Abstract
Erythrocytes release both O2 and a vasodilator, ATP, when exposed to reduced O2 tension. We investigated the hypothesis that ATP release is impaired in erythrocytes of humans with type 2 diabetes (DM2) and that this defect compromises the ability of these cells to stimulate dilation of resistance vessels. We also determined whether a general vasodilator, the prostacyclin analog iloprost (ILO), stimulates ATP release from healthy human (HH) and DM2 erythrocytes. Finally, we used a computational model to compare the effect on tissue O2 levels of increases in blood flow directed to areas of increased O2 demand (erythrocyte ATP release) with nondirected increases in flow (ILO). HH erythrocytes, but not DM2 cells, released increased amounts of ATP when exposed to reduced O2 tension (Po2 < 30 mmHg). In addition, isolated hamster skeletal muscle arterioles dilated in response to similar decreases in extraluminal O2 when perfused with HH erythrocytes, but not when perfused with DM2 erythrocytes. In contrast, both HH and DM2 erythrocytes released ATP in response to ILO. In the case of DM2 erythrocytes, amounts of ATP released correlated inversely with glycemic control. Modeling revealed that a functional regulatory system that directs blood flow to areas of need (low O2-induced ATP release) provides appropriate levels of tissue oxygenation and that this level of the matching of O2 delivery with demand in skeletal muscle cannot be achieved with a general vasodilator. These results suggest that the inability of erythrocytes to release ATP in response to exposure to low-O2 tension could contribute to the peripheral vascular disease of DM2.
Keywords: muscle blood flow, red blood cells, oxygen delivery, prostacyclin
cardiovascular disease accounts for nearly half of deaths in humans with type 2 diabetes (DM2) (31, 41). It is estimated that in 2010 there will be in excess of 300 million cases of DM2 worldwide making this disease a major public health challenge (4). A significant complication of DM2 is impaired vascular function that contributes to a fourfold increased risk for claudication (4) and as much as a 16-fold increased risk for lower limb amputation (12, 30, 43). Although individuals with DM2 have an increased incidence of atherosclerosis in large-conduit vessels (4, 30), there is extensive evidence that microvascular circulatory control is also abnormal in humans with DM2 (23, 24, 27, 35). Although direct studies of the skeletal muscle microcirculation are not possible in humans, such studies have been performed in several animal models of diabetes and reveal reduced convective O2 delivery and diffusive O2 transport that could contribute to a failure of the skeletal muscle vasculature to deliver appropriate amounts of O2 to meet metabolic need both at rest and during exercise (2, 13, 34). Taken together, these reports strongly suggest that, in DM2, O2 delivery to skeletal muscle in amounts required to appropriately meet metabolic need is impaired; although the mechanism(s) by which this impairment occurs have yet to be fully elucidated.
The O2 required to meet the metabolic needs of all tissues is delivered by the erythrocyte, a small, flexible cell containing hemoglobin which, in mammals, is devoid of a nucleus and mitochondria. Recently, it has been demonstrated that this cell is significantly more than a simple O2 transporter, but rather is a complex cell that controls its own distribution within the microcirculation via its ability to release adenosine triphosphate (ATP) in response to physiological stimuli, including exposure to reduced O2 tension (5, 6, 26, 32, 33, 38). This erythrocyte-derived ATP stimulates the synthesis of endothelium-derived vasodilators resulting in local increases in blood flow and, thereby, erythrocyte supply rate, permitting this cell to deliver O2 in amounts required to precisely meet local metabolic need (9–11, 19). Thus, failure of the erythrocyte to release ATP in response to reduced O2 tension could be expected to lead to impaired matching of O2 delivery with need in skeletal muscle and, thereby, contribute to vascular disease. The activity of the heterotrimeric G protein, Gi, is required for ATP release from erythrocytes in response to reduced O2 (32, 33), wheras a second G protein, Gs, is associated with a prostacyclin receptor (IP) (32). It has been shown that Gαi2 expression is decreased in erythrocytes of humans with DM2 and this defect is associated with impairment of both cAMP accumulation and ATP release when these cells are incubated with a direct activator of Gi (21, 39), suggesting that this defect in erythrocyte physiology could contribute to the associated vascular disease. Interestingly, expression of Gs is unaltered in DM2 erythrocytes, suggesting that prostacyclin-induced ATP release might remain intact.
In this study, we evaluated ATP release from erythrocytes of humans with DM2 in response to exposure to reduced O2 tension as well as activation of the IP receptor by the prostacyclin analog, iloprost (ILO). We determined that although the release of ATP in response to ILO is intact in DM2, and is, in fact, greater in individuals with poorer glycemic control, ATP release in response to reduced O2 tension is absent. In addition, we determined that there is a functional consequence of the defect in low O2-induced ATP release as demonstrated by the failure of isolated skeletal muscle resistance vessels to dilate in response to decreases in extraluminal O2 tension when perfused with DM2 erythrocytes. Finally, we use a computational model of O2 transport to address the question of whether vasodilators such as ILO that nonselectively increase blood flow can restore tissue oxygenation to undersupplied regions of tissue. The simulation results highlight the importance of a functional regulatory system that directs blood flow to where it is needed rather than a vasodilator that simply increases total skeletal muscle blood flow.
MATERIALS AND METHODS
Isolation of human erythrocytes.
