Abstract
To accelerate lung development and protect neonates from other early developmental problems, synthetic steroids are administered maternally in the third trimester, exposing fetuses that are candidates for premature delivery to them. However, steroid exposure at this point of gestation may lead to elevated blood pressure [mean arterial pressure (MAP)] during adolescence. We hypothesize that fetal exposure to steroids activates the renin-angiotensin system, inducing an elevation in blood pressure and attenuation of baroreflex sensitivity (BRS) that is angiotensin II dependent in early adulthood. To test this hypothesis, fetal sheep were exposed to betamethasone (Beta) or vehicle (control) administered to ewes at day 80 of gestation and delivered at full term. At 1.8 yr of age, male offspring were instrumented for conscious recording of MAP, heart rate, and measurement of BRS [as low-frequency-α, high-frequency-α, sequence (seq) UP, seq DOWN, and seq TOTAL]. Beta-exposed sheep (n = 6) had higher MAP than control sheep (n = 5) (93 ± 2 vs. 84 ± 2 mmHg, P < 0.01). Acute blockade of angiotensin type 1 receptors with candesartan (0.3 mg/kg iv) normalized MAP in Beta-exposed sheep (85 ± 4 mmHg), with no effect in control sheep (82 ± 3 mmHg). Before angiotensin type 1 blockade, BRS maximum gain was significantly lower in Beta-exposed vs. control sheep (11 ± 3 vs. 26 ± 3 ms/mmHg, P < 0.0.01). However, 45 min after candesartan injection, BRS was increased in Beta-exposed (21 ± 5 ms/mmHg) and control (35 ± 4 ms/mmHg) sheep. Heart rate variability (HRV) and blood pressure variability (BPV) revealed lower HRV (SD of beat-to-beat interval and root mean square of successive beat-to-beat differences in R-R interval duration) and higher BPV (SD of MAP, systolic arterial pressure in low-frequency range) in Beta-exposed sheep. Candesartan partially restored HRV in Beta-exposed sheep and fully corrected BPV. Thus, in utero exposure to synthetic glucocorticoids causes long-lasting programming of the cardiovascular system via renin-angiotensin system-dependent mechanisms.
Keywords: spectral analysis, fetal programming, heart rate variability, blood pressure variability, renin-angiotensin system, hypertension
the intrauterine environment is extremely important in determining the health of the individual later in life (35). It is believed that individual tissues and organs can be programmed in utero during critical periods of development, and that disturbances of normal programming can lead to adverse consequences in adult life (15). Certain drugs as well as genetic and environmental factors can have programming effects on the developing fetus and can lead to increased risks of cardiovascular, metabolic, and neuroendocrine disorders in adulthood (2, 40). Development of many cardiovascular diseases later in life, especially hypertension, has been linked to glucocorticoid exposure in utero (2, 9, 15, 34, 41, 54). Betamethasone (Beta), a drug administered to the mother to accelerate fetal pulmonary maturation in preterm delivery, has been suggested to induce hypertension in adulthood (34, 52). Also, there is growing evidence that some of the programming effects caused by undernutrition are mediated through excessive exposure of the fetus to adrenocortical steroid hormones (23).
During gestation, the renin-angiotensin system (RAS) plays a vital role in the development of the fetus, and levels of RAS components in fetal life and during the newborn period are elevated. Previous studies show that angiotensin type 1 (AT1) and type 2 (AT2) receptors (AT1R and AT2R) are developmentally regulated (1, 19). Numerous studies indicate that the RAS plays an important role in the etiology of hypertension programmed by in utero insult (53). Maternal undernutrition in the rat is associated with marked increases in AT1R expression observed in areas of the brain critical to cardiovascular regulation (38). Significant changes in the RAS in the kidney occur in response to fetal insult. Reduction in intrarenal renin and ANG II is observed at birth in response to maternal protein restriction in the rat (53), followed by postnatal upregulation of the renal AT1Rs and development of hypertension. Importantly, hypertension in many of these programmed models is abolished by systemic blockade of the RAS (8, 24), indicating that the RAS contributes to hypertension programmed in response to maternal nutrient restriction. In sheep, maternal undernutrition at early to midgestation increased mean arterial pressure (MAP), angiotensin converting enzyme (ACE), and AT1R and AT2R expression in the kidney (16, 28), suggesting a possible role of the RAS system in the development of programmed hypertension.
