Abstract
Ischemic preconditioning (IPC) is a powerful phenomenon that provides potent cardioprotection in mammalian hearts; however, the role of endothelial nitric oxide (NO) synthase (eNOS)-mediated NO in this process remains highly controversial. Questions also remain regarding this pathway as a function of sex and ischemic duration. Therefore, we performed extensive experiments in wild-type (WT) and eNOS knockout (eNOS−/−) mice to evaluate whether the infarct-limiting effect of IPC depends on eNOS, ischemic periods, and sex. Classical IPC was induced by three cycles of 5 min of regional coronary ischemia separated by 5 min of reperfusion and was followed by 30 or 60 min of sustained ischemia and 24 h of reperfusion. The control ischemia-reperfusion protocol had 30 or 60 min of ischemia followed by 24 h of reperfusion. Protection was evaluated by measuring the myocardial infarct size as a percentage of the area at risk. The major findings were that regardless of sex, WT mice exhibited robust IPC with significantly smaller myocardial infarction, whereas eNOS−/− mice did not. IPC-induced cardiac protection was absent in eNOS−/− mice of both Jackson and Harvard origin. In general, female WT mice had smaller infarctions compared with male WT mice. Although prolonged ischemia caused significantly larger infarctions in WT mice of both sexes, they were consistently protected by IPC. Importantly, prolonged myocardial ischemia was associated with increased mortality in eNOS−/− mice, and the survival rate was higher in female eNOS−/− mice compared with male eNOS−/− mice. In conclusion, IPC protects WT mice against in vivo myocardial ischemia-reperfusion injury regardless of sex and ischemic duration, but the deletion of eNOS abolishes the cardioprotective effect of classical IPC.
Keywords: endothelial nitric oxide synthase, cardioprotection, ischemic periods, gender, mortality
nitric oxide (NO) is a highly versatile signaling molecule with a wide variety of functions in the cardiovascular system including the regulation of systemic blood pressure, blood flow, regional vascular tone, and cardiac function (31, 32). It is now well recognized that in biological tissues, NO is generated by specific NO synthases (NOSs) that metabolize arginine to citrulline with the formation of NO (14, 32). There are three known isoforms of NOS; among them, neuronal NOS (nNOS or NOS1) and endothelial NOS (eNOS or NOS3) are Ca2+ dependent and constitutively present in the heart (32, 33), whereas inducible NOS (iNOS or NOS2) is Ca2+ independent and can be induced by cytokines or other stimuli (41).
Each of these NOSs has been disrupted by targeted gene deletion in mice to investigate the contribution of each NOS in a variety of experimental models (31, 33). While these murine genetic models continue to be invaluable tools to provide new insights into the pathophysiological role of NOSs and NO, discrepancies have emerged from previous studies (3, 4, 13, 16, 20, 21, 24, 42, 44, 45, 58–61) regarding the role of eNOS in myocardial ischemia-reperfusion (I/R) injury and ischemic preconditioning (IPC) using eNOS knockout (eNOS−/−) mice. Four groups (15, 18, 22, 43) have independently generated mice with targeted deletion of eNOS using different targeting strategies, and all of these mice are hypertensive. eNOS−/− mice from three different sources have been used for myocardial I/R studies; however, there is considerable controversy regarding the role of the eNOS-NO pathway in cardioprotection. Although the precise causes of differences in previous studies are unclear, the inconsistent data in eNOS−/− mice may be ascribed, at least in part, to differences in the animals used and the precise experimental conditions. Differences in sex, background strain, and age could profoundly influence the results obtained. During I/R, eNOS−/− mice exhibited either improved or decreased functional recovery as well as a variable effect on myocardial infarct size. Of note, overexpression of eNOS has been reported to attenuate myocardial reperfusion injury (25).
IPC is a powerful phenomenon that provides effective cardioprotection in mammalian hearts (34). It is now well established that preconditioning results in two distinct phases of cardioprotection against lethal ischemic injury. The first, known as early IPC (classical preconditioning) (9), has an immediate onset but is of limited duration (2–3 h). The second phase, known as delayed preconditioning [second window of protection (SWOP)], occurs 12–24 h after the preconditioning stimulus and persists for 2–3 days (9). Although the eNOS-NO pathway has been shown to be involved in delayed preconditioning (9, 57), its role in early preconditioning remains controversial and poorly understood. In an early study (3) of eNOS−/− mice with the SV129/B6 strain (3), it was reported that the benefit from early preconditioning in isolated hearts is absent with two or three 5-min episodes of preconditioning stimuli but not when the stimulus is increased (four 5-min episodes of preconditioning stimuli). However, recently, using an in vivo model of I/R and IPC in mice with the C57BL/6 strain (20), it has been reported that eNOS is not necessary for early preconditioning by either one or six 4-min episodes of preconditioning stimuli. Interestingly, while eNOS-overexpressing mouse hearts were more tolerant to I/R injury with significantly smaller myocardial infarction (MI), these hearts did not show any early preconditioning effect (11). It is possible that the difference between the early (3) and recent (20) studies may be due to the use of different models (in vitro vs. in vivo) or protocols (number and duration of preconditioning stimuli) or mouse strains (C57BL/6 vs. SV129/B6). In view of this controversy, further investigation is needed to characterize whether the myocardial constitutive eNOS-NO pathway plays a significant role in early preconditioning.
