Abstract
Recently, opioid receptors have been shown to be expressed on group III and IV afferents, which comprise the sensory arm of the exercise pressor reflex. Although the stimulation of opioid receptors in the central nervous system has been shown to attenuate the exercise pressor reflex, the effect on the reflex of their stimulation in the periphery is unknown. We therefore tested the hypothesis that the activation of peripheral μ-opioid receptors attenuates the exercise pressor reflex. The pressor responses to static contraction were compared before and after the injection of the μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly-ol5]enkephalin (DAMGO; 1 μg) into the abdominal aorta of decerebrated rats in which one femoral artery had been occluded 72 h previously (n = 10) and in control rats whose femoral arteries were freely perfused (n = 8). DAMGO attenuated the peak pressor response to contraction in rats whose femoral arteries had been occluded (before: increase of 34 ± 3 mmHg and after: increase of 22 ± 2 mmHg, P = 0.008); the inhibitory effect of DAMGO was prevented by the injection of naloxone (100 μg) into the abdominal aorta (before: increase of 29 ± 5 mmHg and after: increase of 29 ± 5 mmHg, P = 0.646, n = 7). An intravenous injection of DAMGO (1 μg, n = 6) had no effect on the peak pressor response to contraction in both groups of rats. DAMGO had no effect on the peak pressor response to contraction in rats whose femoral arteries were freely perfused (before: Δ 23 ± 4 mmHg, after: Δ 23 ± 3 mmHg, n = 6) but appeared to have a small effect on topography of the response. DAMGO had no effect on the peak pressor response to tendon stretch in both groups of rats (both P > 0.05). We conclude that the stimulation of peripheral μ-opioid receptors attenuates the exercise pressor reflex in rats whose femoral arteries have been ligated for 72 h.
Keywords: static contraction, thin fiber muscle afferents, ischemia, neural control of circulation
the exercise pressor reflex plays an important role in evoking the cardiovascular responses to exercise in both physiological and pathophysiological states (5, 17, 20). The sensory arm of the reflex consists of thinly myelinated group III and unmyelinated group IV afferents (17), the endings of which are stimulated by both mechanical and metabolic stimuli arising in contracting muscles (13, 14). Group III and IV muscle afferents synapse on cells in laminae I and V of the dorsal horn of the spinal cord. These dorsal horn cells, in turn, relay to neural circuits in the both the ventrolateral medulla (6) and nucleus tractus solitarius (6), where they function to increase the sympathetic outflow to the vasculature and heart (34) as well as to decrease the parasympathetic outflow to the heart (18).
The stimulation of opioid receptors is known to inhibit transmission of nociceptive input to the spinal cord by both pre- and postsynaptic mechanisms (7, 12, 15, 26). The former mechanism is often attributed to opioid-induced inhibition of substance P release from the primary afferent terminals synapsing onto dorsal horn cells, whereas the latter is attributed to inhibition of these dorsal horn cells by enkephalin released from spinal interneurons (11, 16, 19). Recently, evidence has shown that opioid receptors are expressed not only on the central terminals of primary afferents but also on the peripheral endings of thinly myelinated and unmyelinated cutaneous sensory fibers (4). These opioid receptors are synthesized in cell bodies in the dorsal root ganglia and are then transported to the periphery (8, 21, 22). The activation of opioid receptors decreases the excitability of peripheral nerve terminals by inhibiting adenylate cyclase, which, in turn, decreases levels of cAMP, causing increased K+ efflux and decreased Ca2+ entry (38, 39, 40).
The analgesic effects of the stimulation of peripheral opioid receptors have been previously reported (22, 36). Using an in vitro glabrous skin-nerve preparation, Wenk et al. (36) examined the effect of morphine, an opioid receptor agonist, on the discharge properties of single afferent fibers innervating normal and inflamed skin. They found that morphine reduced the responses of most nociceptors to noxious mechanical and thermal stimuli in inflamed skin but had little effect on the responses of nociceptors to these stimuli in normal skin. The reduction was found to depend on the concentration of the opioid and was prevented by naloxone, an opioid receptor antagonist. In addition, Tegeder et al. (31) demonstrated that a local infusion of a low dose of morphine-6β-glucuronide, which does not readily penetrate the blood-brain barrier (2), significantly reduced muscle hyperalgesia by a series of concentric and eccentric muscle contractions in human subjects.
