Abstract
Acute myocardial dysfunction is a typical manifestation of septic shock. Experimentally, the administration of endotoxin [lipopolysacharride (LPS)] to laboratory animals is frequently used to study such dysfunction. However, a majority of studies used load-dependent indexes of cardiac function [including ejection fraction (EF) and maximal systolic pressure increment (dP/dtmax)], which do not directly explore cardiac inotropism. Therefore, we evaluated the direct effects of LPS on myocardial contractility, using left ventricular (LV) pressure-volume catheters in mice. Male BALB/c mice received an intraperitoneal injection of E. coli LPS (1, 5, 10, or 20 mg/kg). After 2, 6, or 20 h, cardiac function was analyzed in anesthetized, mechanically ventilated mice. All doses of LPS induced a significant drop in LV stroke volume and a trend toward reduced cardiac output after 6 h. Concomitantly, there was a significant decrease of LV preload (LV end-diastolic volume), with no apparent change in LV afterload (evaluated by effective arterial elastance and systemic vascular resistance). Load-dependent indexes of LV function were markedly reduced at 6 h, including EF, stroke work, and dP/dtmax. In contrast, there was no reduction of load-independent indexes of LV contractility, including end-systolic elastance (ejection phase measure of contractility) and the ratio dP/dtmax/end-diastolic volume (isovolumic phase measure of contractility), the latter showing instead a significant increase after 6 h. All changes were transient, returning to baseline values after 20 h. Therefore, the alterations of cardiac function induced by LPS are entirely due to altered loading conditions, but not to reduced contractility, which may instead be slightly increased.
Keywords: cardiac function, contractility, mice
septic shock is a major complication of infectious processes, characterized by systemic inflammation and cardiovascular collapse. The profound disturbances of cardiocirculatory homeostasis in sepsis affect all of the components of the circulation, including the vascular smooth muscle, the endothelium, as well as the myocardium (22). Impaired cardiac function due to reduced myocardial contractility is indeed a typical manifestation of septic shock (septic cardiomyopathy), whose underlying physiopathology involves a host of potential mechanisms, as recently reviewed (15, 30, 45). Circulating factors, including cytokines (TNF-α, IL-1β, and IL-6), lysozyme C and endothelin-1, as well as high-level nitric oxide (NO) production, consecutive to the expression of inducible NO synthase in the myocardium, can directly inhibit myocyte contractility. Similarly, disturbed intracellular calcium trafficking, with a reduction in systolic intracellular calcium concentration, can impair cardiac function in sepsis. Further potential mechanisms include alterations of myocardial microvascular blood flow, mitochondrial abnormalities, and autonomic dysfunction (15, 30, 45).
Experimentally, the administration of endotoxin [lipopolysacharride (LPS)] to laboratory animals, especially rodents, has been widely used to study the mechanisms of septic cardiomyopathy (34). However, most in vivo studies evaluating the effects of LPS on the heart have relied on load-dependent indexes of cardiac function, including the calculation of the maximal rate of rise of left ventricular (LV) pressure (dP/dtmax) by micromanometers in the LV (53), and the determination of the LV ejection fraction (EF) or fractional shortening by echocardiography (5, 54, 58). Since LPS may alter not only cardiac contractility, but also cardiac loading conditions (12, 26, 43), the use of such indexes may be insufficient to properly assess the direct effects of LPS on the intrinsic performance of the myocardium. It has notably been reported in a large-animal model (dog) that the LPS-induced decrease of LV dP/dtmax was related to decreased preload, whereas cardiac contractility, determined by end-systolic elastance (Ees) obtained from LV pressure-volume (PV) curves, was not reduced, probably through a reflex increase of sympathetic tone (19, 40). Therefore, it appears essential that both LV loading conditions and LV inotropism be assessed when exploring the influence of LPS on cardiac pump function. Miniaturized PV conductance catheters have been developed to quantify LV volumes and load-independent indexes of contractility in mice (37, 38) and thus may represent invaluable tools to study the hemodynamic consequences of LPS in this species. Therefore, the present study was designed to investigate in detail the effects of LPS, at different doses and different time points, on cardiac performance, by separating between indirect effects related to altered loading conditions, and direct effects on LV contractility, using such catheters.
MATERIALS AND METHODS
All procedures complied with the Swiss laws on animal experimentation and were performed with the approval of the local Institutional Animal Care and Use Committee. Adult male BALB/c mice (23–26 g) were used in this study. The animals were housed in cages (4–5 animals/cage), kept in a room maintained at 23 ± 1°C, 55 ± 5% humidity, with a 12:12-h light-dark cycle, and fed ad libitum.
Experimental protocol and hemodynamic measurements.
A total of 70 mice were used in this study. The animals were challenged with an intraperitoneal injection of E. coli LPS (serotype O127:B8) at doses of 1, 5, 10, or 20 mg/kg, dissolved in 200 μl isotonic saline. A group of mice not injected with LPS was used to determine baseline hemodynamic values for control purposes. Mice were then returned to their cages, and after 2, 6, or 20 h, selected groups of mice (n = 5–6 mice/group) were anesthetized with intraperitoneal injections of ketamine (80 mg/kg) and xylazine (10 mg/kg), tracheotomized, and ventilated (mouse ventilator model 687, Harvard Instruments, Holiston, MA) with a tidal volume of 200 μl at 120 strokes/min. The right carotid artery was exposed by blunt dissection. The artery was cannulated with a microtip 1.4-F PV catheter (PVR-1045; Millar Instruments, Houston, TX), taking great care to avoid any bleeding. The catheter was advanced under pressure control into the LV.
After stabilization for 15 min, the pressure and volume signals were continuously recorded with an MPVS-300 PV conductance system (Millar Instruments), coupled with a Powerlab/16SP A/D converter (AD Instruments), stored and displayed on a personal computer. Heart rate and LV systolic pressure (LVSP) and LV end-diastolic pressures (LVEDP) and volumes (LVESV, LVEDV) were measured, and stroke volume (SV), stroke work (SW), EF, and cardiac output (CO) were calculated and corrected, according to in vitro and in vivo volume calibrations with a cardiac PV analysis program (PVAN3.2; Millar Instruments), as detailed previously (37, 38). All volumes were also corrected, according to LV parallel conductance, determined by injecting, at the end of each experiment, a 15-μl bolus of 15% hypertonic saline into the right ventricle via the right jugular vein (38).