Despite evidence implicating the RAS system in fetal programmed hypertension and a role of glucocorticoids and Beta exposure in utero in the development of hypertension later in life, most studies have not paralleled the situation of Beta administration to mothers expecting premature delivery. The present study examined the cardiovascular effects of antenatal Beta treatment in sheep at a dose and time similar to antenatal clinical steroid treatment in mothers expected to have a premature delivery (14). To accomplish the objectives, we measured MAP, heart rate (HR), baroreflex control of HR, HR variability (HRV) and blood pressure variability (BPV) parameters, and the response to acute AT1R blockade.
METHODS
Saline or Beta was administered (intramuscularly, 2 doses of 0.17 mg/kg, 24 h apart) to date-mated pregnant sheep received at the 80th day of gestation. Animals were brought to term (145 days), and after delivery they were farm raised and weaned at 3 mo of age. At 1.8 yr of age, nonneutered male offspring were brought to our American Association for Accreditation of Laboratory Animal Care-approved facility, where they were maintained on a normal diet, with free access to tap water and a 12:12-h light-dark cycle (lights on 7 AM to 7 PM). Sheep were anesthetized with ketamine and isoflurane, and catheters were inserted in the femoral artery and vein for blood pressure recording and drug administration. Sheep were housed in a study cart after the surgical procedure. Five days after surgery, the arterial catheter was connected to pressure transducers. Arterial pressure and HR were recorded and digitized with Acknowledge software (version 3.8.1, BIOPAC, Santa Barbara, CA). Digitized MAP and HR were used for the measurements of baroreflex sensitivity (BRS) [as low-frequency (LF)-α, high-frequency (HF)-α, sequence (seq) UP, seq DOWN, and seq TOTAL], HRV [standard deviation of beat-to-beat interval (SDRR) and root mean square of successive beat-to-beat differences in R-R interval (RRI) duration (rMSSD), and RRI LF (LFRRI)-to-RRI HF (HFRRI) ratio], and BPV [systolic arterial pressure (SAP) spectra in the LF range (LFSAP) and standard deviation of MAP (SDMAP)], both before and 45 min after the injection of 0.3 mg/kg (iv) candesartan (AT1R blocker). Complete blockade of AT1R was tested using ANG II injection (20 ng/kg iv) before candesartan injection and almost 60 min after candesartan injection at the end of the experiment. All experiments were carried out in accordance with the guiding principles for the care and use of animals and were approved by the Institutional Animal Care and Use Committee.
Baroreflex gain.
After 30 min of equilibration, animals received intravenous infusion of phenylephrine and sodium nitroprusside. Phenylephrine (2–16 μg/kg) was used to raise MAP by 30–40 mmHg above baseline in 30 s, and nitroprusside (10–30 μg/kg) to reduce MAP by 30–40 mmHg below baseline in 30 s. The data of each sheep for the MAP-HR relations after infusions of phenylephrine and nitroprusside were individually fitted to a logistic function curve using a nonlinear regression program (GraphPad Prism 4). Four parameters were derived from the following equation: HR = P1/{1 + exp[P2(MAP − P3)]} + P4, where P1 is the range of HR, P2 is the slope coefficient, P3 is the MAP corresponding to one-half the HR range, and P4 is the lower plateau of HR. The sensitivity of the baroreflex was derived as the maximum gain Gmax = (−P1 × P2/4) of the logistic function curve (20, 21, 30). Baroreflex curves were tested both before and 45 min after candesartan infusion.
Frequency domain analysis.
Spontaneous BRS was calculated by the frequency-domain analysis method, as in our laboratory's previous work (3, 44) and reported studies (36), using analysis software designed for large animals (Nevrokard BRS, Medistar, Ljubljana, Slovenia). In brief, BP was digitized at 1,000 Hz (BIOPAC acquisition software, Santa Barbara, CA), and the SAP and RRI files generated from 5 min of continuous recording at the specified time points were analyzed using Nevrokard BRS software. Power spectral densities of SAP and RRI oscillations were computed by 512 points fast Fourier transform and integrated over the specified frequency range (LF: 0.04–0.15 Hz and HF: 0.15–0.4 Hz). A Hanning window was applied, and the spectra of SAP and RRI series, and their squared-coherence modulus, were computed, if the coherence is >0.5, in accordance with reported criteria (36). The square root of the ratio of RRI and SAP powers was computed to calculate LF, HF-α indexes (LF-α, HF-α), as indexes of BRS (36). Power of RRI spectra in LF and HF range (LFRRI and HFRRI) were calculated in normalized units, and the ratio of LFRRI/HFRRI was used as a measure of sympathovagal balance (22). Power of LFSAP was used as a measure of BPV.