Based on these previous studies, we hypothesized that the activation of peripheral opioid receptors desensitizes group III and IV muscle afferents and thereby attenuates the exercise pressor reflex. Therefore, we examined the peripheral effect of a μ-opioid receptor agonist on the exercise pressor reflex in decerebrated rats. As suggested in the previous studies, the role of peripheral opioid receptors in the exercise pressor reflex may be different if the exercising muscles are either stressed or damaged (31, 36). Previously, we (33) have shown that the pressor response to static contraction of a hindlimb whose ipsilateral femoral artery was occluded for 72 h before the start of an experiment was greater than the pressor response to contraction of the contralateral freely perfused hindlimb. Thus, we compared the effect of an intra-arterial injection of a μ-opioid receptor agonist on the pressor reflex to static contraction in rats whose femoral arteries were ligated for 72 h before the experiment with the effect of the opioid agonist on the reflex in control rats whose hindlimb was freely perfused.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Hershey Medical Center of Pennsylvania State University. Adult male rats (Sprague-Dawley, n = 49, weighing between 344 and 565 g) were used in this study. Rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle. Rats were fed a standard diet and tap water ad libitum. Seventy-two hours before an experiment, 29 of 49 animals underwent surgery to induce unilateral femoral artery occlusion according to procedures described elsewhere (23, 37). Briefly, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen; one femoral artery was isolated and then tightly ligated just distal to the inguinal ligament. Using radiolabeled microspheres, it has been shown that this femoral artery ligation procedure reduced blood flow reserve capacity to ∼10–20% of normal but allowed sufficient blood flow to meet resting requirements (23, 37). Rats were allowed to recover 72 h before the experiments were started. Femoral artery occlusion has been reported to have no effect on normal cage activity (30).
Surgical preparation.
On the day of the experiment, rats were anesthetized with a mixture of 4% isoflurane and 100% oxygen. The right jugular vein and common carotid artery were cannulated for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham). Heart rate (HR) was calculated beat to beat from the arterial pressure pulse (Gould Biotach). The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus). Arterial blood gases and pH were measured by an automated blood gas analyzer (model ABL-700, Radiometer). Pco2 and arterial pH were maintained within normal ranges either by adjusting ventilation or through an intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted, and the core body temperature of the animal was maintained at 37–38°C by a water-perfused heating pad and a lamp.
In 37 of 49 experiments, we cannulated (polyethylene-10 tubing) the right femoral artery in a retrograde direction and advanced the tip to the bifurcation of the abdominal aorta. This allowed us to administer drugs into the arterial supply of the left hindlimb. A reversible vascular occluder was placed around the abdominal aorta and the inferior vena cava just above the aortic bifurcation. When tightened, this occluder helped to keep the injectate within the circulation of the left hindlimb.
The rat was placed in a Kopf stereotaxic frame. Dexamethasone (0.2 mg) was injected intravenously just before the decerebration procedure to minimize brain stem edema. The left common carotid artery was tied off, and a precollicular decerebration was performed. All neural tissue rostral to the section was removed. To minimize cerebral hemorrhage, small pieces of oxidized regenerated cellulose (Ethicon, Johnson & Johnson) were placed on the internal skull surface, and the cranial cavity was packed with cotton. In our experiments, rats were decerebrated instead of anesthetized because the preponderance of the evidence indicates that anesthesia prevents the reflex in this species (27, 32, 35).
A laminectomy exposing the lower lumbar and sacral portions of the spinal cord (L1–L5) was performed. The rat was then secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the skin on the back, we formed a pool, which was filled with warm (37°C) mineral oil. The dura was cut and reflected, allowing the visual identification of the spinal roots. The left L4 and L5 ventral roots were identified and cut close to their exits from the spinal cord. The calcaneal bone of a left hindlimb was severed, and the triceps surae muscles were isolated. Once the surgeries had been completed, the anesthesia was withdrawn, and the lungs were ventilated with room air. A minimum recovery period of 90 min was used after decerebration before any experimental protocol was started.
Experimental protocols.
The L4 and L5 ventral roots were placed on shielded stimulating electrodes. The cut end of each calcaneal tendon was attached to a force transducer (model FT 10, Grass), which, in turn, was attached to a rack and pinion. The tendon was stretched so that baseline tension was set between 50 and 100 g. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, >2 times motor threshold) the cut peripheral ends of the L4 and L5 ventral roots. A muscle mechanoreceptor reflex (9) was evoked by stretching the triceps surae muscles by manually turning the rack and pinion, which was attached to the calcaneal tendon. Baseline tension was set between 50 and 100 g. Both muscle contraction and tendon stretch lasted for 60 s. We attempted to match the magnitudes of the tension traces for static contraction and tendon stretch. The order of presentation of the two stimuli was varied randomly.
In 18 of 49 rats, we stimulated μ-opioid receptors by injecting the μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly-ol5]enkephalin (DAMGO; Sigma-Aldrich, 1 μg) retrogradely into the contralateral femoral artery catheter. In seven rats whose left femoral artery had been occluded for 72 h before the experiment, the nonselective opioid receptor antagonist naloxone (100 μg, Sigma-Aldrich) was injected in conjunction with DAMGO (1 μg) retrogradely into the right femoral arterial catheter. In another 12 rats, saline (200 μl) was injected into the contralateral femoral artery catheter as a vehicle control. In these 37 rats, the vascular occluder was inflated before the injection of naloxone and/or DAMGO or saline to trap the injectate in the circulation of the contracted limb. The inflation was maintained for 5 min, after which it was deflated and the hindlimb was reperfused. Fifteen minutes after reperfusion, static contraction was evoked again. In the remaining 12 rats, 1 μg DAMGO was injected into the jugular vein to test whether our findings with the intra-arterial injection of this opioid could be explained by its circulation to the spinal cord. Static contraction was evoked at least 15 min after the injection. All contractions lasted for 60 s.
