Abstract
The goal of this study was to determine whether β1-adrenergic receptor (AR) and β2-AR differ in regulating cardiomyocyte survival and apoptosis and, if so, to explore underlying mechanisms. One potential mechanism is that cardiac β2-AR can activate both Gs and Gi proteins, whereas cardiac β1-AR couples only to Gs. To avoid complicated crosstalk between β-AR subtypes, we expressed β1-AR or β2-AR individually in adult β1/β2-AR double knockout mouse cardiac myocytes by using adenoviral gene transfer. Stimulation of β1-AR, but not β2-AR, markedly induced myocyte apoptosis, as indicated by increased terminal deoxynucleotidyltransferase-mediated UTP end labeling or Hoechst staining positive cells and DNA fragmentation. In contrast, β2-AR (but not β1-AR) stimulation elevated the activity of Akt, a powerful survival signal; this effect was fully abolished by inhibiting Gi, Gβγ, or phosphoinositide 3 kinase (PI3K) with pertussis toxin, βARK-ct (a peptide inhibitor of Gβγ), or LY294002, respectively. This indicates that β2-AR activates Akt via a Gi-Gβγ-PI3K pathway. More importantly, inhibition of the Gi-Gβγ-PI3K-Akt pathway converts β2-AR signaling from survival to apoptotic. Thus, stimulation of a single class of receptors, β2-ARs, elicits concurrent apoptotic and survival signals in cardiac myocytes. The survival effect appears to predominate and is mediated by the Gi-Gβγ-PI3K-Akt signaling pathway.
Keywords: β-adrenergic receptor subtypes, G proteins, apoptosis
Apoptosis has been implicated as a consequence of cardiac ischemic/reperfusion injury and is involved in the transition from cardiac hypertrophy to decompensated heart failure (1–5). In vivo and in vitro studies have shown that enhanced β-adrenergic receptor (AR) signaling induces cardiac myocyte apoptosis via a Gs-mediated, protein kinase A-dependent mechanism (6–8). On the basis of pharmacological evidence, it had been suggested that β1-AR and β2-AR may exert different effects on cardiac apoptosis (9, 10). However, the mechanism underlying the difference between β1-AR and β2-AR remains largely unknown.
Over the past decade, qualitative and quantitative differences between β1-AR and β2-AR in regulating cardiac contractility, Ca2+ handling, and target protein phosphorylation have been systematically documented (refs. 11–13; for review see ref. 14). These differences can be largely explained by the distinct G protein coupling of these β-AR subtypes (14). Specifically, β2-AR activates both Gs and pertussis toxin (PTX)-sensitive Gi protein (Gi2 and Gi3) signaling pathways, whereas β1-AR couples exclusively to the Gs-adenylyl cyclase-cAMP-protein kinase A signaling cascade (11–16). The cellular logic behind the “one receptor-multiple G protein” signaling mechanism is not well understood.
The goal of this study was to determine whether β1-AR and β2-AR differ in regulating cardiac cell survival and apoptosis and, if so, to explore underlying mechanisms, particularly the possible role of β2-AR-coupled Gi signaling. To avoid complicated interactions between β-AR subtypes because of the lack of absolutely selective ligands, we created “pure” β1-AR or β2-AR experimental settings by expressing β1-AR or β2-AR individually in the null background of β1/β2-AR double knockout (DKO) mouse cardiomyocytes by using adult mouse myocyte culture and adenoviral gene transfer techniques recently developed in this laboratory (17). The present results show that stimulation of a single class of G protein-coupled receptors, β2-AR, delivers both antiapoptotic and proapoptotic signals via Gi and Gs signaling pathways.
Methods
Cardiac Myocyte Isolation, Culture, and Adenoviral Infection.
Single cardiac myocytes were isolated from the hearts of 2- to 3-month-old mice with an enzymatic technique, then cultured and infected with adenoviral vectors, as described previously (17). Briefly, myocytes were plated at 0.5 to about 1 × 104/cm2 with MEM containing 1.2 mM Ca2+/2.5% FBS/1% penicillin–streptomycin in the culture dishes precoated with 10 μg/ml mouse laminin. Adenovirus-mediated gene transfer was implemented by adding an appropriate titer of gene-carrying adenovirus.
Three hours after adenoviral infection, myocytes were treated with a variety of agents (including PTX 0.5 μg/ml, SB 203580 10 μM, LY294002 10 μM, or propranolol 10 μM) as indicated, 1 h before the addition of isoproterenol (ISO, 1 μM). All experiments were performed after 24 h of ISO treatment, unless otherwise indicated. All dishes were supplemented with ascorbic acid (100 μM, Sigma) to prevent ISO oxidation.