Sequence method.
Spontaneous BRS calculated by this method is based on quantification of sequences of at least three beats (n) in which SAP consecutively increases (UP sequence) or decreases (DOWN sequence), which are accompanied by changes in the same direction of the RRIs of the subsequent beats (n + 1). To be included in the BRS estimate, each sequence must fulfill the following reported criteria(44): 1) minimal RRI change 3 ms; 2) minimal SAP change 1 mmHg; 3) minimal number of beats, 3 in the sequence; 4) minimal correlation coefficient of 0.85. The software scans the RRI and SAP records, identifies sequences, and then calculates linear correlation between RRI and SAP for each sequence. If the correlation coefficient exceeds a preset critical value (0.85), the regression coefficient (slope) is calculated and accepted. The mean of all individual regression coefficients (slopes), which is a measure of sequence BRS, was then calculated. Overall, three parameters were obtained by this method (sequence UP, DOWN, and TOTAL).
Time domain analysis.
HRV was determined by computing the SDRR and the rMSSD. The SDMAP was used as a measure for BPV (3, 43, 44, 49).
Statistical procedures.
All measurements were expressed as the mean ± SE. All statistical analyses were performed with GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Two-way repeated-measures ANOVA with Student-Newman-Keuls post hoc analysis was used to compare between groups before and after candesartan treatment. A Student's t-test was used to compare variables with only two conditions. The criterion for statistical significance was set at P < 0.05.
RESULTS
Effect on blood pressure, HR, response to ANG II, and left ventricle-to-body weight ratio.
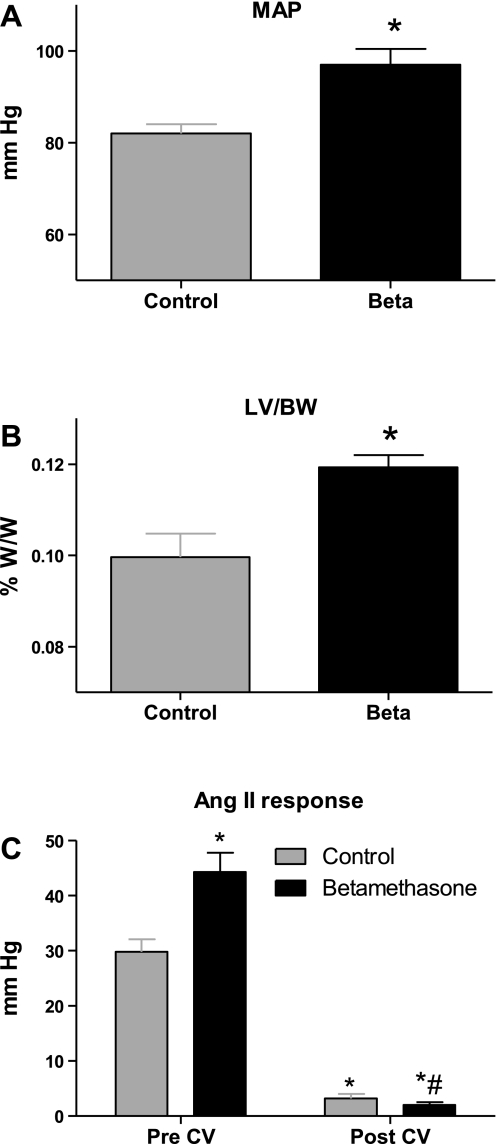
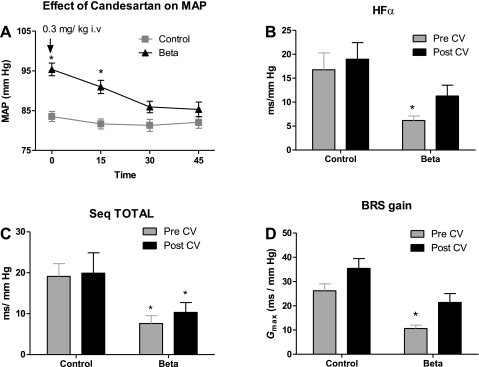
MAP in the Beta-exposed sheep was significantly higher than that in the control group at 1.8 yr of age (Fig. 1A). There was no difference between the two groups at 1.8 yr of age in HR or body weight. Beta exposure in utero caused an enlargement in the left ventricle later in life, and the left ventricle-to-body weight ratio was significantly higher in the Beta-exposed sheep compared with control sheep (Fig. 1B). Beta-exposed sheep had a significantly higher pressor response to ANG II bolus injection (Fig. 1C). This pressor response was completely blocked by the intravenous candesartan treatment, attesting to the complete blockade of AT1R with this dose. Candesartan intravenous administration lowered MAP to levels of the control group at 45 min postinjection (Fig. 2A), without altering HR (not shown).