Therefore, we systematically addressed this issue by taking a four-pronged approach in a well-established murine model of MI. We addressed the following critical questions: 1) Is the constitutive expression of eNOS necessary for early ischemic preconditioning? 2) Does prolonged ischemic duration modulate early IPC? 3) Do sex differences play a role in early IPC? and 4) Does an eNOS gene mutation strategy affect IPC?
MATERIALS AND METHODS
This study was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University, was carried out according to the approved guidelines, and conformed with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
Animals
We used eNOS−/− mice with deletion of either the calmodulin-binding domain (43) (stock no. 002684, Jackson Laboratory) or deletion of the reduced NADP ribose- and adenine-binding sites (22) (Harvard eNOS−/− mice). Details regarding the generation and characterization of Harvard eNOS−/− mice have been previously described (22, 47). Briefly, eNOS−/− mice were derived from a cross between SV129J and C57BL/6 mice and were backcrossed to C57BL/6 mice over 10 generations. Thus, C57BL/6 mice were used as wild-type (WT) controls. For Jackson eNOS−/− mice, C57BL/6 mice (stock no. 00066) were used as WT controls. Mice were housed in an air-conditioned room with 12:12-h light-dark cycle, received standard mouse chow, and drank tap water. Experiments were performed using 10- to 14-wk-old male and female mice.
Experimental Protocol
I/R.
In vivo myocardial I/R protocol was performed as previously described with slight modifications (48). Briefly, mice were anesthetized with a mixture of intraperitoneal ketamine (55 mg/kg) and xylazine (15 mg/kg). After adequate anesthesia and aseptic preparations, the mice were intubated and ventilated with room air by MiniVent (type 845, Harvard Apparatus). The respiratory rate was maintained at 100 breaths/min with a tidal volume of 0.25 ml for a 25-g mouse. The rectal temperature of the mouse was maintained at 37°C by a thermo heating pad. After the chest had been opened and the heart visualized, the left anterior descending coronary artery (LAD) was ligated 2 mm below the tip of the left auricle by a 7-0 silk ligature. A small piece of polyethylene-10 tubing was used to secure the ligature without damaging the artery. Coronary occlusion was confirmed by the dramatic change in color (red to pallor), restricted ventricular motion, and ECG ST segment changes. After 30 or 60 min of LAD coronary artery occlusion, the knot was released to start coronary artery reperfusion, and reperfusion was confirmed by the return of the pink-red color in the previously ischemic area of the left ventricle (LV). The chest was closed in layers with topical penicillin G procaine under the skin. Buprenorphine (0.1 mg/kg) was given subcutaneously to reduce postoperative acute pain. When mice resumed a normal breathing pattern and started walking, the ventilator was taken off, and mice were kept in clean cages with free access to food and water.
IPC.
This procedure was exactly the same as the in vivo myocardial I/R protocol except that there were three cycles of 5-min ischemia and 5-min reperfusion before the 30- or 60-min periods of ischemia.
Myocardial Infarct Size Measurements
MI was measured after 24-h reperfusion as previously described with slight modifications (48). Mice were anesthetized, intubated, and ventilated, and the chest was opened along the previous incision line. Hearts were rapidly excised, flushed with heparinized PBS via the aorta, and then infused/stained with 1% 2,3,5-triphenyltetrazolium chloride (Sigma) for 5 min for the demarcation of the viable and nonviable myocardium within the area at risk (AAR). With coronary reocclusion at the previous site, hearts were infused with 10% phthalo blue to visualize the nonrisk (nonischemic) region. Hearts were frozen, serially sectioned (1 mm thick) using a heart slicer, and fixed in 10% formalin. Both sides of each myocardial slice were photographed, and the area of infarction, AAR, and nonrisk area were determined by computerized planimetry with image-analysis software (Meta Vue, version 6.0). AAR was calculated as a percentage of the total LV area. Infarct size was calculated as a percentage of the LV AAR.
Data Analysis
All results are expressed as means ± SE. Data were analyzed either by a two-tailed Student's t-test for paired data from the same experiment and unpaired data from different experiments or by ANOVA followed by Fisher's post hoc test. Kaplan-Meier survival analysis for the in vivo myocardial I/R and IPC protocols in eNOS−/− mice was performed using GraphPad Prism4 (San Diego, CA) and a log-rank test. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Is the Constitutive Expression of eNOS Necessary for Early Preconditioning?
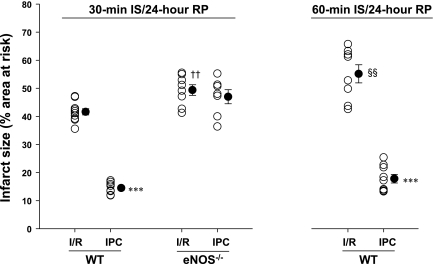
To answer this question, we compared the extent of MI after standard I/R and classical IPC protocols in young male WT and eNOS−/− mice from Jackson Laboratories. There were no differences among the male WT and eNOS−/− mice with respect to body weight (Table 1). Compared with WT mice, infarct size was slightly and significantly larger in eNOS−/− mice after I/R (Fig. 1). Importantly, despite comparable AARs in both groups, IPC significantly reduced myocardial infarct size in WT mice but not in eNOS−/− mice (Fig. 1). These findings demonstrate that constitutively expressed eNOS is required to protect against I/R injury and thus for cardioprotection.
Table 1.
