Abstract
Interest in the health effects of potential endocrine-disrupting compounds (EDCs) that are high production volume chemicals used in consumer products has made exposure assessment and source identification a priority. We collected paired indoor and outdoor air samples in 40 nonsmoking homes in urban, industrial Richmond, CA, and 10 in rural Bolinas, CA. Samples were analyzed by GC-MS for 104 analytes, including phthalates (11), alkylphenols (3), parabens (3), polybrominated diphenyl ether (PBDE) flame retardants (3), polychlorinated biphenyls (PCBs) (3), polycyclic aromatic hydrocarbons (PAHs) (24), pesticides (38), and phenolic compounds (19). We detected 39 analytes in outdoor air and 63 in indoor air. For many of the phenolic compounds, alkylphenols, phthalates, and PBDEs, these represent some of the first outdoor measures and the first analysis of the relative importance of indoor and outdoor sources in paired samples. Data demonstrate higher indoor concentrations for 32 analytes, suggesting primarily indoor sources, as compared with only 2 that were higher outdoors. Outdoor air concentrations were higher in Richmond than Bolinas for 3 phthalates, 10 PAHs, and o-phenylphenol, while indoor air levels were more similar between communities, except that differences observed outdoors were also seen indoors. Indoor concentrations of the most ubiquitous chemicals were generally correlated with each other (4-t-butylphenol, o-phenylphenol, nonylphenol, several phthalates, and methyl phenanthrenes; Kendall correlation coefficients 0.2−0.6, p < 0.05), indicating possible shared sources and highlighting the importance of considering mixtures in health studies.
Short abstract
Paired indoor and outdoor air concentrations of semivolatile endocrine-disrupting chemicals suggest primarily indoor sources, and the most ubiquitous commercial chemicals are correlated across homes.
Introduction
Interest in the health effects of potential endocrine-disrupting compounds (EDCs) has made exposure assessment for these compounds a priority. Many EDCs are high production volume chemicals with consumer uses—for example in plastics, detergents, furniture, and other household and consumer products—making them important indoor contaminants (1,2). The U.S. General Accounting Office has described indoor air as “one of the most serious environmental risks to human health” (3). Because many EDCs are semivolatile organic compounds (SVOCs), they are found in both the gas and condensed phase, and are redistributed from their original source over time to indoor air, house dust, and other indoor surfaces (4). In addition to being a direct route of exposure, indoor air may be a proxy for exposure during product use.
Relatively few studies have evaluated indoor EDC levels, but existing data show variation within and between communities, providing evidence that research in multiple settings will be informative. We previously analyzed indoor air and dust samples from 120 Cape Cod, MA homes for 89 EDCs, including phthalates, alkylphenols, parabens, flame retardants, PCBs, and current-use and banned pesticides (2), providing the first report on indoor concentrations for over 30 compounds. The average home had 19 EDCs in air and 24 in dust (2). In the Cape Cod and other studies, phthalates, which are common in vinyl and other plastics, fragrances, and a range of consumer products, tend to be detected at the highest indoor air concentrations (∼100−1000 ng/m3) with outdoor levels several orders of magnitude lower (1). Indoor levels of nonylphenol, a component of plastics and detergents, appear slightly lower, although data are limited. Indoor levels of PCBs vary dramatically, with highest levels in buildings constructed during the 1950s to 1970s (1). Testing in Cape Cod homes with elevated PCBs in air and dust led to the discovery that a wood floor finish widely marketed in the 1950s and 1960s was the likely source and that residents in these homes had elevated PCBs in their blood (5); high levels have also been reported in European schools and offices built during this period (1). Indoor levels of PBDE flame retardants also vary considerably, with highest levels in California, followed by the rest of the U.S. and then Europe, consistent with patterns of use in furniture (1,6). Pesticide levels indoors are associated with individual use and local practices (7). Cape Cod data may be the only reported air concentrations for parabens and some estrogenic phenols such as o-phenylphenol and 4-t-butylphenol, which have a range of consumer uses (2). For all of these chemical families, outdoor levels tend to be lower than indoor, and concentrations are lower in remote than urban areas (1). Indoor environments may be a source of the compounds measured outside (8).
The Cape Cod study (1999−2000) characterized exposures in a group of predominantly older, white participants in a rural and suburban area in the Northeastern U.S. To evaluate whether household EDC levels vary in communities with different geographic and social characteristics, we extended our research to Richmond, CA, an urban, low-income, minority, industrial community neighboring a large oil refinery in the San Francisco Bay Area. This new study—the Northern California Exposure Study—was conducted in 2006. Silent Spring Institute partnered with a local environmental justice (EJ) organization, Communities for a Better Environment, and researchers at Brown University and University of California, Berkeley, to study exposures relevant to breast cancer and EJ issues (9). A request by the study community advisory committee for a regional and contemporaneous comparison resulted in a limited sampling of 10 homes in Bolinas, CA, a rural, coastal community intended to represent low levels of local industrial and transportation air pollutants.