Fig. 1.
Effect of antenatal betamethasone (Beta) exposure in sheep (1.8 yr of age) on mean arterial pressure (MAP; A) and left ventricle-to-body weight ratio (LV/BW; B). C: vasopressor responses to ANG II bolus injection (20 ng/kg). Effect is shown of acute blockade of the angiotensin type 1 (AT1) receptor by candesartan (0.3 mg/kg iv injection; CV) on the ANG II vasopressor response. W/W, weight/weight. Values are means ± SE. *P < 0.05 vs. control. #P < 0.05 vs. Beta-exposed pre-CV.
Fig. 2.
A: effect of acute AT1-receptor blockade with CV injection (0.3 mg/kg) on MAP. Spontaneous baroreflex sensitivity (BRS) was measured by spectral analysis methods as high-frequency (HF)-α index (B), as sequence all (seq TOTAL; C), and as baroreflex gain (Gmax; D) in control and Beta-exposed sheep. Values are means ± SE. *P < 0.05 vs. control.
Effect on baroreflex.
Spontaneous BRS for HR control measured by spectral analysis as HF-α (parasympathetic arm) was lower in Beta-exposed sheep, indicating impairment in the central control of the circulation (Fig. 2B). There was no effect of Beta exposure on LF-α (sympathetic arm). BRS measured by the time domain parameter seq TOTAL was lower in the Beta-exposed sheep. Candesartan injection increased BRS measured by the spectral analysis method (HF-α) in Beta-exposed sheep to values not different from the control baseline level. There was no effect of candesartan on the BRS measured by sequence method (seq TOTAL) (Fig. 2C).
BRS gain (Gmax) calculated from the logistic function analyses was significantly lower in Beta-exposed sheep (Fig. 2D), with no difference in resting heart range (55 ± 7 beats/min in Beta sheep vs. 51 ± 6 beats/min in Control, n = 5–6). There was also an increase in the set point (P3) of baroreflex curve to a higher MAP (107 ± 7 mmHg in Beta-exposed sheep vs. 84 ± 10 mmHg in control sheep, n = 5–6). Candesartan treatment significantly increased BRS gain in Beta-exposed sheep (Fig. 2D) and lowered the set point in Beta-exposed sheep at 45 min postinjection (Table 1).
Table 1.
Parameters and maximum gain of baroreflex control of heart rate before and after the AT1 receptor antagonist candesartan in control and Beta sheep
| Control | Control Post-CV-11974 | Beta | Beta Post-CV-11974 | |
|---|---|---|---|---|
| n | 5 | 5 | 6 | 6 |
| P1, beats/min | 121.4 ± 9 | 104.7 ± 16 | 125.5 ± 13 | 101.1 ± 17 |
| P2, beats·min−1·mmHg−1 | 0.12 ± 0.013 | 0.18 ± 0.036 | 0.065 ± 0.01* | 0.13 ± 0.02† |
| P3, mmHg | 84 ± 10 | 80 ± 5 | 107 ± 7* | 90 ± 5† |
| P4, beats/min | 51 ± 7 | 52 ± 9 | 55 ± 7 | 64 ± 7 |
| Gmax, beats·min−1·mmHg−1 | −3.8 ± 0.2 | −4.3 ± 0.3 | −1.9 ± 0.3* | −2.9 ± 0.4† |
Values are means ± SE; n, no. of sheep for each condition. Post-CV-11974, after candesartan; Beta, betamethasone; P1, range of heart rate; P2, slope coefficient; P3, mean arterial pressure at midrange; P4, minimum heart rate; Gmax, maximum gain of heart rate.