Blood was obtained from healthy volunteers (n = 18) and patients with DM2 (n = 24) by venipuncture using a syringe containing heparin (500 U) and centrifuged at 500 g at 4°C for 10 min. The plasma, buffy coat, and uppermost erythrocytes were removed by aspiration and discarded. The remaining erythrocytes were washed three times in buffer containing (in mM) 21.0 tris(hydroxymethyl)- aminomethane, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 5.5 glucose, and 0.5% BSA, final pH 7.4. Erythrocytes isolated in this fashion contain less than 1 leukocyte per 50 high-power fields (8–10 leukocytes per mm3) and are devoid of platelets (21). Cells were prepared on the day of use.
Measurement of ATP.
ATP was measured using the luciferin-luciferase assay as described previously (11, 21). A 200-μl sample of erythrocyte suspension (0.04% hematocrit) was injected into a cuvette containing 100 μl of firefly lantern extract (10 mg/ml, FLE 250; Sigma) and 100 μl of a solution of synthetic d-luciferin (50 mg/100 ml; Sigma). The light emitted was detected using a luminometer (Turner Designs). A standard curve was generated for each experiment. Cell counts were obtained by direct counting using a hemocytometer and amounts of ATP measured were normalized to 4 × 108 cells/ml.
Determination of ATP release from erythrocytes in response to exposure to reduced O2 tension.
Isolated erythrocytes were diluted to a 20% hematocrit in a Ringer buffer containing (in mM) 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 11 glucose, 23.8 NaHCO3 with 0.2% dextrose and 0.5% BSA, final pH 7.4 at 37°C in a tonometer equilibrated with 6% CO2. Erythrocytes were equilibrated for 30 min in the tonometer (Instrumentation Laboratory) with a gas mixture containing 15% O2, 6% CO2, balance N2 (normoxia). The gas mixture was then changed to one containing 4.5% O2, 6% CO2, balance N2, followed by 0% O2, 6% CO2, balance N2. The pH, Po2, and Pco2 were determined after a 10-min exposure to each gas mixture using a blood gas analyzer (model pHOx, Nova Biomedical). The amount of ATP released from erythrocytes was determined during normoxia and following the 10-min exposure to each gas mixture.
Isolation, cannulation, and perfusion of arterioles from hamster skeletal muscle.
Male golden hamsters (103 ± 5 g) were anesthetized with pentobarbital sodium (6.5 mg/100 g ip). The right cheek pouch retractor muscle was separated from underlying muscles and a clip was used to secure two ligatures to the muscle. The muscle was cut at its spinal end and placed ventral side up, at its in situ dimensions on a Plexiglas platform and covered with Saran (Dow Corning) to prevent desiccation (42). Unbranched segments of first- and second-order arterioles, ∼1,000 μm in length, were removed, trimmed, and cleared of connective tissue while immersed in cold (4°C) modified Ringer buffer containing (in mM) 144.0 NaCl, 3.0 KCl, 2.5 CaCl2, 1.5 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 2.0 3-[N-morpholino]-propanesulfonic acid (MOPS), 1.21 NaH2PO4, and 1% BSA with pH adjusted to 7.4. The vessel was transferred to a microscope stage-mounted organ bath (2.5 ml) containing the Ringer buffer described above but without albumin. The arterioles were cannulated using glass pipettes mounted on micromanipulators attached to the microscope base. Each end of the vessel was, in turn, aspirated into a holding pipette using a controlled vacuum and cannulated with a perfusion pipette filled with the albumin containing Ringer buffer. Following stabilization, intraluminal pressure was increased to 60 mmHg while the bath temperature was increased to 37°C and the vessel was allowed to develop spontaneous myogenic tone (30–45 min). The vessel was viewed using a Zeiss Axiovert 100 inverted microscope with long working distance objectives (×10 and ×20). The microscope image was recorded using a high-resolution, closed-circuit video system. Vessel diameter was determined off-line using both an automated system (Diamtrax, v3.5) and direct measurement using a video caliper (model 308, Colorado Video). Vessel viability was determined by the demonstration of constriction to pH 7.65 and dilation to pH 6.80. Vessels were perfused with albumin containing Ringer buffer at 3 μl/min using a three-syringe, microinjection pump (model CMA/100, CMA/Microdialysis). The perfusate was then switched by means of a micro-switch to one containing isolated erythrocytes from either healthy humans or humans with DM2 (hematocrit 17.5%) that was oxygenated by equilibration with room air. Care was taken to ensure that the erythrocytes were well-distributed within the syringe. The Po2 in the vessel chamber, the extraluminal O2 tension, was determined using a microelectrode (MI 730, Microelectrodes). Initially, the buffer surrounding the vessel was equilibrated with room air (extraluminal Po2 = 145 ± 2 mmHg). After stability was achieved and vessel diameter was recorded, the extraluminal buffer was replaced with buffer equilibrated with 100% nitrogen (extraluminal Po2 = 18 ± 3 mmHg) and vessel diameter was recorded.
Determination of ATP release from erythrocytes in response to incubation with ILO.
Isolated erythrocytes were diluted to a hematocrit of 20% in wash buffer and incubated with either ILO (1 μM, Cayman) or its vehicle, saline. ATP release was measured 5, 10, and 15 min after administration of ILO and maximal ATP release was reported.
Measurement of total intracellular ATP of erythrocytes.