At the end of each experiment in which we injected naloxone and/or DAMGO into the hindlimb circulation, we injected blue dye retrogradely into the contralateral femoral arterial catheter. In each case, the triceps surae muscles were stained blue, verifying that naloxone and/or DAMGO had access to this muscle group.
Data analysis.
Arterial blood pressure, HR, muscle tension, and the electrocardiogram were recorded with a Spike 2 data-acquisition system (CED) and stored on a computer hard drive (Dell). Mean arterial pressure (MAP) is expressed in mmHg, and HR is expressed in beats per minute. The initial 60-s values were used to compare the differences between baseline and the response to each maneuver. In addition, the time course of MAP and HR responses to static contraction were analyzed at 2 s after the onset of each maneuver and then at each 5-s time point until the maneuver ended. The tension-time index (TTI; in kg·s) was calculated by integrating the area between the tension trace and the baseline level (Spike 2).
All values are expressed as means ± SE. Statistical comparisons were performed with either one-way repeated-measures ANOVA or two-way repeated-measures ANOVA. Post hoc tests were then performed with the Tukey test between individual means. Bonferroni post hoc tests were used to determine significant differences between time course means. The criterion for statistical significance was set at P < 0.05.
RESULTS
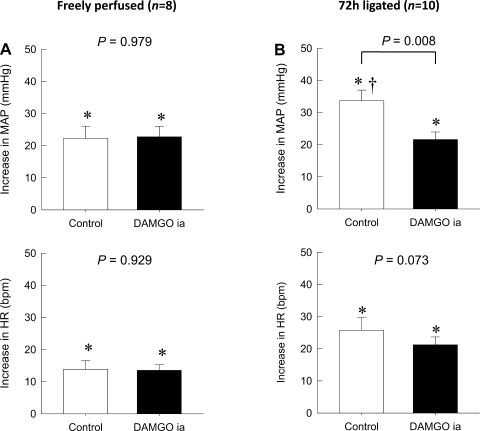
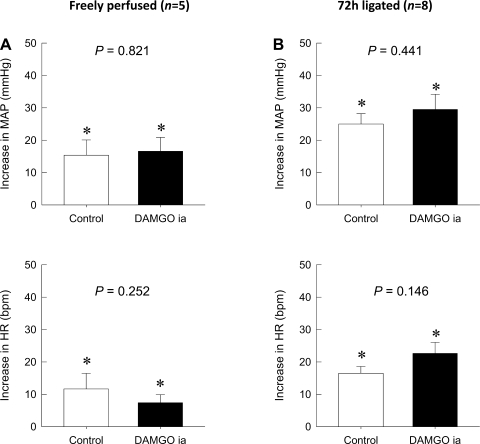
As previously shown (33), the pressor response to static contraction in rats whose femoral artery was ligated 72 h before the start of the experiment was significantly greater than the pressor response in control rats whose hindlimbs were freely perfused (P < 0.05; Fig. 1). The intra-arterial injection of either DAMGO (1 μg) or saline had no significant effect on either baseline MAP or HR in either treatment group (Table 1). Although 5 min of occlusion of the abdominal aorta and inferior vena cava by the inflation of the vascular occluder transiently decreased MAP and increased HR, these parameters returned to their baseline levels within 15 min of the reperfusion period. In control rats (n = 8) whose hindlimb was freely perfused, the average peak pressor response to static contraction (22 ± 4 mmHg) was not affected by the intra-arterial injection of DAMGO (23 ± 3 mmHg, P = 0.979; Fig. 1). Likewise, the peak increase in HR during static contraction (14 ± 3 beats/min) was not affected by the intra-arterial injection of DAMGO (14 ± 2 beats/min, P = 0.929). In contrast, in rats (n = 10) whose femoral artery was occluded 72 h before the start of the experiment, the average peak pressor response to static contraction (34 ± 3 mmHg) was attenuated by the intra-arterial injection of DAMGO (22 ± 2 mmHg, P = 0.008). On the other hand, the peak increase in HR during static contraction (26 ± 4 beats/min) in these rats was not affected by the intra-arterial injection of DAMGO (21 ± 2 beats/min, P = 0.073).
Fig. 1.