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL) and Hoechst Staining.
Nuclear fragmentation was determined in fixed cells (70% alcohol and 30% acetone) either by incubating in 10 μM Hoechst 33342 or by TUNEL staining with an R & D Systems apoptosis detection kit, as previously described (18). The percentage of TUNEL or Hoechst staining positive cells was determined by counting 500–800 cardiac myocytes in 20 randomly chosen fields. Each data point (n = 4≈6) in Figs. 3 and 5 shows the result from 5,000≈8,000 cells.
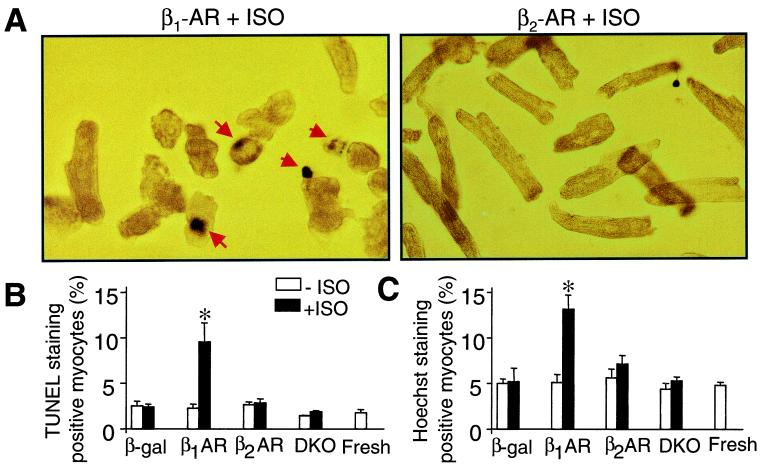
Figure 3.
Stimulation of β1-AR but not β2-AR induces cell apoptosis. (A) Representative micrographs show TUNEL staining in myocytes infected by adeno-β1-AR or adeno-β2-AR and treated with ISO. Apoptotic nuclei appear blue by TUNEL staining with a varying degree of condensation of nuclear chromatin (arrows). (B and C) The average data of TUNEL and Hoechst staining. *, P < 0.01 versus untreated or uninfected myocytes and those infected by adeno-β2-AR (n = 5 to 6 for each group).
Figure 5.
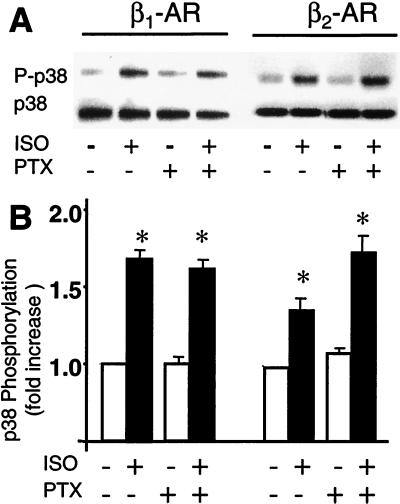
p38 MAPK is not involved in β2-AR-mediated survival effect. (A) Representative Western blots show that stimulation of β1-AR or β2-AR by ISO increases p38 MAPK phosphorylation in a PTX-insensitive manner. (B) The average data (n = 5 to 6, P < 0.01 versus ISO).
DNA Laddering and Cell Death ELISA.
For DNA laddering, 10 μg of DNA was loaded in each lane, and the DNA was size-fractionated on a 1.5% agarose gel in Tris-acetate-EDTA buffer and then stained with ethidium bromide (GIBCO/BRL). For the cell death ELISA, each aliquot of ≈20,000 cells was lysed with 0.25 ml of a lysis buffer (cell death ELISA Plus, Boehringer Mannheim). The extract was then centrifuged, and nucleosomal DNA was assayed according to the manufacturer's instructions.
p38 Mitogen-Activated Protein Kinase (MAPK) Phosphorylation and Akt Activity Assay.
Phosphorylation of p38 MAPK was measured by Western blotting with a phospho-p38 MAPK antibody, as described previously (19). To measure Akt activity, cell lysis was first immunoprecipitated with anti-Akt1 mAb. The precipitated proteins were then incubated with GSK-3 fusion protein in the presence of ATP and kinase buffer to allow precipitated Akt to phosphorylate GSK-3. Phosphorylation of GSK-3 was measured by Western blotting by using a phospho-GSK-3α/β (Ser21/9) antibody.
Materials.
Unless otherwise indicated, all chemicals were purchased from Sigma. Anti-Akt1 mAb, phospho-p38 MAPK, and phospho-GSK-3α/β (Ser21/9) antibodies were purchased from New England Biolabs.