Experimental conditions, area at risk, and infarct area after I/R and IPC
| Protocols | Body Weight, g | Area at Risk, % | Infarct Area, % | No. of Animals |
|---|---|---|---|---|
| Male WT mice | ||||
| 30-min Ischemia | ||||
| I/R | 25 ± 0.6 | 60 ± 2 | 42 ± 1 | 10 |
| IPC | 25.3 ± 0.5 | 59 ± 2 | 14 ± 1a | 8 |
| 60-min Ischemia | ||||
| I/R | 25.5 ± 0.6 | 58 ± 2 | 55 ± 3d | 10 |
| IPC | 26 ± 0.9 | 55 ± 3 | 18 ± 2a | 9 |
| Male eNOS−/−mice | ||||
| 30-min Ischemia | ||||
| I/R | 25 ± 0.6 | 64 ± 2 | 49 ± 2b | 8 |
| IPC | 25 ± 1.1 | 62 ± 2 | 47 ± 3 | 7 |
| 60-min Ischemia | ||||
| I/R | 28 ± 1 | ND | ND | 10 |
| IPC | 26 ± 1 | ND | ND | 11 |
| Female WT mice | ||||
| 30-min Ischemia | ||||
| I/R | 20 ± 0.6 | 57 ± 3 | 32 ± 2g | 7 |
| IPC | 20 ± 0.8 | 56 ± 3 | 17 ± 2a | 7 |
| 60-min Ischemia | ||||
| I/R | 18 ± 0.6 | 62 ± 1 | 42 ± 2d,f | 9 |
| IPC | 19 ± 0.6 | 61 ± 2 | 20 ± 2a | 10 |
| Female eNOS−/−mice | ||||
| 30-min Ischemia | ||||
| I/R | 19 ± 0.6 | 60 ± 3 | 48 ± 3c | 7 |
| IPC | 18.5 ± 0.7 | 63 ± 2 | 45 ± 2 | 7 |
| 60-min Ischemia | ||||
| I/R | 19 ± 0.5 | 63 ± 2 | 58 ± 2c,e | 7 |
| IPC | 19 ± 0.2 | 61 ± 4 | 54 ± 4 | 7 |
Data are presented as means ± SE. WT, wild type; eNOS−/−, endothelial nitric oxide synthase knockout; I/R, ischemia-reperfusion protocol; IPC, ischemia preconditioning protocol; ND, not determined because hearts failed and could not be used for this end point.
P < 0.001 vs. respective I/R;
P < 0.01 and
P < 0.001 vs. respective WT mice with I/R;
P < 0.01 vs. respective WT mice with 30-min I/R;
P < 0.05 vs. female eNOS−/− mice with 30-min I/R;
P <0.01 vs. male WT mice with 30-min I/R;
P <0.001 vs. male WT mice with 60-min I/R.
Fig. 1.
Infarct size (expressed as a percentage of the area at risk) measured in Jackson young male wild-type (WT) and endothelial nitric oxide synthase (eNOS) knockout (eNOS−/−) mice subjected to 30 and/or 60 min of left anterior descending coronary artery (LAD) occlusion [ischemia (IS)] and 24 h of reperfusion (RP) in the absence or presence of ischemic preconditioning (IPC) cycles. ○, Individual values; ●, average values as means ± SE. I/R, ischemia-reperfusion. n = 7–10 mice/group. ***P < 0.001 vs. respective I/R; ††P < 0.01 vs. respective WT mice; §§P < 0.01 vs. WT mice with 30-min I/R. Deletion of the eNOS gene abolished the protective effects of early IPC against myocardial infarction.
Does the Duration of the Lethal Ischemic Period Modulate Early Preconditioning?
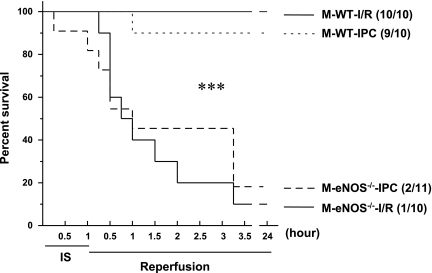
In vivo 30-min LAD ligation is a well-documented, acceptable, and commonly used lethal ischemic insult for myocardial I/R and IPC protocols (20, 42, 59). To investigate whether a more prolonged ischemic period differentially affects the benefit of early preconditioning, we compared myocardial infarct size in male mice after 60-min LAD ligation and 24-h reperfusion. There were no differences in the LV AAR between 30- and 60-min LAD ligation protocols (Table 1). Interestingly, while the myocardial infarct size was increased with 60-min LAD ligation and 24-h reperfusion (Table 1), IPC remained effective in reducing myocardial infarct size in WT mice (Fig. 1). Of note, 60-min LAD ligation and subsequent reperfusion were lethal in eNOS−/− mice compared with WT mice. In WT groups, there were no deaths with the I/R protocol; however, one mouse with IPC died at 1 h of reperfusion (Fig. 2). In eNOS−/− groups (Fig. 2), all but one mouse of the I/R group died within 15 min to 3 h of reperfusion, eight mice with IPC died within 15 min to 3 h of reperfusion, and one mouse died at 60 min of ischemia. The mortality rate was significantly different between WT and eNOS−/− mice (P < 0.001). Thus, it is evident that a potent infarct-limiting effect of early IPC is consistently present in WT mice irrespective of the duration of the ischemic period; however, the IPC effect is lost in eNOS−/− mice and is associated with increased mortality after prolonged ischemia.
Fig. 2.