A known number of erythrocytes, determined by counting, were lysed by dilution 1:8,000 with distilled water. ATP was measured as described above. Values were normalized to ATP concentration per erythrocyte.
Measurement of free hemoglobin.
To exclude the influence of hemolysis in studies where the release of ATP was measured, samples were centrifuged at 500 g at 4°C for 10 min and the presence of free hemoglobin in the supernatant was determined by light absorption at 405 nm. Using this approach, the sensitivity for detection of free hemoglobin is equal to that of the ATP assay. That is, in the absence of increases in free hemoglobin, ATP in the cell suspension cannot be attributed to lysis. If increases in free hemoglobin were detected, the studies were not included.
Computational model of oxygen transport by capillary networks.
Numerical simulations of steady-state O2 transport were performed using an established computational model (16–18) that couples the continuum partial differential equations describing convective transport by flowing blood in the capillaries to those describing oxygen diffusion and consumption in the tissue. The model includes both dissolved and hemoglobin-bound oxygen in the capillaries. Transport between the blood and tissue is described using a flux boundary condition with mass transfer coefficients calculated previously using a discrete erythrocyte model (7). For all O2 transport simulations, an array of 19 parallel capillaries was used to represent a typical capillary network (14, 15) with the placement of capillaries and relative distribution of hemodynamic parameters (erythrocyte velocity and hematocrit) determined from in vivo measurements in the rat extensor digitorum longus muscle (8). The tissue domain surrounding the capillary array was 80 × 331 × 300 μm. Capillary entrance saturations (71.5%) and the tissue O2 consumption rate (6.4 × 10−5 ml O2·ml−1·s−1) were set based on previous experimental data (8).
For the network with baseline blood flow (Q = 4.43 × 10−7 ml/s), average hemodynamic parameters in normal flow capillaries (15/19) were set to match measured values (34). For capillaries with erythrocyte supply rates (proportional to velocity × hematocrit) too high to measure (4/19), hematocrit was set to the average measured value (34) and velocity was set to 1,000 μm/s at baseline. Alterations from baseline blood flow in individual networks (0.25, 0.31, 1.23, and 4 times baseline flow Q) were achieved via uniform changes in erythrocyte velocity in all capillaries.
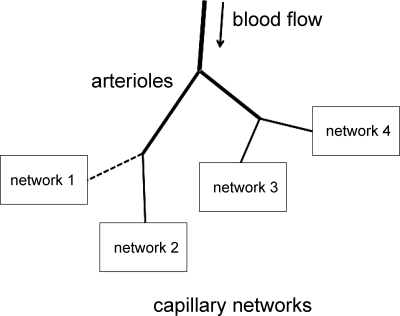
To estimate the effect of changes in local blood flow distribution on tissue Po2, a group of four capillary networks was used to represent a tissue. These four networks can be taken to be supplied by a single arteriole (Fig. 1). Since all four networks considered had the same structure and relative distribution of velocities and hematocrits, and were surrounded by the same amount of O2-consuming tissue, the only factor affecting O2 transport from the different networks was the amount of blood flow that each network received. Four different blood flow distributions were considered as shown in Table 1. To obtain tissue Po2 distributions for each case, calculated tissue Po2 distributions for the appropriate individual networks were combined (averaged) into a single Po2 distribution.
Fig. 1.
Schematic of simulation model. Blood flow to the 4 capillary networks is controlled by upstream arterioles. Nonselective dilation of arterioles will tend to cause uniform increases in flow to all 4 networks, while selective dilation can increase flow to the single undersupplied network only. Dashed line indicates initial undersupply of flow to network 1.
Table 1.
Blood flow distributions and calculated tissue oxygen tension
| Blood Flow |
Tissue Po2, mmHg |
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | Minimum | Means ± SD | |
| Network | |||||||
| Case 1 | 0.25Q | Q | Q | Q | 3.25Q | 37.9 | 46.4 ± 3.2 |
| Case 2 | 0.31Q | 1.23Q | 1.23Q | 1.23Q | 4Q | 39.4 | 47.0 ± 2.7 |
| Case 3 | Q | 4Q | 4Q | 4Q | 13Q | 48.6 | 49.5 ± 1.2 |
| Case 4 | Q | Q | Q | Q | 4Q | 45.5 | 47.9 ± 1.2 |
Q, flow; Po2, oxygen tension.
Data analysis.
Statistical significance among groups was determined using an ANOVA. In the event that the F ratio indicated that a change had occurred, a Fisher's least significant differences test was performed to identify individual differences. A Student's t-test was used where appropriate. Results are reported as means ± SE.
Institutional approval.
The protocol used to obtain blood from humans was approved by the Institutional Review Board of Saint Louis University. Participants gave written, informed consent. All record keeping was in strict compliance with Health Insurance Portability and Accountability Act regulations. The protocol for isolated arteriole studies using hamster vessels was approved by the Animal Care and Use Committee of Saint Louis University.
RESULTS
Characteristics of subjects studied.