P < 0.05 vs. control.
P < 0.05 vs. Beta-exposed pre-candesartan.
Effect on HRV and BPV.
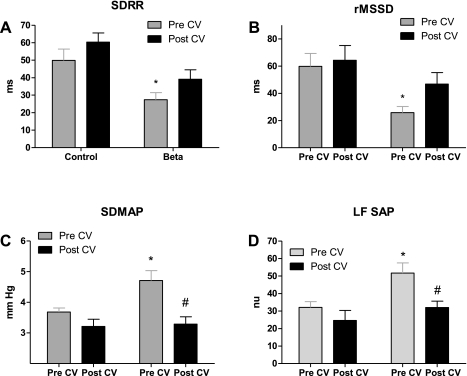
Antenatal Beta exposure significantly lowered HRV measured as either SDRR (Fig. 3A) or rMSSD (Fig. 3B) in the Beta-exposed sheep. Candesartan injection increased HRV in the Beta-exposed sheep to a value that was not different from that of the control sheep. Antenatal Beta exposure significantly increased BPV measured as either SDMAP (Fig. 3C) or LFSAP (Fig. 3D) in the Beta-exposed sheep. Candesartan injection lowered BPV in the Beta-exposed sheep back to a level not different from that of the control sheep.
Fig. 3.
Effect of AT1-receptor blockade with CV on heart rate variability measured by standard deviation of beat-to-beat intervals (SDRR; A) and by root of mean successive differences (rMSSD; B), blood pressure variability (BPV) measured by standard deviation of mean arterial pressure (SDMAP; C), and power of the spectral density of systolic arterial pressure in the low-frequency range (LFSAP) in normalized units (nu; D) in control and Beta-exposed sheep. Values are means ± SE. *P < 0.05 vs. control. #P < 0.05 vs. Beta-exposed pre-CV.
LFRRI/HFRRI, a measure of sympathovagal balance, tended to be greater in Beta-exposed sheep compared with control sheep (1.6 ± 0.5 vs. 0.79 ± 0.2, n = 5–6, P = 0.1), and candesartan treatment of Beta-exposed sheep lowered this ratio to a value similar to that of the control sheep (0.72 ± 0.1).
DISCUSSION
The present study determined the effect of in utero exposure of sheep fetuses to Beta at a clinically relevant dose and time point on the development of higher MAP later in adulthood and the deleterious changes in baroreflex, HRV, and BPV. We also focused on the ability of acute AT1 blockade via a single injection of candesartan to correct these cardiovascular changes. Programming effects of pharmacological and nutritional interventions may be determined by the developmental period during which exposure occurs. Thus it is important to note that the time of the Beta administration in this study differed from that of most previous studies. In fact, the programming effect of Beta or dexamethasone in sheep at either early gestation time (11, 12, 32) or very late gestation time using more than one dose (10, 33, 48) contrasts with our treatment protocol, which mimics the clinical situation of early third-trimester exposure. It has been shown that blood pressure at 14 yr of age was higher in a group of children born preterm whose mothers received Beta before preterm delivery (∼0.7 gestation) than in a similar group who were not exposed to corticosteroids (13). We found that acute AT1R blockade with candesartan lowered the elevated MAP in the Beta-exposed sheep and corrected the impairment in BRS and the attenuation in HRV. Candesartan's ability to lower MAP in Beta-exposed sheep is different from previously reported model of programmed hypertension in sheep, in which another AT1 antagonist, irbesartan, at 5.5 yr of age failed to correct the elevation in MAP due to intrauterine exposure to dexamethasone at the 28th day of gestation (37). This difference could be explained by the glucocorticoids used (Beta vs. dexamethasone), the time of exposure to glucocorticoids (midgestation period in our study vs. early gestation) (37), time of testing the animals (in adulthood 1.8 yr vs. 5.5 yr of age), or differences in the underlying mechanism of this hypertension, such as alterations in expression of specific sodium transporters or endothelium dysfunction (2). The lack of effect of candesartan on MAP in the control sheep is consistent with previous reports in rats, where candesartan had no effect on blood pressure in the normotensive rats (17). Similarly, in conscious sheep, acute AT1R blockade with Losartan did not change MAP, although renal blood flow increased (50). Significant blockade of AT1Rs with the selected dose of candesartan was achieved in our study, since enhanced vasopressor response to ANG II in Beta-exposed compared with Control sheep was abolished by candesartan. This enhanced pressor effect in the Beta-exposed sheep is consistent with reported enhanced vascular responsiveness to chronic ANG II infusion in rats treated in utero with synthetic glucocorticoids (18). Moreover, coronary artery vasoconstriction to ANG II was enhanced in sheep exposed in late gestation (121–124 days) to Beta, which may reflect increased coronary expression of the AT1R (39), which was thought to be due to increased coronary AT1R expression (39). The enhanced pressor effect of ANG II in Beta-exposed sheep could result from increased receptors in the vasculature, impaired endothelium-dependent and -independent nitric oxide-mediated vascular relaxation (2), impaired vascular structure resulting from the hypertension, or the impaired baroreflex in these sheep.
