early signs of diabetic cardiomyopathy have also been reported to have exercise intolerance and a reduced inotropic reserve, suggesting that the β-adrenergic system is affected (43). Thus, a more comprehensive evaluation of the effects of high saturated fat during a β-adrenergic challenge should be the target of future investigation to determine the impact of a nutritional intervention on myocardial function in mild to moderate HF/LV dysfunction.
It is also important to consider that high saturated fat may have deleterious effects under “nonpathological” conditions. We (38) reported previously that a prolonged period (16 wk) of high fat feeding was associated with decreases in mitochondrial respiration (state 3) in normal animals but not animals with mild to moderate HF, suggesting that the normal heart may be susceptible to lipotoxic effects. Ouwens et al. reported that normal rats fed high fat (8 wk) had a hypertrophic-like cardiac phenotype characterized by LV remodeling, decreased FS and EF (30), and impaired recovery from increased workloads (29). Other studies (16, 50), albeit in transgenic models, have also shown that excess myocardial lipid accumulation is accompanied by the generation of toxic lipid intermediates and contractile dysfunction. In contrast, high fat feeding was not associated with any contractile or morphological changes in normal rats or mice after 9 days to 48 wk of feeding (36, 48, 49). These studies argue against the suggestion that the duration of feeding accounts for these disparate results; however, other factors, such as the composition of the dietary fat and associated comorbidities such as diet-induced obesity, have been proposed.
Numerous studies have documented the impact of obesity on cardiac hypertrophy and contractile dysfunction with respect to morbidity and mortality (14, 45). Fang and colleagues (15) reported decreased FS and increased end-systolic diameter and hypertrophy in high fat diet-fed obese mice but not in high-fat diet-fed weight-control mice (15). Other studies (7, 28, 40) have supported the concept that obesity, and not fat feeding itself, is associated with diet-induced cardiac abnormalities. Alternatively, differences reported between studies may reflect variations in the macronutrient content of these diets. Wilson et al. (48) reported that long-term feeding of a Western diet, but not a high-fat diet, was associated with contractile dysfunction. Other studies have reported that neither a high saturated or polyunsaturated fat diet altered cardiac mass or adversely affected cardiac function. A diet high in lard (a mix of saturated and unsaturated fat) did not further compromise cardiac function after infarction-induced HF (27), whereas a high saturated fat diet prevented HF and death associated with chronic hypertension (28, 40, 44). Interestingly, it may be the carbohydrate composition of a diet, and not fats, that have the greater impact based on evidence linking increased glycemic load to greater cardiovascular risk (8), a concept that demands further investigation. Thus, the impact of high fat feeding on contractile function may be dependent on its association with diet-induced obesity or the nutritional composition of the diet rather than the pathophysiological state of the animals.
Genome-wide expression trends were evaluated via microarray analysis in the HF model with high fat feeding to gain insight into the transcriptional changes related to the cardioprotective effect on contractility. It is of interest to note that in the whole model analysis, many genes related to the commonly described “fetal program,” which include changes in the expression of atrial natriuretic factor, VEGF, myosin isoforms, c-myc, c-fos, collagen isoforms, and others, were impacted, but this trend was not apparent when the two HF groups wre compared, highlighting the fact that many of the processes initiated by HF are still present with the addition of high fat. However, pathway analysis of gene expression changes across the two HF groups revealed several biochemical protein cascades with multiple altered gene targets identifying such pathways as those impacted by high fat in the context of infarction. The majority of the significantly altered pathways found to possess multiple gene targets represent cell signaling pathways, and the multiple gene targets within these pathways were primarily downregulated in the HF + SAT group compared with the HF + NC group. The overall decreased expression of multiple genes within a given biochemical cascade constitutes an important observation as these pathways, including apoptosis, TGF-β, TNF-α, and WNT, have been demonstrated to be activated in HF and are involved in the scar formation and remodeling process within the viable (noninfarcted) myocardium (4, 21, 46). The tissue used in the microarray analysis was LV tissue consisting of a heterogenous population of cell types; thus, the origin of the expressional changes seen may represent cell infiltration, especially those relating to the immune response. Taken together, the transcriptional changes occurring with high fat after infarction appear to include decreased expression of genes related to injury response pathways within the myocardium, and this notion is in line with the observed cardioprotection.