Patients with DM2 were identified by physicians in the Endocrinology Clinic of Saint Louis University. Healthy volunteers were faculty and students at Saint Louis University School of Medicine. A history form was completed for each subject that included a detailed listing of all medical conditions and medications, age, and, in the case of humans with DM2, the degree of glycemic control was determined by measurement of hemoglobin A1c (HbA1c) within 4 wk of blood removal. The mean ages for healthy humans (n = 18, 10 males, 8 females) and humans with DM2 (n = 24, 17 males and 7 females) were 34 ± 3 (range 21 to 57) and 56 ± 3 yr (range 29 to 77), respectively. The average HbA1c of all humans with DM2 was 8.4 ± 0.3%. Erythrocyte ATP content in healthy humans and humans with DM2 did not differ and was 3.9 ± 0.5 and 3.2 ± 0.2 mM/cell, respectively. Patients with DM2 were treated with insulin (n = 10); oral hypoglycemic agents (n = 16); lipid-lowering agents (n = 14); antihypertensive drugs including angiotensin-converting enzyme inhibitors (n = 13), β-adrenergic receptor blockers (n = 10), calcium channel blockers (n = 6), thiazide diuretics (n = 6), and aspirin (n = 10). It is not possible to withdraw medications from humans with DM2 for the purpose of this study. However, there are no reports that the medications listed above alter ATP release from erythrocytes.
Effect of exposure to low-O2 tension on ATP release from the erythrocytes of healthy humans and humans with DM2.
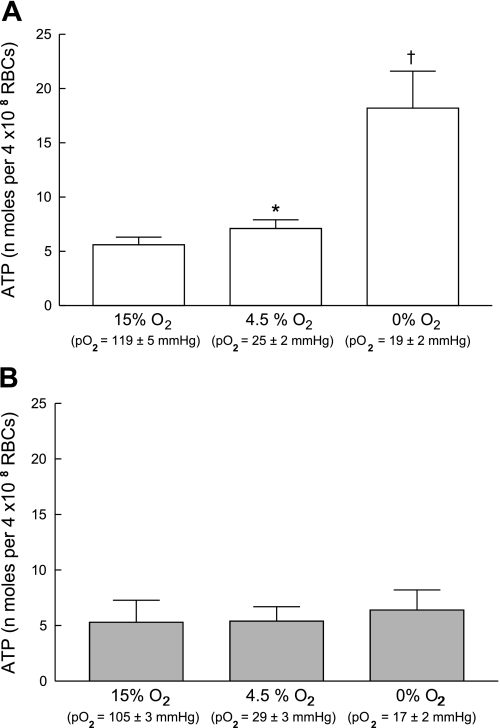
Exposing healthy human erythrocytes to decreased O2 tensions in a tonometer resulted in increases in ATP release (n = 11; Fig. 2A). No gender differences were identified with respect to the magnitude of the response to reduced O2. The release of ATP occurred in a graded fashion such that the lower the O2 tension to which the erythrocytes were exposed, the greater the amount of ATP released. In contrast, cells from humans with DM2 did not release additional ATP when exposed to reduced O2 tension (n = 10, HbA1c = 8.5 ± 0.5; Fig. 2B).
Fig. 2.
Effect of exposure to reduced O2 tension on ATP release from erythrocytes of healthy humans (n = 11; A) and humans with type 2 diabetes (DM2; n = 10; B). In a tonometer, isolated erythrocytes (20% hematocrit) were exposed to gas mixtures containing, sequentially, 15% O2, 6% CO2, balance nitrogen, 4.5% O2, 6% CO2, balance nitrogen and 0% O2, 6% CO2, balance nitrogen. ATP release was determined 30 min after exposure to 15% O2 and 10 min after exposure to 4.5 or 0% O2. Values are means ± SE. *Greater than respective 15% O2 value (P < 0.05). †Greater than respective 15% O2 value (P < 0.01) and respective 4.5% O2 value (P < 0.05). Po2, Oxygen tension of the blood in the tonometer.
Effect of erythrocytes of healthy humans and humans with DM2 on the response of isolated, perfused arterioles to reduced extraluminal O2 tension.
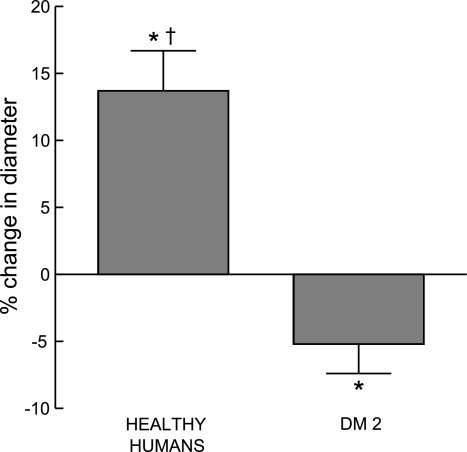
Isolated hamster skeletal muscle arterioles were perfused with buffer containing erythrocytes of healthy humans (n = 7) or humans with DM2 (n = 6, HbA1c = 8.3 ± 0.8). There were no differences between groups in initial vessel diameter or diameter after vessels developed spontaneous myogenic tone (48.1 ± 6.3 and 49.2 ± 3.4 μm, respectively). When arterioles perfused with buffer containing healthy human erythrocytes were exposed to reduced extraluminal Po2 (Po2 = 14 ± 3 mmHg), the vessels dilated with intraluminal diameter increasing by 13.7 ± 3.0% (P < 0.05; Fig. 3). In contrast, when vessels were perfused with buffer containing erythrocytes of humans with DM2, the vessels constricted with intraluminal diameter decreasing by 5.2 ± 2.2% (P < 0.05; Fig. 3) in response to reduced extraluminal Po2 (Po2 = 21 ± 4). The latter response is similar to that seen when buffer-perfused arterioles are exposed to low Po2 (37) and is consistent with the hypothesis that low O2 fails to stimulate release of ATP from DM2 erythrocytes (Fig. 2B) preventing these cells from stimulating vasodilation in response to reduced extraluminal Po2.