We collected paired indoor and outdoor air samples, and house dust. Chemicals of interest included phthalates, alkylphenols, parabens, PBDEs, PCBs, PAHs, pesticides, and other estrogenic phenols such as bisphenol A, o-phenylphenol, and 4-t-butylphenol (2). In general, compounds were included if there was evidence that they are potential EDCs, they were reported to be present in commercial products or building materials, and/or they were compatible with analytical methods being used for these samples. Potential EDCs were identified based on listing as priority substances for investigation of endocrine disruption by the European Commission (10) or based on our review of reports of in vivo or in vitro endocrine activity as cited in our previous paper describing development of this analytical method (11). Target compounds identified as potential EDCs are identified in Table S1 in the Supporting Information. An extended list of PAHs was included to characterize influences of local industry and transportation emission sources. We have separately reported that household dust PBDE levels in CA were higher than any reported to date, and that blood PDBE levels of CA residents were about twice as high as others in the U.S., likely due to the state’s uniquely stringent furniture flammability regulation (6). Our findings for metals and particulates also are reported separately (9).
The objective of this study was to extend our understanding of indoor EDCs to different geographic and demographic areas and to outdoor air. This study is one of the first to characterize indoor EDCs in an urban, industrial, low-income community. Consistent methods between the Cape Cod and Northern California studies facilitate comparisons across these geographically and demographically different communities. The study also uses paired indoor and outdoor air samples to characterize the contribution of indoor and outdoor sources. Simultaneous measurement of many common commercial chemicals is used to determine correlations among target analytes, which highlight mixtures and provide clues about sources. To our knowledge, our reports of outdoor air concentrations are the first for many of these compounds.
Methods
Sampling and Analytical Methods
Forty nonsmoking homes in Richmond and 10 in Bolinas were sampled. Additional information about the study communities and participant selection are described elsewhere (9).
Outdoor and indoor air samples consisting of <7-μm particulate and vapor phases were collected using parallel 160-mm URG personal pesticide sampling cartridges (University Research Glassware; Chapel Hill, NC) at a target flow rate of 8−9 L/min supplied by a flow-controlled pump. Each URG cartridge contained an impactor-equipped inlet (10-μm at 4 L/min) followed by a 25-mm quartz fiber filter and a 3.0-g bed of XAD-2 sandwiched between two 113/16-inch-diameter polyurethane foam (PUF) plugs. Units were placed in a frequently used room within the home and in the backyard, and samples were collected over 24-h periods Monday through Friday. Indoor and outdoor samples were collected simultaneously at 43 homes, and indoor samples were collected in 7 additional homes. Sampler inlets were placed at approximately breathing height and flow rates were measured and recorded at the beginning and end of the 24-h sampling period. At the end of the sampling period, the URG samplers were disconnected from the pump and stored at −4 °C prior to shipping to the laboratory.
Chemical analysis was conducted at the Southwest Research Institute (SWRI) in San Antonio, TX. Two GC/MS analytical methods targeted a total of 104 target compounds, including 70 identified as having potential endocrine activity in the European Commission list of priority substances for investigation of endocrine disruption (10) or in original references cited in our previous work (11). One method measured neutrally extracted pesticides, phthalates, PAHs, PBDEs, and PCBs; the phenols method, which requires derivatization prior to analysis, targeted alkylphenols, parabens, and other phenols and biphenyls identified as EDCs. All samples were analyzed by the neutrals method, and a subset was analyzed by the phenols method (Table S1). Details on sampling pumps, and extraction and analytical techniques are included in Supporting Information.
Quality assurance/quality control (QA/QC) measures were conducted to ensure accuracy and reliability of measurements. To estimate precision we collected four duplicate air samples for each analytical method. To evaluate contamination from laboratory, sampling matrices, and sample handling, we analyzed field blanks (n = 4 neutrals; n = 3 phenols), batch blanks (n = 5), and matrix blanks (n = 5 phenols, 6 neutrals). Matrix spikes (n = 2) and surrogate recoveries were used to characterize accuracy, compound recovery from the matrix, and extraction efficiency. Additional QA/QC information and results are presented in Supporting Information.
Statistical Methods
For each analyte, the method reporting limit (MRL) was defined as the maximum of the analytical detection limit and the 90th percentile of the lab and field blank concentrations. For each individual sample, the MRL varies slightly due to adjustment for sample volume. Values reported by the laboratory as estimated concentrations below the MRL were not included in the detection frequencies in the tables but were treated as estimated values to visualize distributions and for data analysis unless otherwise noted. The sample-specific MRL was used for nondetects except as noted. Sample quantile estimates (e.g., median, 95th percentile) for samples with limited numbers were based on linear interpolation. Differences in detection frequencies were evaluated using the Fisher’s exact test, and differences in median concentrations were evaluated with the nonparametric Wilcoxon rank sum test for chemicals with at least 50% detects >MRL. R was used for all statistical analyses.