Statistical Analysis.
Data were expressed as mean ± SE. Statistical comparisons used one-way ANOVA followed by the Bonferroni procedure for multiple-group comparisons. A P < 0.05 was considered statistically significant.
Results
Stimulation of β2-AR Does Not Induce Cardiac Myocyte Loss.
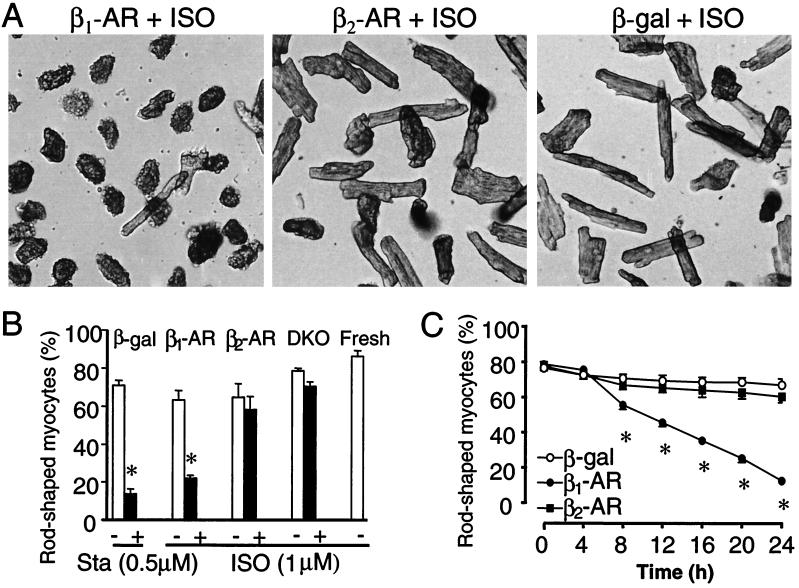
In β1/β2-AR DKO mouse cardiomyocytes infected with either adeno-β1-AR or adeno-β2-AR [multiplicity of infection (moi) 100], the density of β1-AR was similar to that of β2-AR (531 ± 60.7 and 482 ± 56.1 fmol/mg protein, respectively, n = 3). Using the “pure” β1-AR and β2-AR experimental systems, we first individually evaluated effects of either β-AR subtype stimulation on cardiac myocyte survival and death. We used the rod shape to indicate the viability of adult cardiomyocytes because only rod-shaped myocytes retain functional and morphological integrity and because both annexin V and propidium iodide staining are negative in all rod-shaped myocytes (data not shown). As shown in Fig. 1A, ISO (1 μM) markedly reduced the viability of myocytes infected by adeno-β1-AR but not those infected by adeno-β2-AR, as compared with those infected by a control adenovirus, adeno-β-gal at the same moi. Fig. 1B shows that β1-AR stimulation by ISO caused a severe myocyte loss, whereas β2-AR stimulation had no such effect, relative to uninfected myocytes or cells in the absence of ISO, or those infected by adeno-β-gal. Staurosporine (Sta, 0.5 μM), a protein kinase C inhibitor, was used as a positive control; it reduced the percentage of rod-shaped myocytes to an extent similar to that induced by β1-AR stimulation (Fig. 1B). Fig. 1C illustrates the time course of cell loss. The percentage of rod-shaped cells was reduced from ≈80% to ≈20% after 24 h of β1-AR stimulation. In contrast, neither β2-AR stimulation nor adenoviral infection alone (adeno-β-gal) induced any significant cell loss over the same period.
Figure 1.
β1-AR but not β2-AR stimulation induces loss of rod-shaped myocytes. (A) Cardiac myocytes infected by adeno-β1-AR, adeno-β2-AR, or adeno-β-gal (moi 100) were cultured for 24 h in the presence of ISO (1 μM). (B) The average percentage of rod-shaped myocytes was markedly reduced by stimulation of β1-AR but not β2-AR. Sta was used as a positive control. DKO refers to uninfected DKO cells, whereas Fresh refers to freshly isolated cells. (C) The time course of ISO-induced drop of rod-shaped cells in myocytes infected by adeno-β1-AR, adeno-β2-AR, or adeno-β-gal. *, P < 0.01 versus untreated (ISO or Sta) or uninfected myocytes (B) or those infected by adeno-β2-AR or adeno-β-gal (B and C) (n = 4–6 for each data point).
Stimulation of β1-AR but Not β2-AR Induces Myocyte Apoptosis.