Thus, ANG II may also contribute to the altered neural circulatory control in Beta-exposed sheep. 1) ANG II exerts a tonic effect on baroreflex control of HR to shift the curves toward higher pressure levels; 2) ANG II impairs arterial baroreflex function independent of its effects on MAP (42); 3) the peptide acts centrally to modulate vagal control of HR (26); and 4) ANG II at the level of the parasympathetic nerve fibers within the heart may inhibit vagal discharge evoked by stimulation of arterial baroreceptors (25), which would impair parasympathetic control. Thus the RAS components may act at multiple sites and via different mechanisms to contribute to the development of hypertension and the impairment in baroreflex regulation of the circulation via the autonomic nervous system.
The present study does not address directly the sites (central vs. peripheral) where the RAS peptides, especially ANG II, may produce the deleterious hemodynamic effects associated with antenatal Beta or where candesartan intercedes. One site might be the kidney, since others have shown that antenatal exposure to glucocorticoids can increased renal AT1R expression (51). We reported an increase in ACE activity and a reduction in ACE2 activity in proximal tubules and serum of Beta-exposed sheep, which may shift the peptide balance toward ANG II in the kidney or other tissues, exposing these sheep to higher tissue levels of ANG II (46). Alternatively, another possible site of action for increased ANG II is the brain. Although administered systemically, candesartan acts both centrally and peripherally over the 45-min time frame, as it has been shown to inhibit the effects of centrally administered ANG II (17). Indeed, ANG II acts on AT1Rs in the nucleus of the solitary tract (NTS) to attenuate the baroreflex and the improvement of baroreflex control of HR by intravenous candesartan has been shown to be mediated centrally in both spontaneously hypertensive (30) and Wistar-Kyoto rats (29). In sheep, our laboratory has shown that the NTS is a site for candesartan action, and that the ANG II/ ANG-(1–7) balance within this brain region may play a role in the alteration in BRS (47). Our laboratory has preliminary data showing a reduction in ANG-(1–7) receptors (4) and function (45) in the brain dorsal medullary tissue corresponding to the site of baroreflex control, suggesting ANG II actions at the AT1Rs may be unopposed.
We used both classical pharmacological approaches for baroreflex assessment and spectral analysis for spontaneous baroreflex measurements. Together, these methods uniformly revealed an inhibition of the parasympathetic nervous system in Beta-exposed sheep that was reflected by the reduction in the HRV and the shift in sympathovagal balance (LFRRI-to-HFRRI ratio) toward the sympathetic system. Although the action of candesartan in NTS was mainly via improving the parasympathetic system, there was also inhibition in the sympathetic system, as reflected by SDMAP and LFSAP. Possible sites for ANG II/candesartan actions on both parasympathetic and sympathetic functions include the NTS (5), rostral ventrolateral medulla (5), ganglia (27), paraventricular nucleus (6), and vasculature. In addition to alteration in the baroreflex gain in the Beta-exposed sheep, the set point (P3) was higher in these animals, making them less able to mount a significant increase in HR in response to decrease in blood pressure. This set point resetting could be pressure independent, as has been shown in models of chronic ANG II-dependent hypertension (7), and may have contributed to the maintenance of elevated MAP in these animals. In the present study, candesartan treatment lowered both MAP and the set point over the time frame of this experiment, which is consistent with rapid resetting of the set point (31). In fact, the correction of both set point and gain sensitivity components of the reflex is consistent with ANG II modification of baroreceptor function at multiple sites of action.