Survival of young male (M) WT and eNOS−/− mice subjected to 60 min of LAD occlusion and 24 h of reperfusion in the absence or presence of IPC cycles (I/R and IPC, respectively). Number in parentheses indicate survival relative to total experimented mice. Kaplan-Meier survival curve analysis showed markedly decreased survival in eNOS−/− mice compared with WT mice after I/R and IPC. n = 10–11/group. ***P < 0.001 vs. WT mice.
Does a Sex Difference Play a Role in Early Preconditioning?
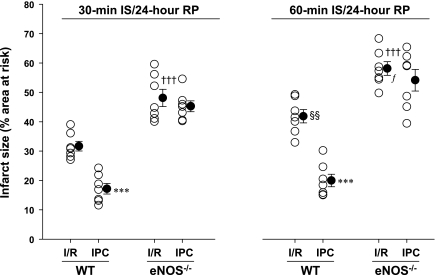
To evaluate whether a sex difference plays a role in early preconditioning, we repeated both 30- and 60-min LAD ligation protocols in female WT and eNOS−/− mice. Female mice were relatively smaller in body weight compared with age-matched male mice (Table 1); however, body weights among female WT and eNOS−/− mice were similar. There were no significant differences in the LV AAR between WT and eNOS−/− mice (Table 1). Interestingly, compared with male WT mice, myocardial infarct sizes were significantly smaller in female WT mice with both I/R protocols (Table 1). However, there were no differences in the infarct size between male and female eNOS−/− mice after 30-min I/R (Table 1). The infarct-limiting effect of early preconditioning was consistently present in female WT mice either with the 30- or 60-min LAD ligation protocol, but IPC-induced cardioprotection was absent in female eNOS−/− mice (Fig. 3).
Fig. 3.
Infarct size (expressed as a percentage of the area at risk) measured in Jackson young female WT and eNOS−/− mice subjected to 30 and 60 min of LAD occlusion and 24 h of reperfusion in the absence or presence of IPC cycles. ○, Individual values; ●, average values as means ± SE. n = 7–10 mice/group. ***P < 0.001 vs. respective I/R; †††P < 0.01 vs. respective WT mice with I/R; §§P < 0.01 vs. WT mice with 30-min I/R; fP < 0.05 vs. eNOS−/− mice with 30-min I/R. Deletion of the eNOS gene abolished the protective effects of early IPC against myocardial infarction.
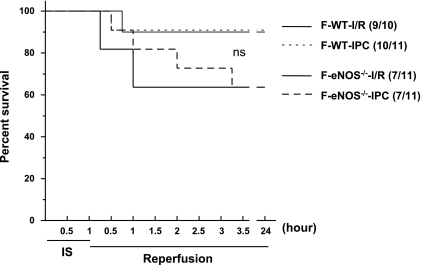
Importantly, 60-min LAD ligation and subsequent reperfusion were associated with increased mortality in eNOS−/− mice compared with WT mice. In WT groups, one mouse with the I/R protocol and one mouse with the IPC protocol died at 45 and 30 min of reperfusion, respectively (Fig. 4). In eNOS−/− groups (Fig. 4), four mice with the I/R protocol died within 15 min to 1 h of reperfusion and four mice with the IPC protocol died within 30 min to 3 h of reperfusion. There were no significant differences in the mortality between female WT and eNOS−/− mice. Thus, the potent infarct-limiting effect of early IPC was also consistently present in female WT mice irrespective of the durations of the ischemic period; however, the IPC effect was lost in female eNOS−/− mice, and there was an increased prevalence of mortality with prolonged ischemia.
Fig. 4.
Survival of young female (F) WT and eNOS−/− mice subjected to 60 min of LAD occlusion and 24 h of reperfusion in the absence or presence of IPC cycles (I/R and IPC, respectively). Numbers in parenthese indicate survival relative to total experimented mice. n = 10–11 mice/group. ns, Not significant.
Does an eNOS Gene Mutation Strategy in Animals Affect IPC?
To answer this question, we repeated the same I/R and IPC protocols in Harvard eNOS−/− mice. The findings were similar to those observed with the Jackson Laboratory eNOS−/− mice (Figs. 1 and 3) in that IPC failed to induce any cardioprotective effects in either male (Fig. 5A) or female (Fig. 5B) eNOS−/− mice. Thus, regardless of the gene mutation strategy, these findings consistently reaffirm that the absence of the eNOS-NO pathway abolishes the benefit of early IPC.
Fig. 5.
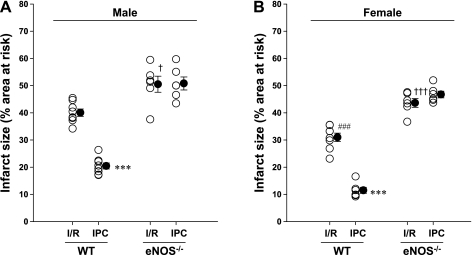
Infarct size (expressed as a percentage of the area at risk) measured in Harvard young male (A) and female (B) WT and eNOS−/− mice subjected to 30 min of LAD occlusion and 24 h of reperfusion in the absence or presence of IPC cycles. ○, Individual values; ●, average values as means ± SE. n = 6–8 mice/group. ***P < 0.001 vs. respective I/R; ###P < 0.001 vs. male WT mice with I/R; †P < 0.05 and †††P < 0.001 vs. respective WT mice with I/R. Deletion of the eNOS gene abolished the protective effects of early IPC against myocardial infarction.