Total effective arterial elastance (Ea), an integrated index of arterial load that is sensitive to resistive and pulsatile load, was calculated as the ratio of LV end-systolic pressure (ESP) and SV and was used as an index of LV afterload (7). We also calculated the ratio of CO to LVSP as an index of systemic vascular resistance (SVR) and LV afterload. The maximum rate of LV pressure increment during isovolumetric contraction (dP/dtmax) was computed from the pressure signal. The inotropic state of the LV was determined by two different, load-independent indexes of contractility. First, the ratio of dP/dtmax divided by end-diastolic volume (Ved), dP/dtmax/Ved, was computed as an isovolumic phase measure of contractility. To determine whether this ratio could have been influenced by preload in the conditions of our study, we determined (in 5 control mice and 20 endotoxemic mice) the potential impact of acute changes in preload during transient inferior vena cava occlusion on the values of dP/dtmax/Ved. Second, the slope of the end-systolic PV relationships (ESPVR) of successive PV loops at rapidly reduced preload (obtained by transiently reducing venous return by compressing the inferior vena cava), was computed as an ejection phase measure of LV contractility (Ees) (17, 33, 48). The zero-pressure intercept of the ESPVR, V0, was used as a correction term, according to the definition: Ees = ESP/(ESV − V0), with ESV representing end-systolic volume. Since we noticed that endotoxemia resulted in concomitant changes of both Ees and V0, we used a further index of contractility by determining, in each mouse, the maximal ESP (ESPmax) reached at a given operating ESV. The values of ESP were then compared with those obtained at matched ESV in control mice and were expressed as percentage of controls.
Statistical analysis.
Results are expressed as means ± SE of n =5–6 mice per group. At each time point (2, 6 and 20 h), statistical analysis was performed by analysis of variance, and post hoc Dunnett test was used to compare the different groups of LPS challenged with the control group. The Dunnett test was only carried out when the overall F value was significant. This conservative approach was adopted in view of the large number of experimental conditions tested. Statistical significance was ascribed to P < 0.05.
RESULTS
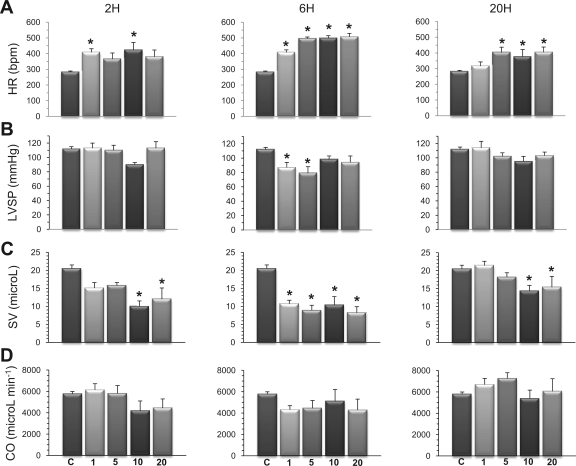
As illustrated in Fig. 1A, all doses of LPS induced a significant increase in HR, mainly after 6 h, which persisted until 20 h, except at the lowest LPS dose of 1 mg/kg. LVSP (Fig. 1B) was maintained, except at 1 and 5 mg/kg at 6 h, where LVSP showed a significant reduction compared with baseline conditions. SV (Fig. 1C) was significantly smaller than in controls, especially after 6 h at all doses of LPS. This alteration persisted after 20 h at LPS doses of 10 and 20 mg/kg. CO (Fig. 1D) was not significantly different at all doses and at all times studied, although it tended to decrease at the 6-h time point.
Fig. 1.
Influence of endotoxin on major hemodynamic variables. Separate groups of mice were challenged with LPS at 1, 5, 10, or 20 mg/kg, and their hemodynamic status was compared with that of a control (C) group of mice after 2, 6, or 20 h. A: all doses of LPS induced a significant increase in heart rate (HR), especially after 6 h. bpm, Beats/min. B: left ventricular (LV) systolic pressure (LVSP) was maintained throughout the experiments, except from significant decreases after 6 h at the doses of 1 and 5 mg/kg. C: stroke volume (SV) decreased significantly after 6 h. D: cardiac output (CO) was maintained, although it tended to decrease with all doses of LPS after 6 h. Values are means ± SE. *P < 0.05 vs. control (ANOVA followed by Dunnett test).
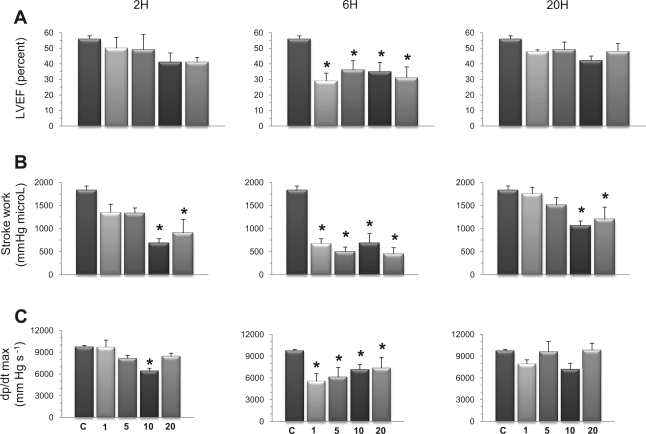
Three load-dependent indexes of LV function were assessed, including LV EF (Fig. 2A), LV SW (Fig. 2B), and dP/dtmax (Fig. 2C). All of these indexes disclosed marked and significant reductions after 6 h at all doses of LPS tested. After 20 h, these changes had mostly resolved, except from SW, which was still significantly depressed in mice challenged with 10 and 20 mg/kg LPS.
Fig. 2.