Effect of an intra-arterial (ia) injection of the selective μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly-ol5]enkephalin (DAMGO) on reflex increases in mean arterial pressure (MAP; in mmHg) and heart rate [HR; in beats/min (bpm)] during static contraction. A: the peak increases of MAP and HR during static contraction in control rats were not affected by the intra-arterial injection of DAMGO. B: the augmented peak increases of MAP during static contraction in rats whose left femoral artery was occluded for 72 h previously were diminished by the intra-arterial injection of DAMGO (P = 0.008). The pressor response to contraction in the ligated rats was significantly greater (†P < 0.05) than that to contraction in their freely perfused counterparts in A. On the other hand, the peak increases of HR during static contraction in these rats were not affected by the intra-arterial injection of DAMGO (P = 0.073). Values are means ± SE. The pressor responses to contraction in each group were significantly increased from their respective baselines (*P < 0.05). The horizontal brackets signify that the responses were significantly different from each other (P < 0.05).
Table 1.
Baseline MAP and HR before and after the administration of DAMGO, saline, and naloxone with DAMGO
| n | MAP, mmHg | HR, beats/min | |
|---|---|---|---|
| Freely perfused group | |||
| Intra-arterial administration (DAMGO treatment) | |||
| Control | 8 | 90.4 ± 6.7 | 436.4 ± 14.4 |
| DAMGO | 8 | 8.14 ± 3.8 | 444.5 ± 16.5 |
| Intravenous adminstration | |||
| Control | 6 | 73.6 ± 11.1 | 470.7 ± 25.8 |
| DAMGO | 6 | 76.3 ± 11.3 | 476.1 ± 17.9 |
| Intra-arterial adminstration (saline treatment) | |||
| Control | 6 | 79.8 ± 4.6 | 491.3 ± 18.4 |
| Saline | 6 | 74.8 ± 2.4 | 493.8 ± 15.3 |
| 72-h occluded group | |||
| Intra-arterial administration (DAMGO treatment) | |||
| Control | 10 | 89.7 ± 4.7 | 404.1 ± 9.0 |
| DAMGO | 10 | 92.7 ± 1.7 | 396.6 ± 10.0 |
| Intravenous administration | |||
| Control | 6 | 87.6 ± 4.6 | 421.5 ± 10.5 |
| DAMGO | 6 | 86.0 ± 5.0 | 410.4 ± 6.9 |
| Intra-arterial administration (saline treatment) | |||
| Control | 6 | 86.1 ± 2.4 | 392.2 ± 16.3 |
| Saline | 6 | 86.2 ± 5.0 | 400.3 ± 13.6 |
| Intra-arterial administration (DAMGO + naloxone treatment) | |||
| Control | 7 | 77.5 ± 4.3 | 406.8 ± 10.0 |
| Naloxone and DAMGO | 7 | 85.6 ± 5.3 | 411.6 ± 12.9 |
Values are means ± SE; n, no. of rats/group. MAP, mean arterial pressure; HR, heart rate; DAMGO, [d-Ala2,N-MePhe4,Gly-ol5]enkephalin; freely perfused group, control rats whose hindlimb was freely perfused; 72-h occluded group, rats whose femoral artery had been ligated 72 h before the start of the experiment.
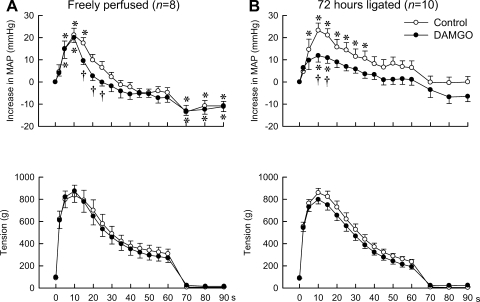
Next, we examined the effect of DAMGO on the topography of the pressor response to static contraction. We did so by plotting at 2 s, 5 s, and every 5-s interval thereafter from the onset of static contraction, the change in MAP from baseline values for 90 s. Before DAMGO was given, static contraction of the hindlimb, regardless of whether its femoral artery had been occluded or not, rapidly increased MAP, effects that reached their peak levels at ∼10 s from the onset of tension development (Fig. 2). Subsequently, the increases in MAP gradually decreased from their peak levels even though the hindlimb muscles continued to contract. DAMGO had minimal statistically significant effects on the topography of the pressor response to static contraction in the rats whose hindlimbs were freely perfused (Fig. 2). Specifically, the only time point that was affected by DAMGO was at 15 s into the contraction period. At this point, the pressor response to contraction was significantly above baseline before DAMGO but was not significantly above baseline after DAMGO.
Fig. 2.
Time courses of the average changes in MAP and triceps surae muscle tension during static contraction before and after the intra-arterial injection of DAMGO. A: although the intra-arterial injection of DAMGO did not attenuate the peak levels of the pressor response to static contraction, it attenuated the subsequent pressor response in rats whose hindlimbs were freely perfused. B: on the other hand, the intra-arterial injection of DAMGO attenuated both the peak and subsequent pressor responses to static contraction in rats whose femoral artery had been ligated for 72 h previously. The time courses of the muscle tension were the same before and after the administration of DAMGO into the hindlimb circulation. Note that the maximum values of the increase in MAP obtained by averaging the consecutive data points were smaller than the averaged peak increase of MAP shown in Fig. 1, because the time points when MAP values reached their peak during static contraction were different in each trial. Values are means ± SE. *MAP values were significantly increased from their respective baselines (P < 0.05). †Values at the same time point were significantly different from each other (P < 0.05).