Indoor sources are considered dominant if indoor concentrations exceed and are uncorrelated with outdoor concentrations. The differences between indoor and outdoor concentrations for paired samples were used to evaluate whether indoor concentrations result from predominantly indoor or outdoor sources, because chemicals with predominantly outdoor sources, assuming penetration near unity, will have indoor−outdoor differences close to zero.
Kendall’s tau rank correlation coefficients, adjusted for censored data, were calculated to investigate the relationships between indoor and outdoor measurements, with p-values obtained from 10,000 bootstrap replications. We used this method because data tended to be left censored due to laboratory detection limits. With high levels of censoring, Pearson and Spearman correlation coefficients calculated with substitution of arbitrary values (DL/2 or DL/sqrt(2)) for censored data have been shown to be poor estimates. Instead, either maximum likelihood estimates or Kendall’s tau rank correlations with adjustment for ties are more accurate, although, in general, Kendall’s tau estimates tend to be lower than corresponding Pearson or Spearman correlations (12). Correlations are presented on two-way scatter plots of indoor and outdoor concentrations for those compounds with at least 10 indoor and outdoor concentrations above the MRL.
Kendall’s tau rank correlation estimates were also used to identify patterns among co-occurring compounds. Correlation estimates were calculated for chemicals with at least 30% detected or estimated concentrations. In addition, factor analyses were conducted to explore potential structures within the data and as a data reduction tool. Factor analyses were on the log concentrations and conducted in R using the “factanal” function, with the number of factors retained determined by examining scree plots and rotated factors calculated using oblique (Promax) rotations. Only those compounds with reported values in 100% of the samples are included in the exploratory factor analysis due to statistical constraints.
Results and Discussion
Indoor and Outdoor Concentrations
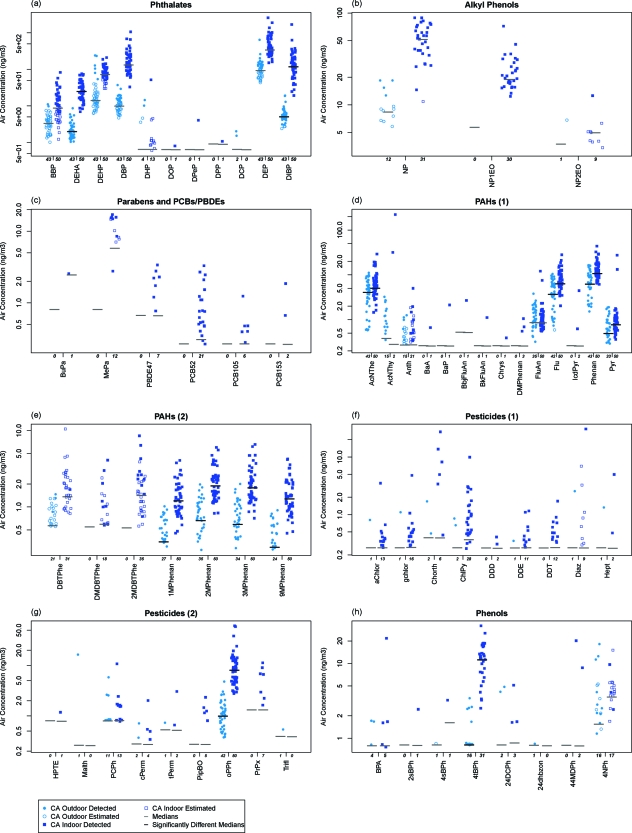
We detected 63 chemicals indoors and 39 outdoors above method reporting limits. Consistent with previous observations, indoor air concentrations were generally higher than outdoor across all chemical classes (1), indicating primarily indoor or mixed indoor and outdoor sources (Figure 1, Table S1). For the 13 chemicals with indoor and outdoor medians above the MRL, 12 were significantly higher indoors (2 phthalates, 8 PAHs, o-phenylphenol, 4-t-butylphenol).
Figure 1.