Endotoxin reduces load-dependent indexes of LV function. Mice receiving an intraperitoneal injection of LPS (1, 5, 10, or 20 mg/kg) were followed for 2, 6, or 20 h. A: LV ejection fraction (LVEF). B: stroke work (SW). C: maximal rate of rise of LV pressure (dP/dtmax). Compared with control mice, all doses of LPS induced significant decreases of EF, SW, and dP/dtmax, mainly after 6 h, with return to baseline values after 20 h, except from SW, which remained decreased at 10 and 20 mg/kg LPS. Values are means ± SE. *P < 0.05 vs. control (ANOVA followed by Dunnett test).
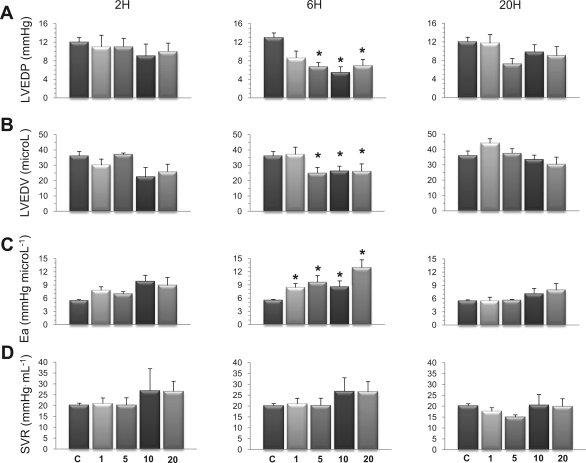
LV loading conditions were evaluated by the determination of LVEDP and LVEDV, both indicators of LV preload, and by the computation of effective Ea and systemic vascular index, both indexes of LV afterload. There was a considerable reduction of LV preload after 6 h at 5, 10, and 20 mg/kg LPS, as indicated by significant decreases of both LVEDP (Fig. 3A) and LVEDV (Fig. 3B). With respect to Ea, a significant increase was noted after 6 h at all LPS doses (Fig. 3C), which may simply be explained by the marked tachycardia induced by endotoxin. Indeed, if we consider that ESP approximates mean arterial pressure, then, ESP = SVR × CO = SVR × (HR × SV), and thus Ea = (ESP/SV) = SVR × HR, implying that, for any given resistance, Ea is directly proportional to HR. Therefore, we calculated SVR by the ratio CO/LVSP as another index of LV afterload. As shown in Fig. 3D, SVR did not show any significant variations among the experimental groups.
Fig. 3.
Effects of endotoxin on the loading conditions of the LV. Indexes of LV preload and afterload were evaluated 2, 6, and 20 h in mice challenged with LPS at 1, 5, 10, or 20 mg/kg. After 6 h, LPS (5 mg/kg and above) induced marked reduction of LV preload, evidenced by significant decreases of LV end-diastolic pressure (LVEDP; A) and volume (LVEDV; B). LPS also significantly increased effective arterial elastance (Ea; C) after 6 h, whereas systemic vascular resistance (SVR; D) remained not statistically different from control mice at all time points. Values are means ± SE. *P < 0.05 vs. control (ANOVA followed by Dunnett test).
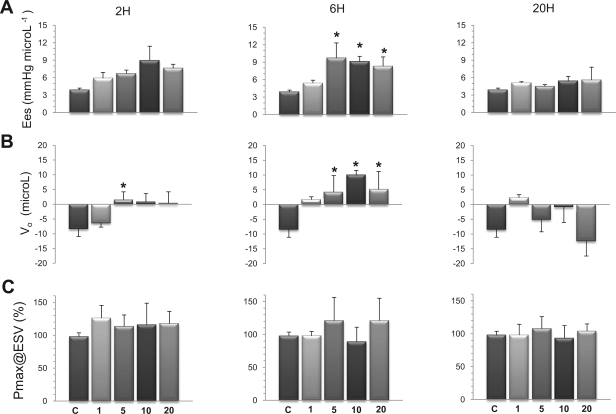
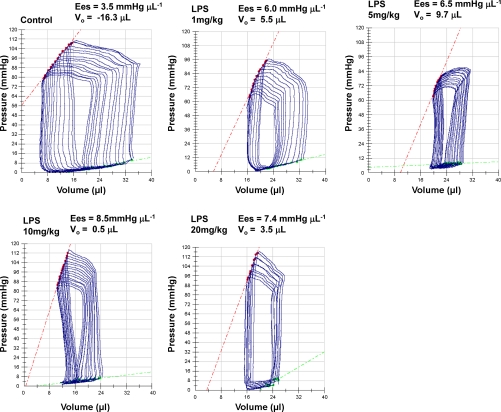
To assess LV inotropism, two load-independent indexes of LV contractility were computed. First, Ees, the slope of the ESPVR, obtained by rapid and transient reduction of preload, which gives information onto LV contractility during the ejection phase of the cardiac cycle, and second, dP/dtmax/Ved, which provides a measurement of LV contractility during the isovolumic contraction phase of the cardiac cycle. As illustrated in Fig. 4A, Ees disclosed significant increases compared with control conditions after 6 h at all doses of LPS, except the smallest one (1 mg/kg). The individual values of Ees (in mmHg/μl) ranged from 3.4 to 4.5 (control), 3.5 to 6.1 (LPS 1), 3.9 to 16.5 (LPS 5), 6.8 to 11.7 (LPS 10), and 5.3 to 13.4 (LPS 20). Importantly, this increase in Ees was associated with a significant shift to the right of the PV curves, as indicated by significant increases of V0 (Fig. 4B). Values of V0 (in μl) were widely distributed among the different groups, as indicated by individual values ranging from −16.3 to −2.5 (control), −11.3 to −7.5 (LPS 1), −13.5 to +16.3 (LPS 5), 0.5 to 13.7 (LPS 10), and −11.3 to 17.9 (LPS 20). Since the increases in both Ees and V0 have opposite effects on ventricular contractility, we determined the ESPmax, at a given operating ESV, as a further index of LV contractility. Values of ESP in endotoxemic mice were expressed as a percentage of values obtained in control mice at matched ESV. A limited number of mice challenged with LPS (n = 5/60) could not be included in this analysis, since their operating range of ESV fell out from the range of ESV in control mice. Using this index, we found no significant differences between the values of ESP measured in control and endotoxemic mice, independently from the dose of LPS and the duration of endotoxemia (Fig. 4C). Representative PV loops of one control mouse and of mice treated with 1, 5, 10, or 20 mg/kg for 6 h, with their respective ESPVRs, are shown in Fig. 5.