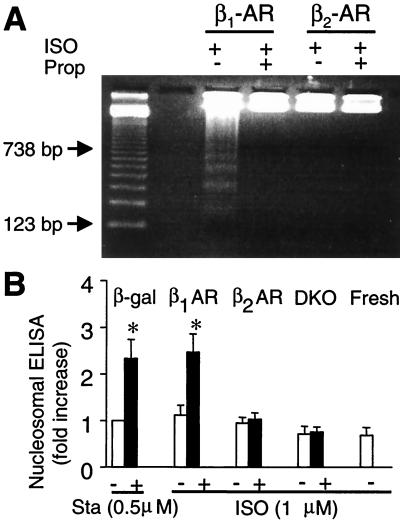
β1-AR-induced cardiac cell loss described above might be mediated by necrosis or apoptosis or both. To determine the role of apoptosis, we measured DNA fragmentation by using cell death ELISA and DNA laddering. Fig. 2A shows that stimulation of β1-AR, but not β2-AR, induced DNA laddering and that the effect of β1-AR stimulation was completely abolished by a β-AR antagonist propranolol (Prop, 10 μM). Similarly, β1-AR stimulation caused a robust increase in DNA fragmentation assayed by cell death ELISA (Fig. 2B). In contrast, β2-AR stimulation had no significant effect as compared with uninfected DKO myocytes or freshly isolated cells (Fig. 2B). These results suggest that stimulation of β1-AR, but not β2-AR, induces myocyte apoptosis. To further confirm the distinct roles of β1-AR and β2-AR in regulating cardiac cell death, we performed both Hoechst and TUNEL staining (Fig. 3). On average, stimulation of β1-AR, but not β2-AR, increased TUNEL and Hoechst staining positive myocytes by 2–3-fold (Fig. 3 B and C).
Figure 2.
Stimulation of β1-AR but not β2-AR increases DNA fragmentation, assayed by DNA laddering (A) or nucleosomal ELISA (B). *, P < 0.01 versus untreated (Sta or ISO) or uninfected myocytes (DKO and Fresh) and those infected by adeno-β2-AR (n = 4–6 for each group).
Role of Gi and Gβγ in β2-AR-Mediated Survival Effect.
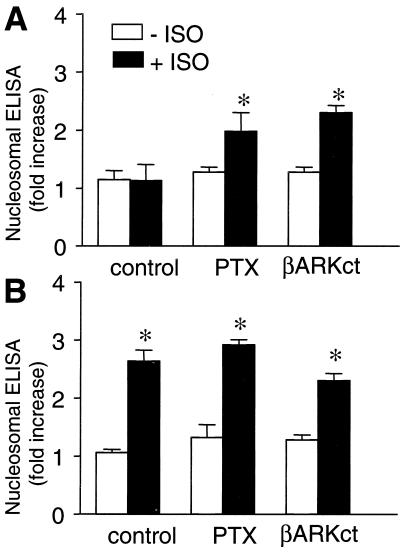
To examine whether the additional coupling of β2-AR to Gi accounts for the differential modulation of cardiac apoptosis by β-AR subtypes, cardiac myocytes were treated with PTX to disrupt Gi signaling. Indeed, PTX treatment permitted β2-AR to induce apoptosis, as evidenced by increased cell loss (data not shown) and DNA fragmentation (Fig. 4A). This result suggests that the β2-AR-coupled Gi pathway delivers a cell survival signal that prevents the concurrent Gs-mediated apoptosis. To further determine which subunits of trimeric Gi proteins are essential to the survival effect, myocytes were co-infected by adeno-β2-AR and adeno-βARK-ct (βARK-ct, a peptide inhibitor of Gβγ signaling; refs. 20 and 21), both at moi 100. Similar to PTX treatment, βARK-ct effectively abolished the β2-AR survival effect and converted β2-AR to apoptotic (Fig. 4A). In contrast, PTX treatment and coexpression of βARK-ct did not alter β1-AR-induced cell loss (data not shown) and DNA fragmentation (Fig. 4B). These results indicate that Gβγ subunits dissociated from Gi proteins are critical components of the β2-AR-mediated survival pathway.
Figure 4.
Involvement of Gi and Gβγ in β2-AR-mediated survival effect. PTX treatment or coexpression of βARK-ct permits β2-AR stimulation by ISO to induce DNA fragmentation (A). These interventions neither blunt nor enhance the effect of β1-AR stimulation (B). *, P < 0.01 versus ISO-untreated myocytes (n = 4–6 for each group).
p38 MAPK Is Not Involved in β2-AR Antiapoptotic Effect.