In contrast to the rats whose hindlimbs were freely perfused, DAMGO had significant effects on the topography of the pressor response to contraction in rats whose femoral artery had been ligated 72 h before the start of the experiment. Specifically, the pressor response to contraction before DAMGO was significantly above baseline values at the 5- to 35-s time points of the contraction periods, whereas the response to contraction after DAMGO was only significantly above baseline values at the 10- and 15-s time points of the contraction period (Fig. 2).
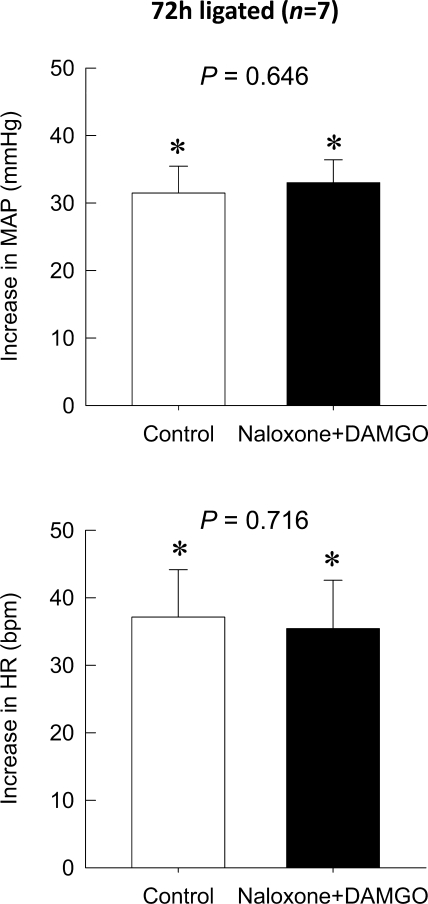
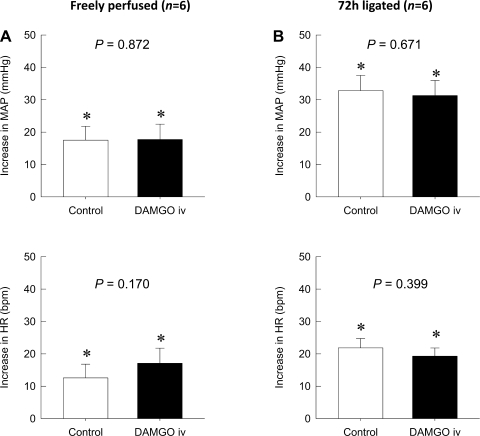
The intra-arterial injection of naloxone with the concomitant administration of DAMGO completely blocked the attenuating effect of DAMGO on the augmented pressor response to static contraction in rats whose left femoral arteries were ligated for 72 h before the start of the experiment (P = 0.646; Fig. 3). Naloxone in conjunction with DAMGO also did not affect the peak HR response to static contraction in the hindlimb whose femoral artery had been occluded 72 h previously (P = 0.716). An intravenous injection of DAMGO at the dose we used in this study (1 μg) did not affect either the pressor or HR responses to static contraction regardless of whether the femoral artery had been occluded for 72 h previously or not (Fig. 4). Likewise, TTIs were not affected by any drugs regardless of its route of administration or whether the femoral artery had been ligated for 72 h before the start of the experiment or not (Table 2).
Fig. 3.
Effect of the coadministration of the μ-opioid receptor antagonist naloxone with DAMGO on the pressor reflex to static contraction in the hindlimb whose femoral artery had been ligated for 72 h. The coadministration of naloxone (100 μg) with DAMGO (1 μg) into the hindlimb circulation antagonized the inhibitory action of DAMGO on the pressor response to static contraction in the hindlimb whose left femoral artery had been occluded for 72 h previously (P = 0.646). Peak increases of HR during static contraction were not affected by the coadministration of naloxone with DAMGO (P = 0.716) in the same rats. Values are means ± SE; n = 7. The pressor responses to contraction were significantly increased from their respective baselines (*P < 0.05).
Fig. 4.
Effect of the intravenous (iv) injection of the selective μ-opioid receptor agonist DAMGO on reflex increases in MAP and HR during static contraction. A: the intravenous injection of DAMGO (1 μg) did not affect the peak increases of both MAP (P = 0.872) and HR (P = 0.170) during static contraction in control rats. B: the intravenous injection of DAMGO (1 μg) also had no effect on the peak increases of MAP (P = 0.671) and HR (P = 0.399) during static contraction in rats whose left femoral artery had been occluded for 72 h previously. Values are means ± SE; n = 6. The pressor and cardioaccelerator responses to contraction in each group were significantly increased from their respective baselines (*P < 0.05).
Table 2.