Fig. 4.
Effects of endotoxin on the end-systolic pressure-volume (PV) relationship of the LV. End-systolic PV relationships were evaluated after 2, 6, and 20 h in mice challenged with LPS at 1, 5, 10, or 20 mg/kg. A: end-systolic elastance (Ees) significantly increased after 6 h at 5 mg/kg LPS and above. B: the zero-pressure intercept of the LV PV relationship (V0) significantly augmented at all doses of LPS after 6 h. C: maximal end-systolic pressure (Pmax) reached at a defined end-systolic volume (ESV), expressed in percentage of values obtained in control mice at matched ESV, was not significantly influenced by LPS. Values are means ± SE. *P < 0.05 vs. control (ANOVA followed by Dunnett test).
Fig. 5.
Representative PV loops in control and endotoxemic mice. Examples are of PV loops obtained in one control mouse and in mice challenged with 1, 5, 10, or 20 mg/kg LPS. The values of Ees and V0 are indicated in each panel. Ees is the slope of the end-systolic PV relationship, indicated by the red dotted lines.
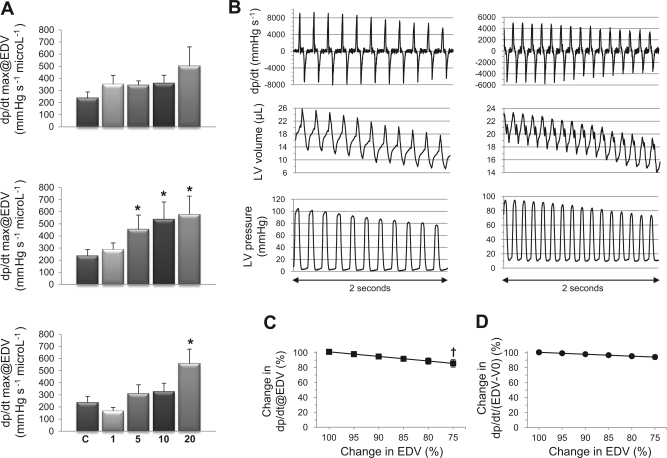
Besides Ees, we also noticed significant increases of dP/dtmax/Ved after 6 h in mice treated with 5, 10, and 20 mg/kg LPS (Fig. 6A). To evaluate whether such increase might have been promoted by the marked reduction of preload in endotoxemic mice, we determined the time course of dP/dtmax/Ved in conditions of rapidly changing preload (in a subset of n = 25 mice). As shown in Fig. 6, B and C, the reduction of Ved (inferior vena cava occlusion) was associated with a concomitant reduction of dP/dtmax, resulting in a slight decrease of the ratio dP/dtmax/Ved (by ∼14% for a 25% decrease of Ved). However, such reduction was not observed when dP/dtmax was normalized by the preload term (Ved − V0) instead of Ved (Fig. 6D). Indeed, if we consider the time-varying elastance, E(t), the ventricular pressure P can be expressed as:
so that
and
where (dE/dt)max is the maximum rate of rise of time-varying elastance during systole. Normalization with Ved implies therefore that:
In conditions of rapidly reduced preload, the progressive reduction of Ved implies that the term (Ved − V0/Ved) becomes smaller. Therefore, even though (dE/dt)max remains unchanged, (dP/dt)max/Ved decreases with preload reduction. To take this point into account, (dP/dt)max was normalized by the term (Ved − V0). As shown in Fig. 6D, the ratio (dP/dt)max/(Ved − V0) remained largely unaffected by preload reduction. These considerations imply, therefore, that the increased values of dP/dtmax/Ved in LPS mice could not be an artifact related to reduced preload. The observation that the contractility index ESPmax/ESV remained constant, while the index dP/dtmax/Ved increased, may be explained by the significant tachycardia triggered by endotoxin. Indeed, in extreme tachycardia, the time (Tmax) to reach end-systole and maximal elastance (Emax) is shortened, whereas the slope of the ESPVR remains largely unaffected (29). Furthermore, it has been shown that the shapes of different curves of time-varying elastance E(t) in different contractile states, if normalized with respect to their respective Emax and Tmax, were reduced to a single curve, En, totally unaffected by the contractile state, loading conditions, or heart rate (47, 50). This normalized En can thus be described as:
and thus,
and
Owing to the constant value of En, (dEn/dt)max is also constant, implying that (dE/dt)max entirely depends on the ratio Emax/Tmax. Since marked tachycardia results in a shortened Tmax with almost no change in Emax, (dE/dt)max also increases. This may explain why dP/dtmax/Ved increased, whereas the ESPVR-related index, ESPmax/ESV did not increase.
Fig. 6.
Effects of LPS on the ratio of dP/dtmax to end-diastolic volume (EDV). Mice received LPS at 1, 5, 10, or 20 mg/kg, and hemodynamic measurements were made after 2, 6, or 20 h. A: maximal pressure increment in the LV during systole (dP/dtmax) divided by EDV (dP/dtmax@EDV) significantly increased at 5, 10, and 20 mg/kg LPS after 6 h compared with control conditions. Top: 2 h; middle: 6 h; bottom, 20 h. B: time course of dP/dt, LV volume, and LV pressure at rapidly decreasing preload in two representative mice (left and right). C and D: influence of preload on dP/dtmax/EDV (C), and on dP/dtmax/EDV-V0 (D), determined during rapid reduction of EDV. Whereas dP/dtmax/EDV slightly decreased with reductions of EDV, the ratio dP/dtmax/EDV-V0 remained unchanged. Values are means ± SE. *P < 0.05 vs. control; †P < 0.05 vs. baseline (ANOVA followed by Dunnett test).