In summary, we found that antenatal exposure of sheep fetuses to Beta at a clinically relevant dose and time programmed the sheep cardiovascular system with the outcomes of elevated MAP, impaired reflex control for HR, reduced HRV, and increased BPV. These hemodynamic changes were corrected by acute AT1R blockade. Antenatal exposure to Beta in humans could have undesirable cardiovascular outcomes later in life. Understanding the mechanisms underlying these changes in an animal model and further elaboration of the effects of chronic AT1R blockade may be beneficial for more directed therapeutic management of young adults exposed to antenatal steroids.
GRANTS
This study was supported by National Institutes of Health Grants HD-47584, HD-17644, HL-56973, and HL-51952.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We acknowledge the help of David Jones and Eric Lesane with the animals.
Present address of H. A. Shaltout: Department of Pharmacology and Toxicology, School of Pharmacy, University of Alexandria, Alexandria 21526, Egypt.
REFERENCES
- 1.Aguilera G, Kapur S, Feuillan P, Sunar-Akbasak B, Bathia AJ. Developmental changes in angiotensin II receptor subtypes and AT1 receptor mRNA in rat kidney. Kidney Int 46: 973–979, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 290: R1–R10, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs cardiovagal baroreflex function at the level of the solitary tract nucleus. Hypertension 54: 1001–1008, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arter A, Nautiyal M, Shaltout HA, Chappell MC, Diz DI. Mas and AT1 receptor expression in adult sheep dorsomedial medulla (Abstract). FASEB J. In press [Google Scholar]
- 5.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull 51: 119–128, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res 599: 223–229, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Brooks VL, Ell KR, Wright RM. Pressure-independent baroreflex resetting produced by chronic infusion of angiotensin II in rabbits. Am J Physiol Heart Circ Physiol 265: H1275–H1282, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Ceravolo GS, Franco MC, Carneiro-Ramos MS, Barreto-Chaves ML, Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Enalapril and losartan restored blood pressure and vascular reactivity in intrauterine undernourished rats. Life Sci 80: 782–787, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3: 19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crossley KJ, Morley CJ, Allison BJ, Polglase GR, Dargaville PA, Harding R, Hooper SB. Blood gases and pulmonary blood flow during resuscitation of very preterm lambs treated with antenatal betamethasone and/or Curosurf: effect of positive end-expiratory pressure. Pediatr Res 62: 37–42, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 94: 149–155, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Dodic M, Peers A, Coghlan JP, May CN, Lumbers E, Yu Z, Wintour EM. Altered cardiovascular haemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci (Lond) 97: 103–109, 1999 [PubMed] [Google Scholar]
- 13.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 98: 137–142, 2000 [PubMed] [Google Scholar]
- 14.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuna G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res 58: 510–515, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction 127: 515–526, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol 565: 137–147, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gohlke P, Von Kugelgen S, Jurgensen T, Kox T, Rascher W, Culman J, Unger T. Effects of orally applied candesartan cilexetil on central responses to angiotensin II in conscious rats. J Hypertens 20: 909–918, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol 188: 435–442, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hu F, Morrissey P, Yao J, Xu Z. Development of AT(1) and AT(2) receptors in the ovine fetal brain. Brain Res Dev Brain Res 150: 51–61, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kent BB, Drane JW, Blumenstein B, Manning JW. A mathematical model to assess changes in the baroreceptor reflex. Cardiology 57: 295–310, 1972 [DOI] [PubMed] [Google Scholar]
- 21.Kumagai H, Averill DB, Ferrario CM. Renal nerve activity in rats with spontaneous hypertension: effect of converting enzyme inhibitor. Am J Physiol Regul Integr Comp Physiol 263: R109–R115, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Laitinen T, Hartikainen J, Niskanen L, Geelen G, Lansimies E. Sympathovagal balance is major determinant of short-term blood pressure variability in healthy subjects. Am J Physiol Heart Circ Physiol 276: H1245–H1252, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sci 60: 1213–1221, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol 110: 223–228, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Lumbers ER, McCloskey DI, Potter EK. Inhibition by angiotensin II of baroreceptor-evoked activity in cardiac vagal efferent nerves in the dog. J Physiol 294: 69–80, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumbers ER, Potter EK. The effects of vasoactive peptides on the carotid cardiac baroreflex. Clin Exp Pharmacol Physiol Suppl 7: 45–49, 1982 [PubMed] [Google Scholar]