Fig. 3.
Effect of reduced extraluminal O2 tension on dilation of isolated skeletal muscle arterioles perfused with erythrocytes. Isolated arterioles were exposed to either extraluminal normoxia (room air, Po2 = 145 ± 2 mmHg) or reduced O2 tension (Po2 = 18 ± 3 mmHg) and perfused with buffer containing well-oxygenated erythrocytes from either healthy humans (n = 7) or humans with DM2 (n = 6, HbA1c = 8.3 ± 0.8). Values are means ± SE. *Different from value during normoxia. †Different from vessels perfused with erythrocytes of humans with DM2 (P < 0.01).
Effect of ILO on ATP release from the erythrocytes of healthy humans and humans with DM2.
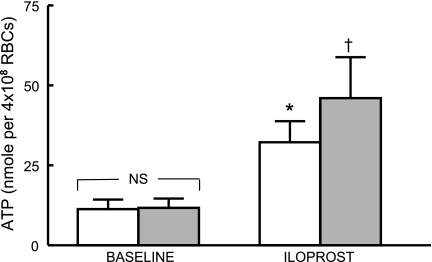
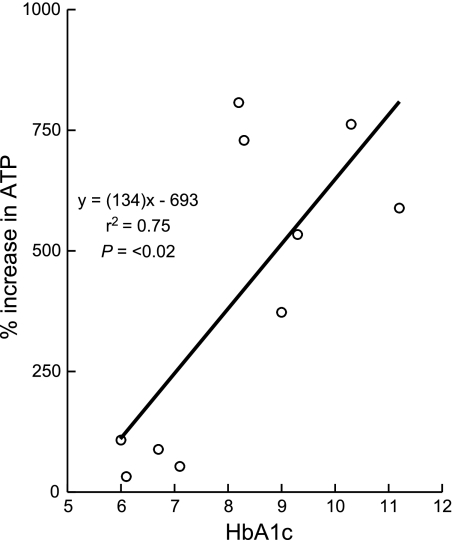
Incubation of isolated erythrocytes (n = 13) with ILO (1 μM) resulted in a 328 ± 92% increase in ATP release (Fig. 4, open bars; P < 0.05). When erythrocytes of humans with DM2 (n = 10, HbA1c = 8.2 ± 0.6) were incubated with the same concentration of ILO, the increase was 408 ± 99% (Fig. 4, filled bars; P < 0.01). Although the amounts of ATP released did not differ between the two groups, when the increase in ATP release from DM2 erythrocytes was compared with HbA1c levels, a significant direct relationship was found (Fig. 5). Thus, ILO-induced ATP release was greater from erythrocytes of humans with higher HbA1c levels, that is, with worse glycemic control.
Fig. 4.
ATP release from erythrocytes of healthy humans (n = 13) and humans with DM2 (n = 10) in the absence (open bars) and presence of iloprost (1 μM, filled bars). Values are means ± SE. *Greater than respective baseline value (P < 0.05). †Greater than respective baseline value (P < 0.01).
Fig. 5.
Linear regression relationship between hemoglobin A1c (HbA1c) and the percent increase in ATP release produced by incubation of erythrocytes of humans with DM2 with iloprost (n = 10). The baseline ATP level was 11.7 ± 3 nmol/108 erythrocytes.
Calculated tissue oxygen distributions for varying capillary network blood flow.
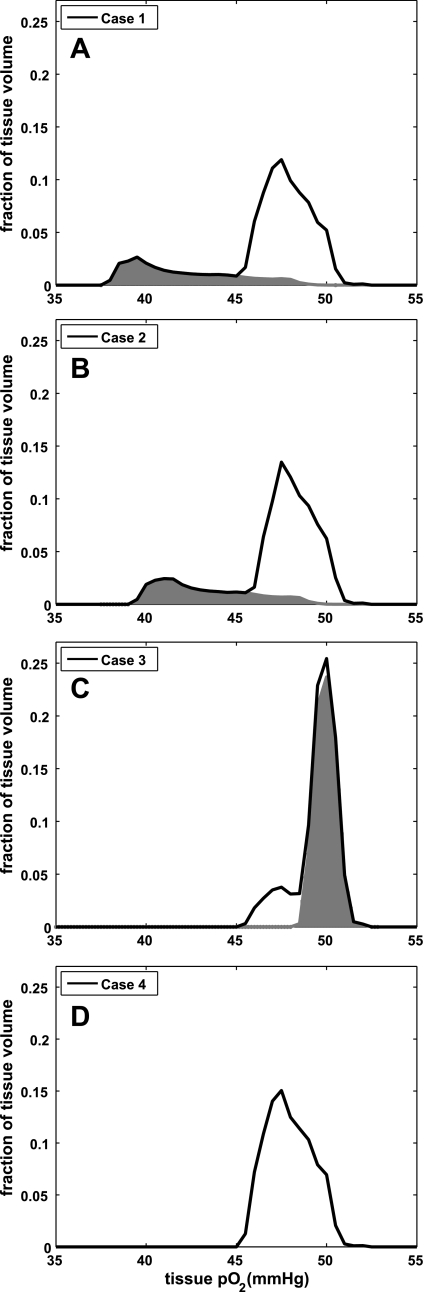
Our computational model allows calculation of the distribution of tissue Po2 values for the four blood flow distributions described above. Figure 6 shows the calculated combined tissue Po2 distributions from the four networks for each of the four cases we tested. For case 1, these calculations show that the undersupply of blood flow to network 1 results in a tissue region with substantially decreased tissue Po2 (Fig. 6A with shaded area showing contribution of network with flow 0.25Q) relative to the tissue supplied by the other three networks. The minimum tissue Po2 for the undersupplied region was 37.9 mmHg (Table 1) compared with 45.5 mmHg for the tissue with adequate blood supply. When total flow to the four networks was returned to baseline (4Q, case 2) by a uniform (i.e., nonselective) flow increase, there remained a substantial region of tissue with abnormally low tissue Po2 (Fig. 6B with shaded area showing contribution of network with flow 0.31Q) and a minimum tissue Po2 only slightly increased by 1.5 mmHg relative to case 1. To eliminate the low-Po2 region by a uniform flow increase, the total flow to the four networks must be increased by a factor of four (to 13Q, case 3). However, this resulted in oversupply of O2 to three of the networks and a large tissue region with abnormally high tissue Po2 (Fig. 6C with shaded area showing contribution of networks with flow 4Q) that increased mean Po2 to 49.5 mmHg. To return tissue Po2 to normal, a selective increase in blood flow to the one undersupplied network was required. When this was done in case 4, minimum tissue Po2 was substantially increased (to 45.5 mmHg; Table 1) without creating regions of abnormally high or low Po2, as indicated by the absence of any shaded areas in Fig. 6D.
Fig. 6.