To define the downstream signaling events of the β2-AR-Gi-Gβγ pathway, we first examined the possible involvement of p38 MAPK because recent studies have reported that in adult rat cardiac myocytes, Gi-initiated antiapoptosis is mediated by activation of this MAPK (22). Fig. 5A shows that stimulation of both β-AR subtypes by ISO (1 μM) significantly increased p38 MAPK activation. However, disrupting Gi signaling with PTX neither enhanced nor reduced p38 MAPK response to either β-AR subtype stimulation (Fig. 5), suggesting that both β-AR subtypes activate p38 MAPK in a Gi-independent manner. Furthermore, β2-AR did not induce myocyte apoptosis even in the presence of a p38 MAPK inhibitor, SB203580 (10 μM) (data not shown), indicating that p38 MAPK activation is not required for the survival effect of β2-AR stimulation in cardiac myocytes.
Inhibiting Phosphoinositide 3 Kinase (PI3K) or Akt Switches β2-AR Signaling from Survival to Apoptotic.
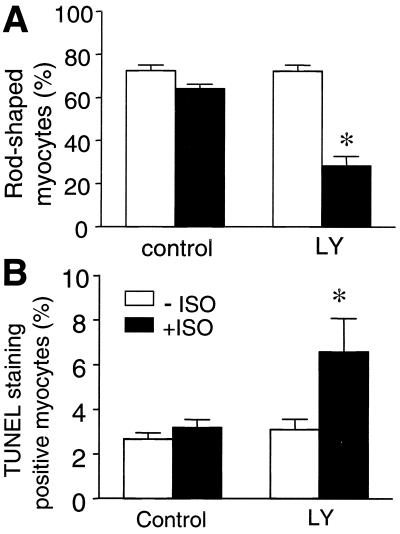
In addition to MAPKs, PI3K has also been implicated as an important cell survival signal in various cell types (23–26). Emerging evidence has shown that stimulation of G protein-coupled receptors is able to activate this kinase (27–29). Thus, we hypothesized that PI3K might be involved in the β2-AR-Gi antiapoptotic effect. Similar to Gi or Gβγ inhibition (Fig. 4), PI3K inhibition by LY294002 (10 μM) unmasked β2-AR-induced apoptotic effect, as manifested by a significant loss of rod-shaped cells and an increase in TUNEL staining positive cells (Fig. 6), whereas LY294002 did not alter β1-AR stimulation (data not shown).
Figure 6.
Inhibition of PI3K by LY 294002 abolishes β2-AR-induced survival effect and unmasks β2-AR-mediated apoptotic effect. β2-AR stimulation by ISO (1 μM) reduces the percentage of the rod-shaped myocytes (A) and increases the TUNEL staining positive cells (B) only in the presence of LY294002 (10 μM). *, P < 0.01 versus the groups in the absence of ISO and the group with ISO but without LY (n = 5 to 6 for each group).
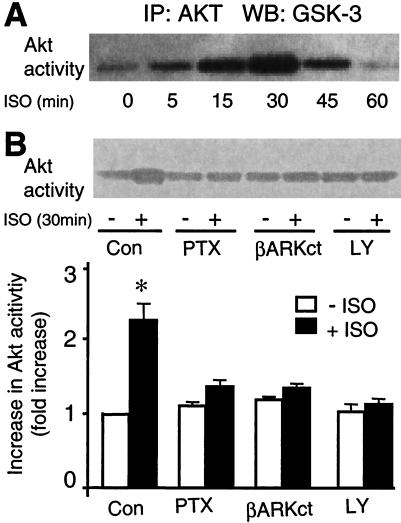
We also examined the possible effect of β2-AR stimulation on the phosphorylation status of an immediate substrate of PI3K, Akt (or protein kinase B), a powerful antiapoptosis factor (23–26, 30). β2-AR stimulation by ISO (1 μM) markedly enhanced Akt activity in a time-dependent manner with the peak at 30 min (Fig. 7A). Fig. 7B shows that β2-AR-induced Akt activation was completely abolished by maneuvers that inhibit the β2-AR-mediated survival signals, including PTX, LY 294002, and βARK-ct. In contrast, β1-AR has no effect on Akt activity (data not shown). These results suggest that PI3K and Akt are necessary downstream events of the β2-AR-coupled Gi signaling underlying the β2-AR-mediated cardiac cell survival effect.
Figure 7.
β2-AR stimulation by ISO increases Akt activation. (A) A typical example of the time course of β2-AR-stimulated Akt phosphorylation. (B) PTX, coexpression of βARK-ct, or LY 294002 fully inhibits the response of Akt to β2-AR stimulation (ISO 1 μM, 30 min). (Upper) A typical example. (Lower) The average data. *, P < 0.01 versus all other groups (n = 5 to 6).