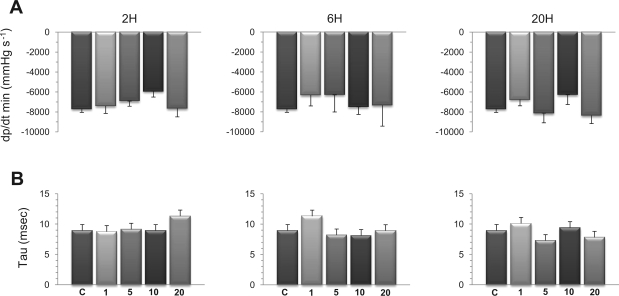
The changes of Ees and dP/dtmax/Ved noted at 6 h were transient, as indicated by their return to baseline values after 20 h, except in mice challenged with the highest dose of LPS (20 mg/kg), in which dP/dtmax/Ved, remained elevated at 20 h. Indexes of diastolic function, including maximal rate of pressure decrease during isovolumic relaxation and the time constant of isovolumic pressure fall during ventricular relaxation are shown in Fig. 7, A and B, respectively. Both indexes did not show any significant variations among the different experimental groups.
Fig. 7.
Effects of LPS on indexes of diastolic function of the LV. Hemodynamic exploration was performed after 2, 6, or 20 h in mice challenged with LPS at 1, 5, 10, or 20 mg/kg. A: maximal rate of pressure decrease during isovolumic relaxation (dP/dtmin). B: Time constant (τ) of isovolumic pressure fall during ventricular relaxation. No statistically significant variations were noted for both indexes. Values are means ± SE.
DISCUSSION
Myocardial contractile failure (“septic cardiomyopathy”) occurs in ∼50% of patients with septic shock and, in its most severe form, is associated with a poor prognosis. Animal models of acute endotoxemia, notably rodents, have been frequently used to study septic cardiomyopathy, given the ability of endotoxin to reproduce many aspects of clinical sepsis. Hemodynamically, acute endotoxemia in rodents is generally characterized by decreased blood pressure with unchanged (normodynamic) or reduced (hypodynamic) CO, together with increased SVR (25, 31, 44, 46, 57). Such pattern resembles the early (unresuscitated) phase of clinical septic shock, but contrasts with the typical hyperdynamic pattern, which develops later in the course of sepsis, following volume resuscitation.
In the present study, mice were challenged with different doses of LPS in the milligram per kilogram range, which are the usual doses used in this species. Mice remained normodynamic, in agreement with prior studies (12, 35, 44, 57), but developed profound reductions of EF, dP/dtmax, and SW 6 h after the injection of endotoxin. These changes had mostly resolved after 20 h, consistent with the transient nature of cardiovascular dysfunction reported both in human and experimental septic shock (21, 32, 39). Such reductions of EF, dP/dt, and SW are typical findings in this model of murine endotoxemia and are usually interpreted as the result of direct negative inotropic effects of endotoxin. It must be underscored, however, that these hemodynamic variables are also heavily influenced by LV loading conditions. During endotoxemia and in the initial stage of sepsis, cardiac preload is reduced secondary to fluid losses and relative hypovolemia due to increased venous capacitance, resulting in a marked decrease of vascular stressed volume. As a result, both fluid losses and reduced stressed volume concur to impair venous return and cardiac filling by decreasing the mean systemic filling pressure, a consequence further aggravated by the reduction of diastolic time due to concomitant tachycardia (12, 26, 43). This makes the basis for the administration of large amounts of fluids in the early phase of sepsis, to restore adequate cardiac filling, CO, and blood pressure (12, 26, 43). Such preload reduction was obvious in our study, as indicated by the significant decrease of LVEDV, 6 h after endotoxin, which is in agreement with similar observations previously made in rodent endotoxemia (12, 24, 57, 58). It must be underscored that animals were given only a limited amount of fluid by intraperitoneal route (∼20 ml/kg), which is not different from the general use in this murine model of endotoxemia (14).
Besides preload, afterload can also be significantly influenced under endotoxic or septic conditions. Reduced SVR due to systemic vasodilation is typically observed in the advanced stages of sepsis (22). This pattern occurs only after the administration of large amounts of fluids (8, 42) and contrasts with the increased vascular resistance that prevails before volume resuscitation, as in the early phases of sepsis and in a majority of endotoxemia models in rodents (3, 12, 14, 18, 34, 44). Our data indicate that endotoxin elicited a significant increase in effective Ea, an integrated index of arterial load that is sensitive to resistive and pulsatile load (7). However, as stated earlier, such rise of Ea may simply reflect the marked tachycardia induced by endotoxin and then does not necessarily indicate that LV afterload was increased. In fact, LV afterload was probably not influenced by endotoxin in the conditions of our study, as indicated by the lack of significant variation of SVR.
The marked reduction of LV preload after LPS does not allow the interpretation that the decreased EF, dP/dtmax, and SW reflect reduced LV contractility. We, therefore, determined the slope of ESPVR, or Ees, which represents an ejection phase measure of LV contractility, as well as the rise of LV pressure during isovolumic systole divided by Ved, dP/dtmax/Ved, which is an isovolumic phase measure of contractility, these two indexes being considered insensitive to altered loading conditions (17, 33, 48). Unexpectedly, we found that both disclosed significant increases after 6 h, indicating that endotoxin did not impair LV contractility and may have, instead, promoted an increase in LV contractile function. Considering first the increase of dP/dtmax/Ved, we ruled out the possibility of an artifact due to the reduction of preload induced by LPS, by showing that this ratio did not increase, but instead tended to decrease, when preload was reduced. Second, with specific respect to Ees, several difficulties of interpretation are illustrated by our data, which need to be further discussed.