DISCUSSION
The results of the present study demonstrate that in vivo IPC provides acute, robust cardioprotection against I/R injury in mice with constitutive eNOS and that this protection is totally lost when there is no functional eNOS in the heart. The associated key findings are that 1) prolonged ischemia (from 30 to 60 min) caused larger infarct size in WT mice; however, they were effectively protected by IPC; 2) prolonged ischemia and subsequent reperfusion were lethal in eNOS−/− mice with premature mortality; 3) female WT but not eNOS−/− mice exhibited smaller infarct size after I/R than male mice; and 4) with different eNOS gene deletion strategies, similar infarct sizes were seen after I/R and a similar abrogation of the protective effect of IPC occurred. Thus, our findings clearly indicate that constitutive eNOS expression in the murine ventricular myocardium is required for in vivo early IPC regardless of the ischemic duration and sex and that the ablation of functional eNOS abolishes the cardioprotective effect of early IPC.
eNOS and IPC of the Heart
IPC is a well-documented phenomenon that provides robust cardioprotection in all mammalian species tested so far as well as in humans (6, 37, 49). NO has been acknowledged to be a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies (7). Several pharmacological studies (7, 17, 39, 63, 64) have clearly shown that elevating NO, by providing a NO donor or decreasing its scavenging by superoxide, just before or at reperfusion is cardioprotective; however, the role of the eNOS-NO pathway is unclear and highly controversial. While the involvement of the endogenous eNOS-NO pathway has been consistently reported in the in vivo infarct-limiting effect of delayed preconditioning (SWOP) (9, 57), its contribution in the in vivo classical (early) preconditioning is poorly understood. Two groups have reported somewhat conflicting results using mice with genetic deletion of eNOS. In the first study (3) with eNOS−/− mice, in an isolated heart model, it has been reported that the eNOS-NO pathway has a role in mediating early preconditioning; however, this effect depended on the magnitude of the preconditioning stimulus. In the second study (20) with eNOS−/− mice, regardless of the preconditioning stimulus, it was reported that the eNOS-NO pathway is not necessary for the early phase of IPC in vivo.
Our data, obtained from male and female mice with an in vivo model of MI, show that deletion of the eNOS-NO pathway abolishes the infarct-sparing effects of early IPC (Figs. 1 and 3). Of note, regardless of the gene mutation strategy, the benefit of early IPC was lost in the absence of functional eNOS (Fig. 5). Therefore, the findings in different studies with eNOS−/− mice and IPC need careful evaluation. It is possible that the experimental design with IPC or other factors might have caused the observed differences. The use of different IPC protocols and techniques is a major difference between the recent report (20) and our study: 1) we used three 5-min episodes of regional ischemia compared with one or six 4-min episodes of regional ischemia, 2) reperfusion was always 24 h in our study compared with 4 or 24 h, 3) a well-established ligation technique was used in our mice with 7-0 silk suture and polyethylene-10 tubing compared with a balloon occluder, and 4) no volume supplement was needed in our study during reperfusion compared with blood from a donor mouse in the recent report (20). Other differences were 1) a ketamine-xylazine mixture was used in our study compared with pentobarbital, 2) male and female were investigated in two separate groups compared with unknown sexes, and 3) young animals (10–14 wk of age) were used in our study compared with the unknown age in the prior study (20). Thus, based on the well-defined systematic approach used, the present findings clearly demonstrate that early preconditioning does not occur in the absence of eNOS, indicating that the eNOS-NO signaling pathway plays an important role in early preconditioning in both sexes.
Ischemic Duration and IPC
Infarct size measurement is an important end point for the quantification of the cardioprotective effects of drugs or interventions. The duration of coronary artery occlusion required for the development of gross myocardial necrosis is species dependent (40). In the canine or bovine heart, it has been demonstrated that the protective effect of IPC is diminished or lost if the duration of index regional ischemia is sustained for 40 min or longer (28). Myocardial infarct size has been reported to be larger in mice with prolonged LAD occlusions (36), and mice with infarct sizes of 60–80% often die during reperfusion (12). Ischemia has been reported to cause duration-dependent increase in NO generation with both NOS-dependent (65) and NOS-independent sources (66), and the increased NO during early reperfusion may react with ROS to form peroxynitrite and thus result in aggravated postischemic injury (52). Importantly, ROS formed during brief preconditioning pulses can reduce the generation of ROS during subsequent index ischemia and reperfusion (26). However, the effect of prolonged ischemia on the efficacy and related mechanisms of in vivo IPC is largely unknown.
Interestingly, despite significantly the larger infarct size with prolonged LAD ligation (Table 1), IPC effectively reduced infarct size to a similar extent irrespective of the duration of sustained ischemia in both male and female WT mice. Furthermore, with functional eNOS, both WT male and female mice exhibited much higher survival rates than sex-matched eNOS−/− mice. This suggests that functional eNOS prevents myocyte injury caused by cardiac pump failure and secondary death after prolonged ischemic durations. With either ischemic duration, the protective effect of IPC was abrogated by eNOS disruption with increased infarct sizes, and higher mortality was noted with longer ischemic periods. Thus, the decreases in infarct size seen with our in vivo IPC protocol are due to the NOS-dependent cardioprotection triggered by IPC, and this protection persists even with marked prolonged ischemic stress, which would otherwise result in massive infarction and death.