In addition to mediating changes in the remodeling processes of the heart, high fat feeding altered the expression of genes related to energy metabolism. Overall, the expression of genes relating to fatty acid metabolism were upregulated, and two of these genes in particular, Pdk4 and acyl-CoA thioesterase 1 (cte1), showed similar fold changes in response to diet as our previous work (39) using quantitative PCR, suggesting cross-platform agreement. The findings presented here regarding gene expression changes further support the notion that energy metabolism with the high saturated fat diet favors fatty acid oxidation, as Pdk4 regulates the entry of pyruvate generated from the glycolytic pathway into the tricarboxylic acid cycle, generating reducing equivalents for oxidative phosphorylation. An upregulation in fatty acid metabolism genes might have been anticipated as the treatment is the pathway substrate, but it also serves to highlight a potential mechanism as to how cardiac function is maintained. HF is commonly accompanied by an increased reliance on glucose oxidation, which has been shown in a number of studies (38, 41, 46) at both the level of expression, transcription factor activation, and enzyme activity. Our data suggest that altering the circulating availability of fatty acids may be beneficial to the injured heart through a reversal of the increased reliance on glucose oxidation. Also, the majority of the energy metabolism genes upregulated HF + SAT group primarily reside within the mitochondrion. Indeed, many of the proteins involved in energy metabolism subsequent to glycolysis are housed in mitochondria, but expansion of this list to account for any gene localized to the mitochondrion resulted in genes that are not directly related to metabolism. Rather, this may indicate not only a shift in the metabolic machinery housed in mitochondria but also a change in the overall composition of the mitochondrial population that could be beneficial after infarction. Whether this is a cause of, or a result of, high fat protection requires further investigation.
In summary, our results show that high fat feeding improves myocardial function at rest and during physiological stress in mild to moderate HF/LV dysfunction but may negatively impact the contractile reserve under nonpathological conditions. Additionally, differentially expressed genes in the HF/LV dysfunction group fed high saturated fat fell under the category of energy metabolism; that these genes/pathways are classically downregulated with HF alone suggests that high fat is cardioprotective after infarction by sustaining a normal metabolic phenotype while preventing the increased expression of hallmark mediators of cardiac injury. Taken together, these results offer novel insights into the molecular mechanism through which high fat confers cardioprotection subsequent to myocardial injury.
GRANTS
This work was supported by American Heart Association-National Office Scientist Development Grant 0535361N and by National Heart, Lung, and Blood Institute Grant HL-081857.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors gratefully acknowledge Mary H. Sailors (Department of Epidemiology, University of Alabama, Birmingham, AL) for the expert assistance in the analysis of the gene array data.