TTIs during static contraction
| TTI, kg·s |
|||
|---|---|---|---|
| n | Before | After | |
| Freely perfused group | |||
| Intra-arterial DAMGO | 8 | 24.8 ± 3.6 | 24.3 ± 3.9 |
| Intravenous DAMGO | 6 | 19.6 ± 1.5 | 18.7 ± 1.7 |
| Intra-arterial saline | 6 | 17.4 ± 2.1 | 17.3 ± 2.2 |
| 72-h occluded group | |||
| Intra-arterial DAMGO | 10 | 26.8 ± 1.9 | 23.2 ± 1.7 |
| Intravenous DAMGO | 6 | 22.0 ± 1.7 | 18.3 ± 2.0 |
| Intra-arterial saline | 6 | 23.5 ± 1.9 | 20.2 ± 4.2 |
| Intra-arterial naloxone and DAMGO | 7 | 28.8 ± 2.7 | 27.7 ± 3.0 |
Values are means ± SE; n, no. of rats/group. TTI, tension-time index.
We next examined the effect of DAMGO (1 μg), injected into the femoral artery, on the reflex pressor response to tendon stretch (28). The intra-arterial injection of DAMGO (1 μg) had no significant effect on either baseline MAP or HR in either treatment group (Table 3). DAMGO also had no effect on either the pressor or cardioaccelerator responses to stretch in either the freely perfused limbs or in those whose femoral arteries were ligated for 72 h before the start of the experiments (Fig. 5). TTIs during tendon stretch were not different before and after the administration of DAMGO (Table 3).
Table 3.
Baseline MAP and HR immediately before tendon stretch and TTIs during tendon stretch before and after the intra-arterial administration of DAMGO
| n | MAP, mmHg | HR, beats/min | TTI, kg·s | |
|---|---|---|---|---|
| Freely perfused group | ||||
| Control | 5 | 86.6 ± 7.1 | 427.8 ± 11.8 | 32.9 ± 3.4 |
| Intra-arterial DAMGO | 5 | 85.9 ± 13.3 | 424.8 ± 12.8 | 34.1 ± 0.4 |
| 72-h occluded group | ||||
| Control | 8 | 85.1 ± 3.5 | 401.3 ± 17.5 | 31.9 ± 1.2 |
| Intra-arterial DAMGO | 8 | 89.4 ± 3.6 | 391.9 ± 14.4 | 33.9 ± 0.7 |
Values are means ± SE; n, no. of rats/group.
Fig. 5.
Effect of the intra-arterial injection of DAMGO on reflex increases in MAP and HR during tendon stretch. A and B: peak increases of MAP and HR during tendon stretch were not affected by the intraarterial injection of DAMGO in both groups [freely perfused hindlimb (A) or 72-h ligated (B) groups]. Values are means ± SE. The pressor responses to tendon stretch in each group were significantly increased from their respective baselines (*P < 0.05).
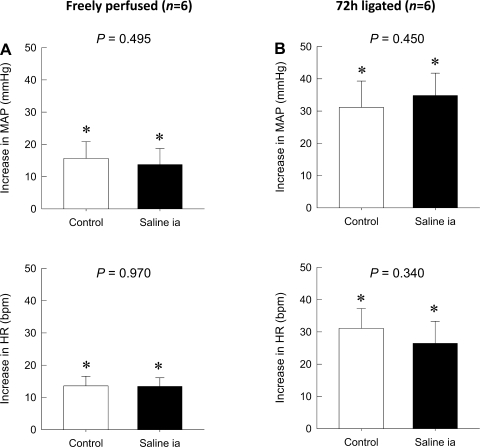
Finally, neither peak pressor nor cardioaccelerator responses to static contraction were altered by the intra-arterial injection of saline regardless of whether or not the femoral artery had been occluded for 72 h previously or not (P > 0.05; Fig. 6).
Fig. 6.
Effect of the intra-arterial injection of saline on reflex increases in MAP and HR during static contraction. A: the intra-arterial injection of saline (200 μl) did not affect the peak increases in both MAP and HR during static contraction in control rats. B: the intra-arterial injection of saline (200 μl) also had no effect on the peak increases in MAP and HR during static contraction in rats whose left femoral artery had been occluded for 72 h previously. Values are means ± SE; n = 6. The pressor and cardioaccelerator responses to contraction in each group were significantly increased from their respective baselines (*P < 0.05).
DISCUSSION
We found that DAMGO, a μ-opioid receptor agonist, injected into the arterial supply of a hindlimb whose femoral artery had been ligated for 72 h before the start of the experiment, significantly attenuated the exercise pressor reflex. In contrast, DAMGO had only a minimal effect on the reflex in rats whose hindlimbs were freely perfused. We also found that the attenuation by DAMGO of the exercise pressor reflex in the rats whose femoral arteries were ligated was prevented by naloxone, an opioid receptor antagonist. Moreover, the reflex was not attenuated when DAMGO was injected intravenously, a finding that indicates that DAMGO did not exert its attenuating effect by circulating to the spinal cord or brain stem. These findings indicate that the stimulation of μ-opioid receptors accessible from the femoral arterial supply to the hindlimb muscles was responsible for the attenuation of the exercise pressor reflex. Thus, the simplest conclusion to draw from our findings is that DAMGO stimulated μ-opioid receptors on the endings of group III and IV muscle afferents to attenuate the exercise pressor reflex.