Sex and IPC
Coronary artery disease is the leading cause of death in both men and women. The risk and prevalence of acute MI increase progressively with age, and the levels in females approach those in males after the fifth decade of life (1, 35). Recent animal studies (2, 27, 51) have suggested that there are sex differences in the myocardial response to acute I/R injury, but the underlying mechanisms remain unclear. While the deletion of eNOS raises the blood pressure to a similar magnitude with a concurrent decrease in the heart rate in both male and female eNOS−/− mice (43), the NOS pathway has been shown to play an important role in mediating cardioprotection in females (5, 8). We observed that myocardial infarct sizes were significantly smaller in female WT mice compared with male WT mice, whereas infarct sizes in female eNOS−/− mice were comparable with those of male eNOS−/− mice (Table 1). This suggests that the signaling pathways mediated by myocardial eNOS are largely responsible for the relative resistance to I/R injury seen in female WT mice compared with male WT mice.
There are no prior reports on in vivo myocardial I/R with female eNOS−/− mice; however, one in vitro I/R study (45) has shown significantly reduced postischemic myocardial contractile function in eNOS−/− mice compared with WT mice. This observation was made with 20-min global ischemia and 40-min reperfusion. Using a well-documented in vivo infarction model with different ischemic periods, we consistently observed larger MIs in female eNOS−/− mice than in female WT mice (Fig. 3). Importantly, IPC effectively reduced the infarct size to an identical extent irrespective of sustained ischemia in WT mice but not in eNOS−/− mice (Fig. 3). With prolonged I/R and IPC protocols, there was increased mortality in female eNOS−/− mice compared with female WT mice. Taken together, these findings demonstrate that the eNOS-NO pathway plays an important role in the myocardial protection of female mice against I/R-related injury.
eNOS, Prolonged Ischemia, Sex, and Mortality
The important role of the eNOS-NO signaling pathway in myocardial protection was clearly evident in protocols with prolonged ischemia, where increased mortality was prevalent in eNOS−/− mice compared with WT mice (Figs. 2 and 4). These findings suggest that functional eNOS is critically important not only for IPC but also for the survival of animals in the setting of prolonged myocardial ischemia followed by reperfusion. Increased mortality with chronic MI has been reported in eNOS−/− mice with decreased capillary density and increased myocyte width (38). It is well known that eNOS-derived NO possesses potent vasodilatory, antiplatelet, and anti-inflammatory effects (14, 31, 33), and, importantly, NO selectively reduces the endothelial expression of adhesion molecules and proinflammatory cytokines (10). It has been further reported that increased myocardial deposition of activated platelets plays an important role in myocardial I/R injury and that it is dependent on the duration of the preceding coronary occlusion (56). Interestingly, after I/R and IPC, the survival rate was significantly higher in female eNOS−/− mice (Fig. 4) compared with male eNOS−/− mice (Fig. 2). The sex differences in the mortality also indicate that young males with a severely impaired eNOS-NO pathway might be at the greater risk of mortality than young females when the myocardium is under prolonged ischemic stress. If our observations in mice can be extrapolated to humans, we predict that men with advanced endothelial dysfunction may be at increased risk for experiencing more severe and life-threatening MI than otherwise similar women. Recently, the INTERHEART study (1) on the risk factors for MI in women and men demonstrated that the probability of an acute MI occurring before the age of 60 yr is higher in men than in women. Importantly, the Heart Disease and Stroke Statistics Update of 2010 of the American Heart Association reported that all age mortality in 2006 from MI was higher in males than in females (54% vs. 46%) (30).
Possible Signaling Pathways in NOS-NO-Mediated Cardioprotection
eNOS-derived NO plays a pivotal role in the control of myocardial O2 consumption and modulation of mitochondrial respiration (50). We have previously demonstrated that while permanent deletion of eNOS or NOS inhibition does not alter baseline in vivo myocardial O2 levels and O2 consumption rates from those values in WT control mice, in the postischemic myocardium O2 consumption is decreased, resulting in marked myocardial hyperoxygenation. This is prevented with eNOS deletion or inhibition (60). Importantly, compared with nonpreconditioned WT hearts, IPC prevented in vivo hyperoxygenation in the postischemic myocardium of WT mice with the preservation of mitochondrial O2 consumption (62), and this improved oxidative status with IPC was associated with decreased formation of nitrotyrosine and smaller MI. In contrast, IPC had no significant effect on postischemic myocardial oxygenation in eNOS−/− mice (62). While the large burst of ROS formation that occurs after prolonged ischemia and reperfusion causes myocardial injury (63, 64), a small burst of NO, superoxide, and secondary ROS formation during brief preconditioning episodes can reduce the generation of subsequent high levels of NO and peroxynitrite formation during subsequent sustained ischemia and reperfusion and can act as a survival signal in acute IPC (26, 53, 54). Thus, our previous studies in concert with the present findings demonstrate that the constitutive eNOS-derived NO pathway is required for IPC-induced generation of the critical cardioprotective survival signal for the maintenance of postischemic myocardial O2 consumption after sustained I/R. With regard to the second window of IPC, the cardiac myocyte-specific constitutive expression of iNOS has been shown to protect the heart against I/R injury by decreasing postischemic ROS and by preventing mitochondrial permeability transition pore (MPTP) opening (55). It is worthy to note that iNOS-mediated cyclooxygenase-2 activation has been reported to confer cardioprotection secondary to the arachidonic acid metabolites PGI2 and PGE2 (19, 23, 29). Thus, several mechanisms could account for the cardioprotective effects of NO after acute IPC, and these include the following: 1) downregulation of intraischemic myocardial O2 consumption (62), 2) preservation of mitochondrial respiration and normal levels of myocardial oxygenation upon reperfusion (62), 3) suppression of the high levels of NO and peroxynitrite formation upon reperfusion (26, 50, 53, 60, 62), 4) activation of mitochondrial ATP-sensitive K+ channels (6); 5) inhibition of the MPTP (55), and 6) inhibition of cell death pathways (39). Importantly, regardless of the window (early or delayed) of protection, cardioprotective mechanisms of NO seem to converge on the mitochondrial targets leading to reduced apoptosis and necrosis.