Our computation of Ees was based on the concept of linear elastance developed by Suga and Sagawa (49). To be valid, this concept requires, first, that the shape of the isochronous lines connecting data acquired at the same time instant after the onset of systole, notably the ESPVR, be linear. This condition appears not fulfilled in the mouse heart, in which the PV isochrones disclose a curvilinear, but not linear, shape, as recently demonstrated by Claessens et al. (9). Second, the linear elastance concept requires that V0, the zero pressure intercept of the end-systolic PV relationship, remain constant (9, 23). This was clearly not the case in our study, as indicated by a significant shift to the right of V0 in endotoxemic mice, especially at the 6-h time point. Such an increase in V0 points to a reduced contractility, which, therefore, opposes the enhanced contractile function that would be anticipated from the increase of Ees (23). To understand the net effect of concomitant changes of Ees and V0, we, therefore, determined the ESPmax obtained at a given operating ESV in each mouse, and the values were expressed as a percentage of the ESP obtained at matched ESV in control mice. Using this method, we found no significant differences between control and LPS-treated mice, implying that the opposite influences of the increased Ees and increased V0 canceled out each other, so that the final outcome was an unchanged contractile function. Taking these limitations into account, our findings indicate that, in the conditions of our study, the overall effect of LPS on cardiac contractility was either neutral or slightly positive (as suggested by the increased values of dP/dtmax/Ved). It is plausible that contractility was maintained through intense reflex sympathetic activation in response to endotoxemia, as suggested by previous investigators (40, 51). Furthermore, it is worth noting that the increased heart rate per se may have contributed to maintain LV inotropism, given the known positive influence of heart rate on LV performance (13). As exposed earlier, it is also worth mentioning that the important tachycardia induced by LPS may explain why the index dP/dtmax/Ved increased, whereas the ESPVR-related index ESPmax/ESV did not.
Our conclusions may appear surprising at first glance, but are in agreement with data previously reported by others. In a study in anesthetized calves, Constable (10) reported that endotoxin increased LV contractility (Ees), while reducing mean systemic arterial pressure, CO, and LV SW, indicating that circulatory dysfunction, rather than LV dysfunction, predominates during acute endotoxemia in this model. In dogs, endotoxin (1 mg/kg) induced significant reductions in preload and dP/dtmax, whereas Ees was either unchanged (40) or significantly increased (19). In this model, Ees decreased only in the terminal phase, supporting the concept that the early depression of cardiovascular performance in endotoxic dogs is due to decreased preload but not cardiac dysfunction, and that myocardial contractility is only depressed as a terminal event (19, 40). In a recent study in mice, animals challenged with 25 mg/kg endotoxin remained normodynamic, with reduced dP/dtmax and unchanged load-independent indexes of contractility, but with reduced preload, as shown by decreased LV diastolic pressure, despite slightly reduced LV diastolic compliance (thus indicating reduced LV filling) (6). In humans, it is also noteworthy that minute amounts of endotoxin (4 ng/kg) elicited a hypotensive hyperdynamic state in volunteers, with a significant increase in load-independent indexes of LV contractility during the first 3 h (20, 28).
At variance with our results and the above-discussed studies, several investigators did report on reduced inotropic state of the LV in endotoxemic conditions in vivo. In rats challenged with 10 mg/kg endotoxin, a reduced Ees was found after 6 h. This effect was not ascribed to a direct effect of endotoxin, but to a marked decrease of myocardial oxygenation, implying that the reduced contractility was due to myocardial ischemia (52). A reduced Ees has also been reported in rabbits challenged with very low doses of LPS (600 μg/kg), and occurring 36 h after the endotoxin challenge (2). In mice, two studies reported a decreased Ees 6–7 h after endotoxin, but it is noteworthy that both studies used very high doses of endotoxin (40–50 mg/kg) (11, 16), suggesting that LV dysfunction may have occurred as a preterminal event. Furthermore, in one study, interpretation of the data is made difficult, given the extremely and unusually low LV volumes measured in control animals. Indeed, according to the data, baseline SV was 6.5 μl on average (11), which is 70–80% smaller than normal SV measured by conductance catheters in mice, suggesting improper positioning of the catheter in the LV. It is also important to consider that differences between these results and ours may, at least partly, reflect variable responses to endotoxin of different mouse strains, as well described in the literature (27).
Some additional limitations of our study require further discussion. We used a standard calibration procedure for the conversion of conductance-derived data into volume, according to the initial description by Baan et al. (1). The accuracy of this method requires a linear conductance-volume relationship, a condition that is not fulfilled due to the nonuniformity of the electrical field generated by conductance catheters, resulting in a nonlinear conductance-volume relationship (56). Therefore, a new method for nonlinear conversion has been recently proposed by Wei et al. (56). However, with specific respect to the dimensions of the mouse heart, the volume offset introduced by linear conversion appears only limited, and nonlinear conversion seems to provide only minor improvements (36). A second technical limitation of conductance volumetry is the determination of parallel conductance, related to the presence of conducting material (mainly myocardial tissue) outside the LV pool. Such parallel conductance is usually determined by transiently changing blood conductivity using a bolus injection of hypertonic saline, as performed in our study. According to this method, a constant value of parallel conductance is subtracted from the total conductance to calculate the volume of the LV cavity. This method has been criticized, due to the time-varying nature of parallel conductance, which changes dynamically with changes of LV size during the cardiac cycle (4, 41). New methods for the determination of dynamic parallel conductance have been recently proposed to overcome this problem, by determining not only the capacitive, but also the resistive properties of the myocardium (55).
Although the ability of endotoxin to impair the contractile function of cardiomyocytes is a well-established fact, its study in vivo is made difficult by the multiple actions of LPS on hemodynamics, and by the inherent limitations of the animal model used. Our present data indicate that, in the usual model of acute murine endotoxemia, the cardiac hemodynamic abnormalities entirely result from severe alterations of LV loading conditions, but not from direct negative inotropic properties of endotoxin. The interpretation of load-dependent indexes of contractility in this setting as indicators of contractile failure may be largely misleading. Therefore, we recommend that load-independent indexes of contractility, as well as proper measurements of LV preload and afterload, be systematically determined to avoid inappropriate conclusions.