Footnotes
Supplemental Material for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 88: 389–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barger PM, Kelly DP. PPAR signaling in the control of cardiac energy metabolism. Trends Cardiovasc Med 10: 238–245, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 115: 3213–3223, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bujak M, Frangogiannis NG. The role of TGF-β signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74: 184–195, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Carroll JF, Jones AE, Hester RL, Reinhart GA, Cockrell K, Mizelle HL. Reduced cardiac contractile responsiveness to isoproterenol in obese rabbits. Hypertension 30: 1376–1381, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail 14: 82–88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res 79: 269–278, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6: 292–300, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol 4: R7, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Low-carbohydrate/high-fat diet attenuates pressure overload-induced ventricular remodeling and dysfunction. J Card Fail 14: 327–335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem 281: 24254–24269, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation 105: 2923–2928, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol 295: H1206–H1215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARα-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 100: 1226–1231, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 295: 655–666, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hsich E, Gorodeski EZ, Starling RC, Blackstone EH, Ishwaran H, Lauer MS. Importance of treadmill exercise time as an initial prognostic screening tool in patients with systolic left ventricular dysfunction. Circulation 119: 3189–3197, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu FB. Diet and cardiovascular disease prevention the need for a paradigm shift. J Am Coll Cardiol 50: 22–24, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, Granger CB, McMurray JJ, Solomon SD. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Circulation 116: 627–636, 2007 [DOI] [PubMed] [Google Scholar]
- 21.LaFramboise WA, Bombach KL, Dhir RJ, Muha N, Cullen RF, Pogozelski AR, Turk D, George JD, Guthrie RD, Magovern JA. Molecular dynamics of the compensatory response to myocardial infarct. J Mol Cell Cardiol 38: 103–117, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med 122: 1106–1114, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, Karanja N, Lefevre M, Rudel L, Sacks F, Van Horn L, Winston M, Wylie-Rosett J. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 114: 82–96, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Lopaschuk GD, Folmes CD, Stanley WC. Cardiac energy metabolism in obesity. Circ Res 101: 335–347, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111: 2837–2849, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, Chandler MP, Hoit BD. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol 287: H2049–H2053, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, Hoit BD, Stanley WC, Chandler MP. Effects of chronic activation of peroxisome proliferator-activated receptor-α or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol 290: H1899–H1904, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive Dahl salt-sensitive rat. Clin Exp Pharmacol Physiol 32: 825–831, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Ouwens DM, Boer C, Fodor M, de Galan P, Heine RJ, Maassen JA, Diamant M. Cardiac dysfunction induced by high-fat diet is associated with altered myocardial insulin signalling in rats. Diabetologia 48: 1229–1237, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ouwens DM, Diamant M, Fodor M, Habets DD, Pelsers MM, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, Boer C, Coort SL, Glatz JF, Luiken JJ. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 50: 1938–1948, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107: 896–904, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panchal AR, Stanley WC, Kerner J, Sabbah HN. β-Receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail 4: 121–126, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation 105: 2303–2308, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Plante E, Lachance D, Drolet MC, Roussel E, Couet J, Arsenault M. Dobutamine stress echocardiography in healthy adult male rats. Cardiovasc Ultrasound 3: 34, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raher MJ, Thibault HB, Buys ES, Kuruppu D, Shimizu N, Brownell AL, Blake SL, Rieusset J, Kaneki M, Derumeaux G, Picard MH, Bloch KD, Scherrer-Crosbie M. A short duration of high-fat diet induces insulin resistance and predisposes to adverse left ventricular remodeling after pressure overload. Am J Physiol Heart Circ Physiol 295: H2495–H2502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Remondino A, Rosenblatt-Velin N, Montessuit C, Tardy I, Papageorgiou I, Dorsaz PA, Jorge-Costa M, Lerch R. Altered expression of proteins of metabolic regulation during remodeling of the left ventricle after myocardial infarction. J Mol Cell Cardiol 32: 2025–2034, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, Chandler MP. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol 46: 883–890, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, Stoll MS, Minkler PE, Fujioka H, Hoit BD, Young ME, Hoppel CL, Chandler MP. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res 79: 331–340, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol 292: H1498–H1506, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94: 2837–2842, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 14: 281–287, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Scognamiglio R, Avogaro A, Casara D, Crepaldi C, Marin M, Palisi M, Mingardi R, Erle G, Fasoli G, Dalla Volta S. Myocardial dysfunction and adrenergic cardiac innervation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol 31: 404–412, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Sharma N, Okere IC, Duda MK, Johnson J, Yuan CL, Chandler MP, Ernsberger P, Hoit BD, Stanley WC. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens 20: 403–409, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Sowers JR. Obesity as a cardiovascular risk factor. Am J Med 115, Suppl 8A: 37S–41S, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res 86: 939–945, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Trifunovic B, Woodiwiss AJ, Norton GR. Preload recruitable stroke work in the presence of intact cardiovascular reflexes in rats fed a diet high in unsaturated fats. Experientia 51: 5–10, 1995 [PubMed] [Google Scholar]
- 48.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J 406: 457–467, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 82: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]