Discussion
β2-AR Stimulation Elicits Concurrent Antiapoptotic and Proapoptotic Effects.
The present results provide unequivocal evidence that in cultured adult mouse ventricular myocytes, although β1-AR consistently induces cardiac apoptosis, β2-AR activates concurrent proapoptotic and antiapoptotic effects with the survival effect predominating. The differential regulation of cardiac cell survival and cell death by these β-AR subtypes is largely accounted for by the additional coupling of β2-AR to PTX-sensitive Gi proteins. Our conclusion is based on several independent lines of evidence. First, β2-AR induces myocyte loss and myocyte apoptosis only after Gi inhibition with PTX. Second, β2-AR, but not β1-AR, activates Akt, a key regulator of cell survival, in a PTX-sensitive manner (see below). Thus, β2-AR activates concurrent antiapoptotic and proapoptotic effects because of its dual coupling to Gi and Gs proteins.
The Gi-Gβγ-PI3K-Akt Signaling Pathway, Rather Than p38 MAPK, Mediates the β2-AR Antiapoptotic Effect.
We have further elucidated that the β2-AR antiapoptotic effect is mediated by the β2-AR-coupled Gi-Gβγ-PI3K-Akt signaling pathway. Inhibiting β2-AR-activated Gi proteins, Gβγ signaling, or PI3K activity with PTX, βARK-ct, and LY294002, respectively, completely abolishes β2-AR-stimulated Akt activation and, more important, converts β2-AR signaling from survival to apoptotic. Therefore, PI3K constitutes an important downstream messenger of the β2-AR-coupled Gi proteins, which protects myocytes against the Gs-mediated apoptosis via activating the survival factor, Akt.
In regard to Akt-mediated survival effects, several mechanisms might be involved. These include phosphorylation of BAD, releasing antiapoptotic Bcl family members (31), phosphorylation and inactivation of caspase-9 (32), and phosphorylation of the forkhead transcription factor, FKHL1 (33). In addition, recent evidence demonstrates that Akt-mediated antiapoptotic effect occurs at the level of cytochrome c, upstream of caspase 9 (34, 35). Further studies are required to define the exact downstream events of the β2-AR-initiated, Akt-dependent survival signaling.
The present study provides several lines of evidence that argue against the previous proposal that β-AR stimulation activates p38 MAPK in a Gi-dependent manner and that the activated p38 MAPK results in an antiapoptotic effect (22). First, both β1-AR and β2-AR increase p38 MAPK activation, which cannot be abolished by PTX, suggesting that p38 MAPK is activated via the β-AR-Gs signaling pathway (19). Second, inhibiting p38 by SB 203580 (10 μM) cannot block the β2-AR survival effect, indicating that p38 MAPK activation is not related to the β2-AR/Gi-mediated antiapoptotic effect in adult mouse cardiac myocytes. The reason for the discrepancy between the present results and the previous study (22) is presently unclear.
Opposing Effects of β-AR Subtypes on Cardiac Apoptosis: Pathophysiological Relevance.
β1-AR is the predominant β-AR subtype in the heart in terms of receptor density and modulation of cardiac contractility. In contrast to the concurrent survival and apoptotic effects following β2-AR stimulation, β1-AR stimulation robustly and consistently increases cardiac myocyte apoptosis. In fact, chronic stimulation of these β-AR subtypes in the heart also elicits strikingly different phenotypes in mouse transgenic models. Overexpression of cardiac β1-AR by 5≈40-fold induces cardiac hypertrophy, apoptosis, and fibrosis within a few weeks after birth and heart failure within several months (36, 37). In contrast, overexpression of cardiac β2-AR by 100≈200-fold does not induce hypertrophy or heart failure, at least up to the age of 1 year (38–40). However, overwhelming expression of β2-AR (e.g., 350≈1,000-fold) induces pathological phenotypes (39, 40), perhaps caused by a mechanical and metabolic overload because of spontaneous β2-AR activation. Given the different phenotypes of cardiac-specific overexpression of β1-AR versus β2-AR and the distinct, even opposing effects of these β-AR subtypes on cardiac myocyte apoptosis demonstrated in the present study, it is reasonable to assume that the apoptotic effects induced by in vivo ISO infusion (8) is mainly mediated by β1-AR activation. The opposing effects of β-AR subtypes on cardiac myocyte death may also explain, at least in part, the inverse relationship between the plasma level of norepinephrine (with higher affinity for β1-AR than for β2-AR) and the survival in patients with chronic heart failure (41) and the salutary effects of β-AR blockade on morbidity and mortality in heart failure patients (42).
β2-AR-Mediated Survival Effect: Therapeutic Implications.