Results of oxygen transport simulation of a tissue supplied by 4 discrete capillary networks for 4 different flow distributions among the networks. The solid line in each graph shows the combined tissue Po2 distributions for all 4 networks, while the shaded areas show the contribution from the subset of networks that are under- or oversupplied. A: tissue Po2 distribution is presented for 1 network undersupplied at 25% of normal blood flow (0.25Q, shaded area) and the remaining 3 networks each with normal blood supply (Q) for a total blood flow to the simulated tissue of 3.25Q. B–D: results of 3 different ways to adjust the blood supply in an attempt to restore tissue Po2 levels. B and C: represent flows that occur in response to a vasodilator that uniformly increases flow in all arterioles while D represents a regulatory system that directs flow where it is needed. B: total flow to the 4 networks was increased to normal (4Q) with a uniform 23% (100% × 4/3.25) increase in flow to each network. Flow to the undersupplied network (shaded area) increased to 0.31Q. C: flow to the undersupplied network was restored to normal by uniformly increasing flow to all networks by 4-fold such that total flow increased to 13Q, with 3 networks receiving an oversupply (4Q, shaded area). D: represents the case where blood supply to the undersupplied network was increased to normal while blood supply to the other 3 networks was maintained at normal (total flow = 4Q) and hence represents the normal tissue Po2 distribution. Using a vasodilator cannot restore tissue oxygenation to normal since a uniform increase in flow results in some regions either undersupplied (B, shaded area) or oversupplied (C, shaded area).
DISCUSSION
The erythrocyte, by releasing ATP when exposed to reduced O2 tension, can participate in the regulation of the matching of O2 supply with demand in skeletal muscle (2, 13, 27, 32, 34). It was shown previously that erythrocytes of patients with DM2, a condition associated with decreased muscle blood flow both at rest (35) and during exercise (10), demonstrate a selective decrease in the expression of Gαi2 (39, 40), the heterotrimeric G protein required for reduced O2 tension-induced ATP release (32, 33). Here, we extend these studies with the demonstration that erythrocytes of humans with DM2, in contrast to cells of healthy humans, fail to release ATP in response to exposure to this physiological stimulus (Fig. 2). In addition, we demonstrate that although isolated skeletal muscle arterioles perfused with erythrocytes from healthy humans dilate when exposed to low extraluminal Po2, arterioles perfused with DM2 erythrocytes do not (Fig. 3).
Direct studies of oxygen transport in the skeletal muscle microcirculation are not possible in humans. However, studies performed in several animal models of DM2 (2, 13, 34) demonstrate 1) reduced O2 delivery (2, 13), 2) reduced capillary erythrocyte flux (2), and 3) reduced convective O2 delivery and diffusive O2 transport (34), suggesting an impairment in O2 delivery relative to metabolic need. Vasodilation in response to both pharmacological and physiological stimuli has been shown to be altered in humans with DM2 with defects in both endothelium-dependent and -independent mechanisms proposed (1, 20, 22, 29, 44, 46, 47). In addition, it has been suggested that there is reduced nitric oxide (NO) synthesis (29, 44), increased NO degradation (1, 46), and/or abnormalities in the vascular smooth muscle (47) in these individuals. Although there is evidence in support of each of these, none appears sufficient to explain the failure to match O2 delivery with metabolic need in skeletal muscle in humans with DM2. Our demonstration that isolated skeletal muscle arterioles perfused with DM2 erythrocytes fail to dilate when exposed to reduced extraluminal O2 tension suggests that a defect in erythrocyte physiology that limits ATP release could contribute to the impairment in microvascular oxygen supply in DM2.