Little is known both about the effects of opioids on the discharge properties of group III and IV muscle afferents. Likewise, little is known about the effects of opioids in the periphery either on alleviating pain arising from skeletal muscle or on attenuating the exercise pressor reflex. In contrast, the role played by opioids on cutaneous and articular nociception has received considerable attention. Consequently, interpreting our findings in muscle based on what is known about skin might be informative and might allow us to draw some useful parallels. In particular, our finding that DAMGO had only modest effects on the exercise pressor reflex in freely perfused hindlimbs seems to parallel the lack of effect of opioids on cutaneous nociception in noninflamed skin (22). In general, opioids applied to healthy tissues have been shown to have substantially less analgesic effects than when they are applied to damaged tissue (29). Taking these findings into consideration, we speculate that stimulation of peripheral opioid receptors expressed on the group III and IV muscle afferents would play a role in alleviating the augmented exercise pressor reflex only when the contracted muscles are stressed or damaged.
Although four major subtypes (δ, κ, μ, and nociceptin/orphanin FQ) of opioid receptors have identified to date, the functional differences among these receptors are still not well characterized. Inflammation in tissues such as the skin and joints is known to increase μ-opioid receptor numbers in dorsal root ganglion cells. For example, an injection of the rat hindpaw with Freund's complete adjuvant increased the mRNA in dorsal root ganglion cells for μ-opioid receptors but had no effect on the mRNA for δ-opioid receptors (24). Moreover, axonal transport of μ-opioid receptors from the cell nucleus in the dorsal root ganglion to sensory endings in the periphery was enhanced after the hindpaw injection of Freund's complete adjuvant. In noninjected control rats, μ-opioid receptor RNA levels have been shown to be about seven times higher than δ-opioid receptor RNA levels in lumbar dorsal root ganglion cells (3, 24), a finding that provides a strong rationale for examining, in our experiments, the effect of μ-opioid receptor agonists, such as DAMGO, on the exercise pressor reflex.
Recently, μ-opioid receptors on cutaneous sensory nerves in mice have been shown to be distributed exclusively on peptidergic (i.e., substance P containing) C-fiber afferents, whereas δ-opioid receptors have been shown to be distributed exclusively on nonpeptidergic Aδ- and C-fiber afferents (25). Moreover, the selective distribution of opioid receptors was found on both the peripheral and central terminals of these thin fiber afferents. In these mice, the stimulation of μ-opioid receptors with DAMGO reduced responsiveness to noxious heat stimulation but had no effect on responsiveness to noxious mechanical stimulation. In contrast, the stimulation of δ-opioid receptors with SCN80, a potent and highly selective δ-opioid receptor agonist, reduced responsiveness to noxious mechanical stimulation but had no effect on responsiveness to noxious heat stimulation (25). The above findings concerning cutaneous sensation might provide a useful context with which to view our results concerning static contraction, which is both a mechanical and metabolic stimulus to group III and IV muscle afferents, and stretch, which is solely a mechanical stimulus, to these thin fiber afferents (28). Extending the findings of Scherrer et al. (25) to those of our own, we offer the speculation that DAMGO attenuated the augmented exercise pressor reflex in limbs whose femoral arteries were ligated by inhibiting the responsiveness of group IV metaboreceptors (13, 14) to contraction. We further speculate that DAMGO had no effect on the responsiveness of group III mechanoreceptors (13, 14) to contraction. This last conclusion, nevertheless, needs some qualification. Specifically, only about half of the group III mechanoreceptors that respond to tendon stretch also respond to static contraction (9). Consequently, tendon stretch is not an adequate tool with which to test the responsiveness of group III mechanoreceptors to static contraction. Nevertheless, the fact that DAMGO had no effect on the pressor reflex evoked by tendon stretch is consistent with the theory postulated by Scherrer et al. (25): that μ-opioid receptors are not found on mechanoreceptors.
One limitation of our findings is that they only apply to the effect of peripheral μ-opioid receptors on the exercise pressor reflex. Among others, δ- and κ-opioid receptors may also have a role to play in the periphery in attenuating the reflex. In particular, δ-opioid receptors may be important in controlling input from group III mechanoreceptors. Indeed, an injection of a specific agonist to δ-opioid receptors onto the surface of the spinal cord near the entry point of the dorsal roots has been found to attenuate the exercise pressor reflex (10). The possibility exists that this δ-opioid receptor agonist acted presynaptically to decrease neurotransmitter and/or neuromodulator release from the terminals of group III mechanoreceptors responding to static contraction. It remains to be shown that the peripheral stimulation of δ-opioid receptors on the sensory endings of thin fiber muscle afferents can also attenuate the reflex.