In the light of the distinct functional roles of β1-AR and β2-AR in modulating cardiac cell survival and cell death, it seems plausible to consider the use of enhanced β2-AR signaling (and inhibited β1-AR stimulation) to improve the performance of failing hearts. Previous in vivo and in vitro studies have provided appealing evidence to support this hypothesis. First, in vitro overexpression of β2-AR by 15-fold using adenoviral gene transfer restores β-AR responsiveness and contractility in cardiac myocytes from pacing-induced failing rabbit hearts (43). Second, crossing transgenic mice overexpressing cardiac β2-AR by 30-fold (a low level) with transgenic mice overexpressing Gqα not only improves cardiac performance but also prevents cardiac hypertrophy in the Gqα overexpression heart failure model (39). Parenthetically, extremely high levels of β2-AR overexpression (e.g., 350≈1,000-fold) induce pathological phenotypes and fail to rescue the genetic mouse heart failure model (39, 40). Furthermore, our recent studies show that β2-AR stimulation protects cardiac myocytes from a range of apoptotic assaults, including hypoxia or reactive oxygen species-induced apoptosis (18). Finally, the beneficial effect of β2-AR in the context of heart failure is further evidenced by the analysis of polymorphisms of β2-AR in chronic heart failure patients. The prognosis of heart failure patients with the Ile-164 polymorphism (with reduced β2-AR signaling efficacy) relative to patients without the β2-AR variant is much worse, and they are significantly more likely to receive earlier aggressive interventions or cardiac transplantation (44).
In summary, stimulation of β2-AR concurrently activates cardiac survival and apoptotic signals in adult mouse cardiac myocytes, with the survival signal predominating. Inhibition of the novel survival pathway, β2-AR-Gi-Gβγ-PI3K-Akt, converts β2-AR stimulation from survival to apoptotic. In contrast, stimulation of β1-AR induces apoptosis of cardiac myocytes regardless of the functional status of Gi proteins. The striking differences in the effects of β1-AR and β2-AR on myocyte apoptosis may have important pathological relevance and points toward novel therapeutic strategies involving selectively enhancing β2-AR subtype signaling in the context of chronic heart failure.
Acknowledgments
We thank Drs. Edward G. Lakatta, Mike Crow, and Heping Cheng for critical comments, and Mr. Bruce Ziman for excellent technical support.
Abbreviations
- PI3K
phosphoinositide 3 kinase
- AR
adrenergic receptor
- PTX
pertussis toxin
- DKO
double knockout
- ISO
isoproterenol
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- MAPK
mitogen-activated protein kinase
- moi
multiplicity of infection
- Sta
staurosporine
References
- 1.Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt U, Semigran M J, Dec G W, Khaw B A. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara J A, Quaini E, Di Loreto C, Beltrami C A, Kratjewski S, et al. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 3.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert S R, Cigola E, Anversa P. J Mol Cell Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 4.Teiger E, Than V D, Richard L, Wisnewsky C, Tea B S, Gadoury L, Tremblay J, Schwartz K, Hamet P. J Clin Invest. 1996;97:2891–2897. doi: 10.1172/JCI118747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Bing O H, Long X, Robinson K G, Lakatta E G. Am J Physiol. 1997;272:H2313–H2319. doi: 10.1152/ajpheart.1997.272.5.H2313. [DOI] [PubMed] [Google Scholar]
- 6.Geng Y J, Ishikawa Y, Vanter D E, Wagner T E, Bishop S P, Vatner S F, Homcy C J. Circ Res. 1999;84:34–42. doi: 10.1161/01.res.84.1.34. [DOI] [PubMed] [Google Scholar]
- 7.Communal C, Singh K, Pimentel D R, Colucci W S. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 8.Shizukuda Y, Buttrick P M, Geenen D L, Borczuk A C, Kitsis R N, Sonnenblick E H. Am J Physiol. 1998;275:H961–H968. doi: 10.1152/ajpheart.1998.275.3.H961. [DOI] [PubMed] [Google Scholar]