In addition to exposure to reduced O2 tension, human erythrocytes also release ATP in response to receptor-mediated activation of the Gs-coupled IP receptor (36). Previously, it was shown that, in contrast to Gαi2, expression of Gαs and adenylyl cyclase type II is not decreased in erythrocytes of humans with DM2. Therefore, we determined whether ATP release in response to the PGI2 analog, ILO, was present in DM2 erythrocytes. As shown in Fig. 4, ATP release in response to ILO was not reduced in DM2 erythrocytes. Interestingly, ATP release from DM2 erythrocytes tended to be greater than from erythrocytes of healthy humans (Fig. 4). To investigate this further, we correlated the percent increase in ATP release with hemoglobin HbA1c levels, a measure of glycemic control. It was determined that as HbA1c increased (glycemic control worsened), the amount of ATP release from DM2 erythrocytes increased (Fig. 5). Investigation of the mechanism responsible for the increased response to ILO is beyond the scope of this study but could involve alterations in the IP receptor or in several components of the signaling pathway for ATP release. Mechanism notwithstanding, these findings suggest that PGI2 analogs could be of value in the treatment of the peripheral vascular disease associated with DM2. However, pharmacological activation of the erythrocyte IP receptor throughout the circulation would result in release of ATP from erythrocytes perfusing both metabolically active skeletal muscle as well as inactive muscle with less O2 demand. To what extent the resulting general vasodilation would reverse the oxygen supply defect in DM2 remains to be determined.
To begin to address this important question, we employed a computational model of oxygen transport in a simulated skeletal muscle supplied by four discrete capillary networks under conditions where one of the four capillary networks is initially undersupplied with blood flow. We set flow into this network at 25% of normal while the other three networks received normal flow. This meant that the total blood flow to the simulated tissue was 81% of normal. Levels of O2 in the undersupplied region of tissue were substantially lower than those found in the other regions of the tissue (shaded area, Fig. 6A). One might expect that using a vasodilator to restore total flow to normal levels would be sufficient to restore tissue oxygenation, but as the simulation shows (Fig. 6B, shaded area), this would result in only a slight improvement in tissue oxygenation. Flow increased to this region from 25 to only 31% of normal because the reduced flow was localized and not uniform across the four networks, and hence could not be corrected by a uniform flow increase. If one knew how much the one region was undersupplied, one might apply vasodilators more aggressively to increase O2 supply to supranormal levels. In the simulation, a fourfold increase in total flow was needed to restore tissue oxygenation to the undersupplied region but with the consequence of grossly oversupplying the other three networks with a substantial increase in tissue oxygenation in these areas (shaded area, Fig. 6C). Clearly, restoring a functional regulatory system that matches flow to where it is needed yielded the optimal result (Fig. 6D). Walley (45) reached a similar conclusion with his theoretical approach to investigate the impact of a mismatch between O2 supply and demand on critical O2 extraction, concluding that, as the heterogeneity between the ratio of supply and demand increased (loss of O2 regulation), O2 supply dependency occurred at higher and higher O2 supply rates.
Although the true situation in a tissue may differ substantially from what we simulated here, this simple model highlights why it is critically important for an O2 regulatory system not only to maintain total flow to a skeletal muscle but also to direct the flow to where it is needed. Failure of the erythrocyte to release ATP in response to a decrease in O2 levels in DM2 may have significant consequences for tissue oxygenation. Computational modeling predicts that restoration of the ability of erythrocytes to release ATP in response to reduced O2 tension would be of greater value in restoring O2 delivery to meet metabolic need in skeletal muscle than would general vasodilators. Thus, the erythrocyte, by virtue of its ability to direct blood flow to areas of increased tissue O2 need, could be considered a novel target for the development of therapeutic approaches to treat the peripheral vascular disease of DM2.
GRANTS
This work is supported by National Institutes of Health Grants HL-064180, HL-089094, and HL-089125 and American Diabetes Association Grant RA-133.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank J. L. Sprague for inspiration.