Conclusions
In conclusion, we performed a systematic evaluation of IPC in age-matched animals with reference to ischemic duration and sex and demonstrated that constitutive eNOS expression in the LV myocardium is required for the in vivo cardioprotective effects of the early phase of IPC. This is in agreement with the previous observation (11) that eNOS-overexpressing mouse hearts resist I/R injury as well as a recent report (46) in pigs showing that cardioselective eNOS gene transfer also protects the heart against I/R injury. The cardioprotective role of eNOS in the present study further reaffirms the importance of the eNOS-mediated signaling pathway in the process of early IPC-induced cardioprotection regardless of sex even after severe ischemic stress. These results suggest that pharmacological preconditioning based on the enhancement and modulation of eNOS function and NO production before the onset of ischemia may be highly effective in conferring myocardial protection and minimizing related morbidity and mortality.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-63744, HL-65608, and HL-38324 (to J. L. Zweier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of F. Yang: The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi 530021, People's Republic of China.
REFERENCES
- 1.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J 29: 932–940, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bae S, Zhang L. Gender differences in cardioprotection against ischemia/reperfusion injury in adult rat hearts: focus on Akt and protein kinase C signaling. J Pharmacol Exp Ther 315: 1125–1135, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bell RM, Yellon DM. The contribution of endothelial nitric oxide synthase to early ischaemic preconditioning: the lowering of the preconditioning threshold. An investigation in eNOS knockout mice. Cardiovasc Res 52: 74–280, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bell R, Yellon DM. Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol 35: 185–193, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Petranka J, Yamamura K, London RE, Steenbergen C, Murphy E. Gender differences in sarcoplasmic reticulum calcium loading after isoproterenol. Am J Physiol Heart Circ Physiol 285: H2657–H2662, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Annu Rev Physiol 62: 79–109, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res 70: 231–239, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Cross HR, Murphy E, Steenbergen C. Ca2+ loading and adrenergic stimulation reveal male/female differences in susceptibility to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 283: H481–H489, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann NY Acad Sci 962: 18–41, 2002 [DOI] [PubMed] [Google Scholar]
- 10.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1: 60–68, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Toit EF, Genade S, Carlini S, Moolman JA, Brunner F, Lochner A. Efficacy of ischemic preconditioning in the eNOS overexpressed working mouse heart model. Eur J Pharmacol 556: 115–120, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Eckle T, Grenz A, Köhler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systemic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol 291: H2533–H2540, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Flogel U, Decking UK, Godecke A, Schrader J. Contribution of NO to ischemia-reperfusion injury in the saline-perfused heart: a study in endothelial NO synthase knockout mice. J Mol Cell Cardiol 31: 827–836, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–18, 1989 [PubMed] [Google Scholar]
- 15.Godecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Godecke S, Schrader J. Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res 82: 186–194, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Gonon AT, Erbas D, Broijersen A, Valen G, Pernow J. Nitric oxide mediates protective effect of endothelin receptor antagonism during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 286: H1767–H1774, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Gourine AV, Bulhak AA, Gonon AT, Pernow J, Sjöquist PO. Cardioprotective effect induced by brief exposure to nitric oxide before myocardial ischemia-reperfusion in vivo. Nitric Oxide 7: 210–216, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Gregg AR, Schauer A, Shi O, Liu Z, Lee CG, O'Brien WE. Limb reduction defects in endothelial nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol 275: H2319–H2324, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res 68: 18–25, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Li Q, Wu WJ, Tan W, Zhu X, Mu J, Bolli R. Endothelial nitric oxide synthase is not necessary for the early phase of ischemic preconditioning in the mouse. J Mol Cell Cardiol 44: 496–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hannan RL, John MC, Kouretas PC, Hack BD, MAtherne GP, Laubach VE. Deletion of endothelial nitric oxide synthase exacerbates myocardial stunning in an isolated mouse heart model. J Surg Res 93: 127–132, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377: 239–242, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Inserte J, Molla B, Aguilar R, Través PG, Barba I, Martín-Sanz P, Boscá L, Casado M, Garcia-Dorado D. Constitutive COX-2 activity in cardiomyocytes confers permanent cardioprotection. Constitutive COX-2 expression and cardioprotection. J Mol Cell Cardiol 46: 160–168, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd'Heuil D, Huang PL, Lefer DJ. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol Heart Circ Physiol 276: H1567–H1573, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 286: H276–H282, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kevin LG, Camara AKS, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol 284: H566–H574, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kher A, Wang M, Tasi BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko M, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock 23: 1–10, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Kim DJ, Kim H, Park JI, Shim TS, Rah BJ, Kim HD. Relation between ischemic preconditioning and the duration of sustained ischemia. J Korean Med Sci 10: 121–131, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, Dawn B, Hunt G, Cui C, Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-κB-dependent pathway. Circulation 116: 1577–1584, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lioyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function. Ten years after, and continuing. Circ Res 93: 388–398, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991 [PubMed] [Google Scholar]