- 27.Ma X, Chapleau MW, Whiteis CA, Abboud FM, Bielefeldt K. Angiotensin selectively activates a subpopulation of postganglionic sympathetic neurons in mice. Circ Res 88: 787–793, 2001 [DOI] [PubMed] [Google Scholar]
- 28.MacLaughlin SM, Walker SK, Kleemann DO, Tosh DN, McMillen IC. Periconceptional undernutrition and being a twin each alter kidney development in the sheep fetus during early gestation. Am J Physiol Regul Integr Comp Physiol 298: R692–R699, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 275: R1611–R1619, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Matsumura K, Averill DB, Ferrario CM. Role of AT1 receptors in area postrema on baroreceptor reflex in spontaneously hypertensive rats. Brain Res 850: 166–172, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Moreira ED, Ida F, Krieger EM. Extent of rapid baroreceptor resetting during first hours of hypertension. Am J Physiol Heart Circ Physiol 257: H711–H716, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology 143: 4455–4463, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Moss TJ, Sloboda DM, Gurrin LC, Harding R, Challis JR, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol 281: R960–R970, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol 28: 957–961, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Nuyt AM, Alexander BT. Developmental programming and hypertension. Curr Opin Nephrol Hypertens 18: 144–152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol Heart Circ Physiol 268: H1606–H1612, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Peers A, Campbell DJ, Wintour EM, Dodic M. The peripheral renin-angiotensin system is not involved in the hypertension of sheep exposed to prenatal dexamethasone. Clin Exp Pharmacol Physiol 28: 306–311, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res 55: 1042–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Roghair RD, Lamb FS, Bedell KA, Smith OM, Scholz TD, Segar JL. Late-gestation betamethasone enhances coronary artery responsiveness to angiotensin II in fetal sheep. Am J Physiol Regul Integr Comp Physiol 286: R80–R88, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Seckl JR. Physiologic programming of the fetus. Clin Perinatol 25: 939–962, 1998 [PubMed] [Google Scholar]
- 41.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci 1032: 63–84, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Segar JL, Minnick A, Nuyt AM, Robillard JE. Role of endogenous ANG II and AT1 receptors in regulating arterial baroreflex responses in newborn lambs. Am J Physiol Regul Integr Comp Physiol 272: R1862–R1873, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Sgoifo A, de Boer SF, Westenbroek C, Maes FW, Beldhuis H, Suzuki T, Koolhaas JM. Incidence of arrhythmias and heart rate variability in wild-type rats exposed to social stress. Am J Physiol Heart Circ Physiol 273: H1754–H1760, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Shaltout HA, Abdel-Rahman AA. Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther 314: 1328–1337, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Evidence of Ang-(1–7) deficiency in antenatal betamethasone-treated young adult sheep. Hypertension 52: E107–E107, 2008 [Google Scholar]
- 46.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension 53: 404–408, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaltout HA, Rose JC, Chappell MC, Averill DB, Diz DI. Modulation of baroreflex sensitivity by endogenous angiotensins in lamb solitary tract nucleus (Abstract). FASEB J. In press [Google Scholar]
- 48.Smith LM, Ervin MG, Wada N, Ikegami M, Polk DH, Jobe AH. Antenatal glucocorticoids alter postnatal preterm lamb renal and cardiovascular responses to intravascular volume expansion. Pediatr Res 47: 622–627, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Stein PK, Bosner MS, Kleiger RE, Conger BM. Heart rate variability: a measure of cardiac autonomic tone. Am Heart J 127: 1376–1381, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Ullman J, Eriksson S, Rundgren M. Effects of losartan, prazosin and a vasopressin V1-receptor antagonist on renal and femoral blood flow in conscious sheep. Acta Physiol Scand 171: 99–104, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Wintour EM, Johnson K, Koukoulas I, Moritz K, Tersteeg M, Dodic M. Programming the cardiovascular system, kidney and the brain–a review. Placenta 24: S65–S71, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol 291: R1069–R1075, 2006 [DOI] [PubMed] [Google Scholar]
- 53.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res 49: 460–467, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol 289: R955–R962, 2005 [DOI] [PubMed] [Google Scholar]