In any event, we have shown that the stimulation of peripheral μ-opioid receptors on thin fiber muscle afferents plays a role in attenuating the exercise pressor reflex in rats whose femoral arteries have been occluded for 72 h. We have also shown that these peripheral μ-opioid receptors play a very modest role in attenuating the reflex in freely perfused muscles. The preparation we used in our experiments is the same as the one developed by Terjung and colleagues (23, 30) and is viewed by some as an animal model of peripheral vascular disease. To the extent that this is the case, then the stimulation of μ-opioid receptors in the periphery may prove useful in the treatment of the pain and excessive cardiovascular responses induced by exercise in patients with peripheral vascular disease (1).
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant RO1-AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Jennifer Probst and Sarah Simmonds for technical assistance.
REFERENCES
- 1.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bickel U, Schumacher OP, Kang YS, Voigt K. Poor permeability of morphine 3-glucuronide and morphine 6-glucuronide through the blood-brain barrier in the rat. J Pharmacol Exp Ther 278: 107–113, 1996 [PubMed] [Google Scholar]
- 3.Buzas B, Cox BM. Quantitative analysis of mu and delta opioid receptor gene expression in rat brain and peripheral ganglia using competitive polymerase chain reaction. Neuroscience 76: 479–489, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res 764: 126–132, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Go VLW, Yaksh TL. Release of substance P from the cat spinal cord. J Physiol 391: 141–167, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan AH, Ableitner A, Stein C, Herz A. Inflammation of the rat paw enhances axonal transport of opioid receptors in the sciatic nerve and increases their density in the inflamed tissue. Neuroscience 55: 185–195, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Kaufman MP. Attenuation of reflex pressor and ventilatory responses to static muscular contraction by intrathecal opioids. J Appl Physiol 68: 2466–2472, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Howe JR, Yaksh TL, Go VL. The effect of unilateral dorsal root ganglionectomies or ventral rhizotomies on alpha 2-adrenoceptor binding to, and the substance P, enkephalin, and neurotensin content of, the cat lumbar spinal cord. Neuroscience 21: 385–394, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature 268: 549–551, 1977 [DOI] [PubMed] [Google Scholar]
- 13.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 15.Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci 25: 3651–3660, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Wu L, Li YQ. Opioid peptides modulate the response of neurons of the superficial laminae of the rat spinal dorsal horn to GABA. Biochem Biophys Res Commun 307: 730–736, 2003 [DOI] [PubMed] [Google Scholar]
- 17.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McWilliam PN, Yang T. Inhibition of cardiac vagal component of baroreflex by group III and IV afferents. Am J Physiol Heart Circ Physiol 260: H730–H734, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Meintjes AF, Nobrega ACL, Fuchs IE, Ally A, Wilson LB. Attenuation of the exercise pressor reflex. Effect of opioid agonist on substance P release in L-7 dorsal horn of cats. Circ Res 77: 326–334, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Mousa SA, Zhang Q, Sitte N, Ji R, Stein C. β-Endorphin-containing memory-cells and mu-opioid receptors undergo transport to peripheral inflamed tissue. J Neuroimmunol 115: 71–78, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Nunez S, Lee JS, Zhang Y, Bai G, Ro JY. Role of peripheral μ-opioid receptors in inflammatory orofacial muscle pain. Neuroscience 146: 1346–1354, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience 129: 473–479, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 137: 1148–1159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered μ- and δ-, but not κ-, opioid receptor agonists. J Pharmacol Exp Ther 302: 1146–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Smith SA, Mitchell GS, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Stein C, Zollner C. Opioids and sensory nerves. Handb Exp Pharmacol 495–518, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Taylor JC, Li Z, Yang HT, Laughlin MH, Terjung RL. α-Adrenergic inhibition increases collateral circuit conductance in rats following acute occlusion of the femoral artery. J Physiol 586: 1649–1667, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lotsch J. Peripheral opioid analgesia in experimental human pain models. Brain 126: 1092–1102, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Toney GM, Mifflin SW. Mediators of contraction-evoked skeletal muscle depressor response in anesthetized rats. J Appl Physiol 81: 578–585, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64: 592–599, 1989 [DOI] [PubMed] [Google Scholar]
- 35.Vissing J, Wilson LB, Mitchell JH, Victor RG. Static muscle contraction reflexly increases adrenal sympathetic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol 261: R1307–R1312, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Wenk HN, Brederson JD, Honda CN. Morphine directly inhibits nociceptors in inflamed skin. J Neurophysiol 95: 2083–2097, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Zollner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest 118: 1065–1073, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M. Painful inflammation-induced increase in μ-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol 64: 202–210, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Zollner C, Stein C. Opioids. Handb Exp Pharmacol 31–63, 2007 [DOI] [PubMed] [Google Scholar]