REFERENCES
- 1.Bagi Z, Koller A, Kaley G. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 285: H1404–H1410, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Behnke BJ, Kinding CA, McDonough P, Poole DC, Sexton WL. Dynamics of microvascular oxygen pressure during rest-contraction transition in skeletal muscle of diabetic rats. Am J Physiol Heart Circ Physiol 283: H926–H932, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res 56: 43–53, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Collison DJ, Rea R, Donnelly R. Masterclass series in peripheral arterial disease. Vasc Med 9: 307–310, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Dazel HH, Westfall DP. Receptors for adenine nucleotides and nucleosides: subclassification, distribution and molecular characterization. Pharmacol Rev 46: 449–466, 1994 [PubMed] [Google Scholar]
- 6.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol 278: H1294–H1298, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Eggleton CD, Vadapalli A, Roy TK, Popel AS. Calculations of intracapillary oxygen tension distributions in muscle. Math Biosci 167: 123–143, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Ellis CG, Goldman D, Hanson M, Stephenson AH, Milkovich S, Benlamri A, Ellsworth ML, Sprague RS. Defects in oxygen supply to skeletal muscle in prediabetic ZDF rats. Am J Physiol Heart Circ Physiol 298: H1661–H1670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand 168: 551–559, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35–41, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Fowkes FG. Epidemiological research on peripheral vascular disease. J Clin Epidemiol 54: 863–868, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC. Impaired dilation of skeletal muscle microvessels to reduce oxygen tension in diabetic obese Zucker rats. Am J Physiol Heart Circ Physiol 281: H1568–H1574, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Goldman D, Bateman RM, Ellis CG. Effect of sepsis on skeletal muscle oxygen consumption and tissue oxygenation: interpreting capillary oxygen transport data using a mathematical model. Am J Physiol Heart Circ Physiol 287: H2535–H2544, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Goldman D, Bateman RM, Ellis CG. Effect of decreased O2 supply on skeletal muscle oxygenation and O2 consumption during sepsis: role of heterogeneous capillary spacing and blood flow. Am J Physiol Heart Circ Physiol 290: H2277–H2285, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Goldman D, Popel AS. Computational modeling of oxygen transport from complex capillary networks. Relation to the microcirculation physiome. Adv Exp Med Biol 471: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Goldman D, Popel AS. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J Theor Biol 206: 181–194, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Goldman D, Popel AS. A computational study of the effect of vasomotion on oxygen transport from capillary networks. J Theor Biol 209: 189–199, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Alonzo J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Goodfellow J, Ramsey MW, Luddington LA, Jones CJ, Coates PA, Dunstan F, Lewis MJ, Owens DR, Henderson AH. Endothelium and inelastic arteries: an early marker of vascular dysfunction in noninsulin dependent diabetes. BMJ 312: 744–745, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol 295: H786–H793, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol 92: 10j–17j, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Jaap AJ, Hammersley MS, Shore AC, Tooke JE. Reduced microvascular hyperaemia in subjects at risk of developing type 2 (noninsulin-dependent) diabetes mellitus. Diabetologia 37: 214–216, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Jaap AJ, Shore AC, Tooke JE. Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycemia. Diabetologia 40: 238–243, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol 1072: 161–168, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Kingwell BA, Formosa M, Muhlmann M, Bradley SJ, McConell GK. Type 2 diabetic individuals have impaired leg blood flow responses to exercise: Role of endothelium-dependent vasodilation. Diabetes Care 26: 899–904, 2003 [DOI] [PubMed] [Google Scholar]
- 28.McCullough WT, Collins DM, Ellsworth ML. Arteriolar responses to extracellular ATP in striated muscle. Am J Physiol Heart Circ Physiol 272: H1886–H1891, 1997 [DOI] [PubMed] [Google Scholar]
- 29.McVeigh GE, Brennan GM, Johnston BJ, McGrath LT, Henery WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and -independent vasodilation in patients with type 2 (noninsulin-dependent) diabetes mellitus. Diabetologia 35: 771–776, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Melher P, Jeffers B, Estacio R, Schrier R. Association of hypertension and complications in NIDDM. Am J Hypertens 10: 152–161, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H, the WHO Multinational Study Group. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44: S14–S21, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol 286: H940–H945, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. NO Inhibits signal transduction pathway for ATP release from erythrocytes via its action on heterotrimeric G protein Gi. Am J Physiol Heart Circ Physiol 287: H748–H754, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI, Poole DC. Effects of type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol 291: H2439–H2444, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Regensteiner JG, Popylisen S, Bauer TA, Lindenfeld J, Gill E, Smith S, Oliver-Pickett CK, Reusch JE, Weil JV. Oral l-arginine and vitamin E and C improve endothelial function in women with type 2 diabetes. Vasc Med 8: 169–175, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogues stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation 15: 461–471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 61: 183–190, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 285: H693–H700, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ. Reduced expression of Gi in erythrocytes of humans with diabetes type 2 is associated with impairment of both cAMP generation and ATP release. Diabetes 55: 3588–3593, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Ricketts G, Lonigro A. Expression of the heterotrimeric G protein Gi and ATP release are impaired in erythrocytes of humans with diabetes mellitus. Adv Exp Med Biol 588: 207–216, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Stern MP. The effect of glycemic control in the incidence of macrovascular complications of diabetes. Arch Fam Med 7: 155–162, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Sullivan SM, Pittman RN. Hamster retractor muscle: a new preparation for intravital microscopy. Microvasc Res 23: 329–335, 1982 [DOI] [PubMed] [Google Scholar]
- 43.Uusitipa M, Niskanen L, Siitonen O, Pyorala K. 5-Year incidence of atherosclerotic vascular disease in relation to gender, risk factors, insulin level and abnormalities in lipoprotein composition in noninsulin dependent diabetic and nondiabetic individuals. Circulation 82: 27–36, 1990 [DOI] [PubMed] [Google Scholar]
- 44.vanEtten RW, de Koning EJP, Verhaar MC, Gaillard CAJM, Rabelink TJ. Impaired NO-dependent vasodilation in patients with type II (noninsulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia 45: 1004–1010, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Walley KR. Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: theory. J Appl Physiol 81: 885–894, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with noninsulin-dependent diabetes mellitus. J Am Coll Cardiol 27: 567–574, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Yugar-Toledo JC, Tanus-Santos JE, Sabha M, Sousa MG, Cittadino M, Tácito LHB, Moreno H., Jr Uncontrolled hypertension, uncompensated type II diabetes, and smoking have different patterns of vascular dysfunction. Chest 125: 823–830, 2004 [DOI] [PubMed] [Google Scholar]