- 9.Communal C, Singh K, Sawyer D B, Colucci W S. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 10.Zaugg M, Xu W, Lucchinetti E, Shafiq S A, Jamali N Z, Siddiqui M A. Circulation. 2000;102:344–350. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 11.Xiao R P, Ji X, Lakatta E G. Mol Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- 12.Xiao R P, Avdonin P, Zhou Y-Y, Cheng H, Akhter S A, Eschenhagen T, Lefkowitz R J, Koch W J, Lakatta E G. Circ Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Kuschel M, Zhou Y Y, Cheng H, Zhang S J, Chen Y, Lakatta E G, Xiao R P. J Biol Chem. 1999;274:22048–22052. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- 14.Xiao R-P, Cheng H, Zhou Y-Y, Kuschel M, Lakatta E G. Circ Res. 1999;85:1092–1100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- 15.Daaka Y, Luttrell L M, Lefkowitz R J. Nature (London) 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 16.Pavoine C, Magne S, Sauvadet A, Pecker F. J Biol Chem. 1999;274:628–637. doi: 10.1074/jbc.274.2.628. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y-Y, Wang S Q, Zhu W Z, Chruscinski A, Kobilka B K, Ziman B, Wang S, Lakatta E G, Cheng H, Xiao R-P. Am J Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 18.Chesley A, Ohtani S, Asai T, Xiao R P, Lunberg M S, Lakatta E G, Crow M T. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 19.Zheng M, Zhang S-J, Zhu W, Ziman B, Kobilka B K, Xiao R P. J Biol Chem. 2000;275:40635–40640. doi: 10.1074/jbc.M006325200. [DOI] [PubMed] [Google Scholar]
- 20.Koch W J, Hawes B E, Inglese J, Luttrell L M, Lefkowitz R J. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 21.Koch W J, Hawes B E, Allen L F, Lefkowitz R J. Proc Natl Acad Sci USA. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Communal C, Colucci W S, Singh K. J Biol Chem. 2000;275:19395–19400. doi: 10.1074/jbc.M910471199. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy S G, Wagner A J, Conzen S D, Jordan J, Bellacosa A, Tsichlis P N, Hay N. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 24.Fujio Y, Nguyen T, Wencker D, Kitsis R N, Walsh K. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsui T, Li L, del Monte F, Fukui Y, Franke T F, Hajjar R J, Rosenzweig A. Circulation. 1999;100:2373–2379. doi: 10.1161/01.cir.100.23.2373. [DOI] [PubMed] [Google Scholar]
- 26.Hemmings B A. Science. 1997;275:628–630. doi: 10.1126/science.275.5300.628. [DOI] [PubMed] [Google Scholar]
- 27.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind J S. J Biol Chem. 1998;273:19080–19085. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 28.Naga Prasad S V, Esposito G, Mao L, Koch W J, Rockman H A. J Biol Chem. 2000;275:4693–4698. doi: 10.1074/jbc.275.7.4693. [DOI] [PubMed] [Google Scholar]
- 29.Hawes B E, Luttrell L M, van Biesen T, Lefkowitz R J. J Biol Chem. 1996;271:12133–12136. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 30.Franke T F, Kaplan D R, Cantley L C, Toker A. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 31.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 32.Datta S R, Brunet A, Greenberg M E. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 33.Romashkova J A, Makarov S S. Nature (London) 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy S G, Kandel E S, Cross T K, Hay N. Mol Cell Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita E, Jinbo A, Matuzaki H, Konishi H, Kikkawa U, Momoi T. Biochem Biophys Res Commun. 1999;264:550–555. doi: 10.1006/bbrc.1999.1387. [DOI] [PubMed] [Google Scholar]
- 36.Engelhardt S, Hein L, Wiesmann F, Lohse M J. Proc Natl Acad Sci USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bisognano J D, Weinberger H D, Bohlmeyer T J, Pende A, Raynolds M V, Sastravaha A, Roden R, Asano K, Blaxall B C, Wu S C, et al. J Mol Cell Cardiol. 2000;32:817–830. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 38.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 39.Dorn G W, II, Tepe N M, Lorenz J N, Koch W J, Liggett S B. Proc Natl Acad Sci USA. 1999;96:6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liggett S B, Tepe N M, Lorenz J N, Canning A M, Jantz T D, Mitarai S, Yatani A, Dorn G W, II. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 41.Cohn J N, Levine T B, Olivari M T, Garberg V, Lura D, Francis G S, Simon A B, Rector T. N Engl J Med. 1984;311:819–823. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 42.Eichhorn E J, Bristow M R. Circulation. 1996;94:2285–2296. doi: 10.1161/01.cir.94.9.2285. [DOI] [PubMed] [Google Scholar]
- 43.Akhter S A, Skaer C A, Kypson A P, McDonald P H, Peppel K C, Glower D D, Lefkowitz R J, Koch W J. Proc Natl Acad Sci USA. 1997;94:12100–12105. doi: 10.1073/pnas.94.22.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liggett S B, Wagoner L E, Craft L L, Hornung R W, Hoit B D, McIntosh T C, Walsh R A. J Clin Invest. 1998;102:1534–1539. doi: 10.1172/JCI4059. [DOI] [PMC free article] [PubMed] [Google Scholar]