GRANTS
This work was supported, in part, by a grant from the Swiss National Fund for Scientific Research (Nr 320000/118174) and a grant from the CARDIOMET Foundation, University of Lausanne (Grant Nr 2007-R07–15) to L. Liaudet.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Baan J, van der Velde ET, de Bruin HG, Smeenk GJ, Koops J, van Dijk AD, Temmerman D, Senden J, Buis B. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation 70: 812–823, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Barraud D, Faivre V, Damy T, Welschbillig S, Gayat E, Heymes C, Payen D, Shah AM, Mebazaa A. Levosimendan restores both systolic and diastolic cardiac performance in lipopolysaccharide-treated rabbits: comparison with dobutamine and milrinone. Crit Care Med 35: 1376–1382, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Batkai S, Pacher P, Jarai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol Heart Circ Physiol 287: H595–H600, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boltwood CM, Jr, Appleyard RF, Glantz SA. Left ventricular volume measurement by conductance catheter in intact dogs. Parallel conductance volume depends on left ventricular size. Circulation 80: 1360–1377, 1989 [DOI] [PubMed] [Google Scholar]
- 5.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res 102: 1239–1246, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Buys ES, Cauwels A, Raher MJ, Passeri JJ, Hobai I, Cawley SM, Rauwerdink KM, Thibault H, Sips PY, Thoonen R, Scherrer-Crosbie M, Ichinose F, Brouckaert P, Bloch KD. sGCα1β1 attenuates cardiac dysfunction and mortality in murine inflammatory shock models. Am J Physiol Heart Circ Physiol 297: H654–H663, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirinos JA, Rietzschel ER, De Buyzere ML, De Bacquer D, Gillebert TC, Gupta AK, Segers P. Arterial load and ventricular-arterial coupling: physiologic relations with body size and effect of obesity. Hypertension 54: 558–566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cholley BP, Lang RM, Berger DS, Korcarz C, Payen D, Shroff SG. Alterations in systemic arterial mechanical properties during septic shock: role of fluid resuscitation. Am J Physiol Heart Circ Physiol 269: H375–H384, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Claessens TE, Georgakopoulos D, Afanasyeva M, Vermeersch SJ, Millar HD, Stergiopulos N, Westerhof N, Verdonck PR, Segers P. Nonlinear isochrones in murine left ventricular pressure-volume loops: how well does the time-varying elastance concept hold? Am J Physiol Heart Circ Physiol 290: H1474–H1483, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Constable PD. Acute endotoxemia increases left ventricular contractility and diastolic stiffness in calves. Shock 12: 391–401, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Davani EY, Boyd JH, Dorscheid DR, Wang Y, Meredith A, Chau E, Singhera GK, Walley KR. Cardiac ICAM-1 mediates leukocyte-dependent decreased ventricular contractility in endotoxemic mice. Cardiovasc Res 72: 134–142, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fishman D, Liaudet L, Lazor R, Perret CH, Feihl F. L-canavanine, an inhibitor of inducible nitric oxide synthase, improves venous return in endotoxemic rats. Crit Care Med 25: 469–475, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Freeman GL, Little WC, O'Rourke RA. Influence of heart rate on left ventricular performance in conscious dogs. Circ Res 61: 455–464, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Hollenberg SM. Mouse models of resuscitated shock. Shock 24, Suppl 1: 58–63, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth 104: 3–11, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Ichinose F, Buys ES, Neilan TG, Furutani EM, Morgan JG, Jassal DS, Graveline AR, Searles RJ, Lim CC, Kaneki M, Picard MH, Scherrer-Crosbie M, Janssens S, Liao R, Bloch KD. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circ Res 100: 130–139, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kass DA, Maughan WL, Guo ZM, Kono A, Sunagawa K, Sagawa K. Comparative influence of load versus inotropic states on indexes of ventricular contractility: experimental and theoretical analysis based on pressure-volume relationships. Circulation 76: 1422–1436, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Knuefermann P, Nemoto S, Misra A, Nozaki N, Defreitas G, Goyert SM, Carabello BA, Mann DL, Vallejo JG. CD14-deficient mice are protected against lipopolysaccharide-induced cardiac inflammation and left ventricular dysfunction. Circulation 106: 2608–2615, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Kober PM, Thomas JX, Jr, Raymond RM. Increased myocardial contractility during endotoxin shock in dogs. Am J Physiol Heart Circ Physiol 249: H715–H722, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Bunnell E, Lynn M, Anel R, Habet K, Neumann A, Parrillo JE. Experimental human endotoxemia is associated with depression of load-independent contractility indices: prevention by the lipid a analogue E5531. Chest 126: 860–867, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lancel S, Petillot P, Favory R, Stebach N, Lahorte C, Danze PM, Vallet B, Marchetti P, Neviere R. Expression of apoptosis regulatory factors during myocardial dysfunction in endotoxemic rats. Crit Care Med 33: 492–496, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med 345: 588–595, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lanoye L, Segers P, Tchana-Sato V, Rolin S, Dogne JM, Ghuysen A, Lambermont B, Hanson J, Desaive T, Verdonck P, D'Orio V, Kolh P. Cardiovascular haemodynamics and ventriculo-arterial coupling in an acute pig model of coronary ischaemia-reperfusion. Exp Physiol 92: 127–137, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Liaudet L, Feihl F, Rosselet A, Markert M, Hurni JM, Perret C. Beneficial effects of L-canavanine, a selective inhibitor of inducible nitric oxide synthase, during rodent endotoxaemia. Clin Sci (Lond) 90: 369–377, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Losser MR, Forget AP, Payen D. Nitric oxide involvement in the hemodynamic response to fluid resuscitation in endotoxic shock in rats. Crit Care Med 34: 2426–2431, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Magder S, Vanelli G. Circuit factors in the high cardiac output of sepsis. J Crit Care 11: 155–166, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Mahieu T, Park JM, Revets H, Pasche B, Lengeling A, Staelens J, Wullaert A, Vanlaere I, Hochepied T, van Roy F, Karin M, Libert C. The wild-derived inbred mouse strain SPRET/Ei is resistant to LPS and defective in IFN-beta production. Proc Natl Acad Sci U S A 103: 2292–2297, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathru M, Pollard V, He G, Varma TK, Ahmad M, Prough DS. Left ventricular diastolic filling characteristics are not impaired but systolic performance was augmented in the early hours of experimental endotoxemia in humans. Shock 25: 338–343, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Maughan WL, Sunagawa K, Burkhoff D, Graves WL, Jr, Hunter WC, Sagawa K. Effect of heart rate on the canine end-systolic pressure-volume relationship. Circulation 72: 654–659, 1985 [DOI] [PubMed] [Google Scholar]