- 33.Mungrue IN, Husain M, Stewart DJ. The role of NOS in heart failure: lessons from murine genetic models. Heart Fail Rev 7: 407–422, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Reddy KS. Cardiovascular disease in non-western countries. N Engl J Med 350: 2438–2440, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Redel A, Jazbutyte V, Smul TM, Lange M, Eckle T, Elitzschig H, Roewer N, Kehl F. Impact of ischemia and reperfusion times on myocardial infarct size in mice in vivo. Exp Biol Med 233: 84–93, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rezkalla SH, Kloner RA. Preconditioning in humans. Heart Fail Rev 12: 201–206, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz T, Lindsey ML, Vancon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation 104: 1286–1291, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res 61: 402–413, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Schwarz ER, Somoano Y, Hale SL, Kloner RA. What is the required period for assessment of myocardial infarct size using triphenyltetrazolium chloride staining in the rat? J Thromb Thrombolysis 10: 181–187, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Shah AM. Inducible nitric oxide synthase and cardiovascular disease. Cardiovasc Res 45: 148–155, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in eNOS-deficient mice. Am J Physiol Heart Circ Physiol 282: H2422–H2426, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA 93: 13176–13181, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumeray MS, Rees DD, Yellon DM. Infarct size and nitric oxide synthase in murine myocardium. J Mol Cell Cardiol 32: 35–42, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Picht E, Ginsburg KS, Bers DM, Steenbergen C, Murphy E. Hypercontractile female hearts exhibit increased S-nitrosylation of the L-type Ca2+ channel α1 subunit and reduced ischemia/reperfusion injury. Circ Res 98: 403–411, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Szelid Z, Pokreisz P, Liu X, Vermeersch P, Marsboom G, Gillijins H, Pellens M, Verbeken E, Van de Werf F, Collen D, Janssens SP. Cardioselective nitric oxide synthase 3 gene transfer protects against myocardial reperfusion injury. Basic Res Cardiol 105: 169–179, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Talukder MA, Fujiki T, Morikawa K, Motoishi M, Matsuo Y, Hatanaka M, Tsutsui M, Takeshita A, Shimokawa H. Endothelial nitric oxide synthase-independent effects of an ACE inhibitor on coronary flow response to bradykinin in aged mice. J Cardiovasc Pharmacol 44: 557–563, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Talukder MA, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ transport properties improves postischemic cardiac function and Ca2+ handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol 293: H2418–H2428, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Tomai F, Crea F, Chiariello L, Gioffre PA. Ischemic preconditioning in humans: models, mediators, and clinical relevance. Circulation 100: 559–563, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Trochu JN, Bouhour JB, Kaley G, Hintze TH. Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism. Implications in health and disease. Circ Res 87: 1108–1117, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 288: E321–E326, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Wang P, Zweier JL. Measurement of nitric oxide and peroxynitrite generation in the postischemic heart. J Biol Chem 271: 29223–29230, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Wang P, Zweier JL. Ischemic preconditioning decreases nitric oxide (NO) formation and NO mediated injury in the postischemic heart (Abstract). Circulation 96: 8, I-72, 1997 [Google Scholar]
- 54.Wei G, Zweier JL. Endothelial nitric oxide synthase is essential for acute ischemic preconditioning (Abstract). FASEB J 15: 4, A465, 2001 [Google Scholar]
- 55.West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, Keith RJ, Schrader J, Guo Y, Conklin DJ, Prabhu SD, Zweier JL, Bolli R, Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 44: 1970–1978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y, Huo Y, Toufektsian MC, Ramos SI, Ma Y, Tejani AD, French BA, Yang Z. Activated platelets contribuye importantly to myocardial reperfusion injury. Am J Physiol Heart Circ Physiol 290: H692–H699, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase-pSer-signal transducers and activators of transcription 1/3 pathway. Circulation 116: 535–544, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamakuchi M, Greer JJM, Cameron SJ, Matsushita K, Morrell CN, Talbot-Fox K, Baldwinn WM, 3rd, Lefer DJ, Lowenstein CJ. HMG-CoA reductase inhibitors inhibit endothelial exocytosis and decrease myocardial infarct size. Circ Res 96: 1185–1192, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang XP, Liu YH, Sheseley EG, Bulagannawar M, Liu F, Carretero OA. Endothelial nitric oxide gene knockout mice. Cardiac phenotypes and the effects of angiotensin-converting enzyme inhibitor on myocardial ischemia-reperfusion injury. Hypertension 34: 24–30, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation 111: 2966–2972, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Zhao X, Chen YR, He G, Zhang A, Druhan LJ, Strauch AR, Zweier JL. Endothelial nitric oxide synthase (NOS3) knockout decreases NOS2 induction, limiting hyperoxygenation and conferring protection in the postischemic heart. Am J Physiol Heart Circ Physiol 292: H1541–H1550, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Zhu X, Liu B, Zhou S, Chen YR, Deng Y, Zweier JL, He G. Ischemic preconditioning prevents in vivo hyperoxygenation in postischemic myocardium with preservation of mitochondrial oxygen consumption. Am J Physiol Heart Circ Physiol 293: H1442–H1450, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Zweier JL. Measurement of superoxide-derived free radicals in the reperfused heart. J Biol Chem 263: 1353–1357, 1988 [PubMed] [Google Scholar]
- 64.Zweier JL, Talukder MAH. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res 70:181–190, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Zweier JL, Wang P, Kuppusamy P. Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J Biol Chem 270: 304–307, 1995 [DOI] [PubMed] [Google Scholar]
- 66.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995 [DOI] [PubMed] [Google Scholar]