- 30.Merx MW, Weber C. Sepsis and the heart. Circulation 116: 793–802, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Mulder MF, van Lambalgen AA, van den Bos GC, Thijs LG. The fall of cardiac output in endotoxemic rats cannot explain all changes in organ blood flow: a comparison between endotoxin and low venous return shock. Shock 5: 135–140, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Natanson C, Fink MP, Ballantyne HK, MacVittie TJ, Conklin JJ, Parrillo JE. Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. J Clin Invest 78: 259–270, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemoto S, DeFreitas G, Mann DL, Carabello BA. Effects of changes in left ventricular contractility on indexes of contractility in mice. Am J Physiol Heart Circ Physiol 283: H2504–H2510, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Nemoto S, Vallejo JG, Knuefermann P, Misra A, Defreitas G, Carabello BA, Mann DL. Escherichia coli LPS-induced LV dysfunction: role of toll-like receptor-4 in the adult heart. Am J Physiol Heart Circ Physiol 282: H2316–H2323, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Nicholson SC, Hahn RT, Grobmyer SR, Brause JE, Hafner A, Potter S, Devereux RB, Nathan CF. Echocardiographic and survival studies in mice undergoing endotoxic shock: effects of genetic ablation of inducible nitric oxide synthase and pharmacologic antagonism of platelet-activating factor. J Surg Res 86: 198–205, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Nielsen JM, Kristiansen SB, Ringgaard S, Nielsen TT, Flyvbjerg A, Redington AN, Botker HE. Left ventricular volume measurement in mice by conductance catheter: evaluation and optimization of calibration. Am J Physiol Heart Circ Physiol 293: H534–H540, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107: 896–904, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 100: 483–490, 1984 [DOI] [PubMed] [Google Scholar]
- 40.Pinsky MR, Rico P. Cardiac contractility is not depressed in early canine endotoxic shock. Am J Respir Crit Care Med 161: 1087–1093, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Porterfield JE, Kottam AT, Raghavan K, Escobedo D, Jenkins JT, Larson ER, Trevino RJ, Valvano JW, Pearce JA, Feldman MD. Dynamic correction for parallel conductance, GP, and gain factor, α, in invasive murine left ventricular volume measurements. J Appl Physiol 107: 1693–1703, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricard-Hibon A, Losser MR, Kong R, Beloucif S, Teisseire B, Payen D. Systemic pressure-flow reactivity to norepinephrine in rabbits: impact of endotoxin and fluid loading. Intensive Care Med 24: 959–966, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345: 1368–1377, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Rosselet A, Feihl F, Markert M, Gnaegi A, Perret C, Liaudet L. Selective iNOS inhibition is superior to norepinephrine in the treatment of rat endotoxic shock. Am J Respir Crit Care Med 157: 162–170, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med 35: 1599–1608, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Sennoun N, Meziani F, Dessebe O, Cattan V, Collin S, Montemont C, Gibot S, Asfar P, Ramaroson A, Regnault V, Slama M, Lecompte T, Lacolley P, Levy B. Activated protein C improves lipopolysaccharide-induced cardiovascular dysfunction by decreasing tissular inflammation and oxidative stress. Crit Care Med 37: 246–255, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Senzaki H, Chen CH, Kass DA. Single-beat estimation of end-systolic pressure-volume relation in humans. A new method with the potential for noninvasive application. Circulation 94: 2497–2506, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Sodums MT, Badke FR, Starling MR, Little WC, O'Rourke RA. Evaluation of left ventricular contractile performance utilizing end-systolic pressure-volume relationships in conscious dogs. Circ Res 54: 731–739, 1984 [DOI] [PubMed] [Google Scholar]
- 49.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res 35: 117–126, 1974 [DOI] [PubMed] [Google Scholar]
- 50.Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circ Res 32: 314–322, 1973 [DOI] [PubMed] [Google Scholar]
- 51.Szabo C, Mitchell JA, Thiemermann C, Vane JR. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol 108: 786–792, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokunaga C, Bateman RM, Boyd J, Wang Y, Russell JA, Walley KR. Albumin resuscitation improves ventricular contractility and myocardial tissue oxygenation in rat endotoxemia. Crit Care Med 35: 1341–1347, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Ullrich R, Scherrer-Crosbie M, Bloch KD, Ichinose F, Nakajima H, Picard MH, Zapol WM, Quezado ZM. Congenital deficiency of nitric oxide synthase 2 protects against endotoxin-induced myocardial dysfunction in mice. Circulation 102: 1440–1446, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Zingarelli B, O'Connor M, Zhang P, Adeyemo A, Kranias EG, Wang Y, Fan GC. Overexpression of Hsp20 prevents endotoxin-induced myocardial dysfunction and apoptosis via inhibition of NF-kappaB activation. J Mol Cell Cardiol 47: 382–390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei CL, Valvano JW, Feldman MD, Nahrendorf M, Peshock R, Pearce JA. Volume catheter parallel conductance varies between end-systole and end-diastole. IEEE Trans Biomed Eng 54: 1480–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Wei CL, Valvano JW, Feldman MD, Pearce JA. Nonlinear conductance-volume relationship for murine conductance catheter measurement system. IEEE Trans Biomed Eng 52: 1654–1661, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation 111: 97–105, 2005 [DOI] [PubMed] [Google Scholar]
- 58.You W, Min X, Zhang X, Qian B, Pang S, Ding Z, Li C, Gao X, Di R, Cheng Y, Liu L. Cardiac-specific expression of heat shock protein 27 attenuated endotoxin-induced cardiac dysfunction and mortality in mice through a PI3K/Akt-dependent mechanism. Shock 32: 108–117, 2009 [DOI] [PubMed] [Google Scholar]