Endocrine-disruptor concentrations in outdoor and indoor air in California: (a) phthalates; (b) alkylphenols; (c) parabens and PCB/PBDE; (d) PAHs (group 1); (e) PAHs (group 2); (f) pesticides; and (g) phenols. Comparison of chemical distributions in outdoor and indoor air for both study communities illustrates that indoor concentrations are typically higher. Chemicals are included if ever detected. Abbreviations are matched to full names in tables. Concentrations are not blank corrected. Medians include estimated and nondetect (at detection limit) values. Numbers below each graph reflect the total number of samples with detects (estimated or >MRL). Medians are marked on the plots, and those that differ significantly between indoors and outdoors are marked in black (p < 0.05) for chemicals with at least 50% of values >MRL. Fluoranthene is the only chemical with sufficient data for the Wilcoxon and no significant difference between indoor and outdoor medians. Note log-scale on y-axis.
Indoor concentrations of dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), and diethyl phthalate (DEP) were higher than any other compounds, with maxima above 1 μg/m3. Figure 1 compares distributions of indoor and outdoor air concentrations for each chemical for California homes. In the Cape Cod study, indoor levels of these phthalates, as well as nonylphenol (NP) and o-phenylphenol, were also the most abundant compounds (Table S1). Levels of DEP, NP, and o-phenylphenol appear somewhat lower in the CA study (Cape medians: 590, 110, and 70 ng/m3 vs CA: 330, 53, and 8.5 ng/m3, respectively), possibly due to changing product formulation over the past few years.
Indoor−outdoor differences for paired data were mostly positive across all chemical groups, with many significantly positive, further supporting dominant indoor sources. Of 25 chemicals with at least 50% detected pairs, indoor−outdoor differences were significantly positive for 22, and not different from zero for 3 (anthracene, fluoranthrene, and pentachlorophenol). Consistent with previous studies, PAHs showed some negative difference values, indicating significant outdoor contributions (Figure S1).
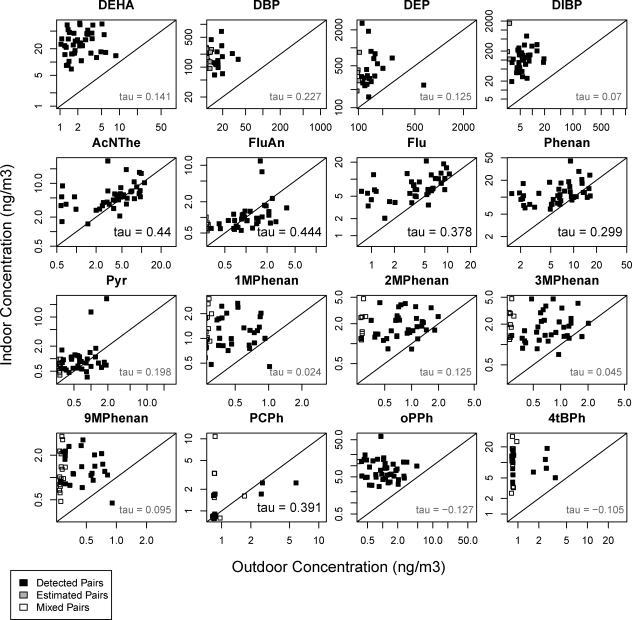
Figure 2 also illustrates paired indoor and outdoor concentrations. Of the 16 chemicals with adequate detection frequencies (>10 detected pairs), 4 PAHs (acenaphthene, fluoranthene, fluorene, and phenanthrene) and pentachlorophenol showed significant correlations between indoor and outdoor air (Kendall’s tau 0.3−0.4; p < 0.05), indicating that outdoor PAH levels are an important determinant of indoor concentrations, as reported by others (200).
Figure 2.
Scatter plot of paired indoor and outdoor concentrations in Richmond and Bolinas with Kendall’s tau correlation estimates. Correlation estimates in bold indicate significant correlation between outdoor and indoor concentrations (p < 0.05) for 4 PAHs and pentachlorophenol. Abbreviations are matched to full names in tables. Compounds with at least 10 indoor/outdoor detected (estimated or >MRL) pairs are included.
Outdoor air concentrations were higher in Richmond than Bolinas for 3 phthalates, 10 PAHs, and o-phenylphenol (Table S2), suggesting industrial or transportation sources. Specifically, medians were significantly higher in Richmond for all 6 chemicals with sufficient detects (at least 50% >MRL at both sites), including bis(2-ethylhexyl) adipate (DEHA), 3 PAHs, and o-phenylphenol, (p < 0.05), as well as diisobutyl phthalate (DIBP) (p < 0.1) and bis(2-ethylhexyl) phthalate (DEHP) based on estimated values. In addition, 7 other PAHs were detected more frequently in Richmond (p < 0.1) and at higher concentrations.
Indoor air levels were similar between communities, except that differences observed outdoors were also seen indoors (Table S3). For example, of 18 chemicals with sufficient detects (at least 50% >MRL in both communities), medians were significantly different for 7, with highest concentrations in Richmond (DIBP, and DEHP and 5 PAHs). Both indoor and outdoor concentrations were higher in Richmond for DEHP, DIBP, and several PAHs, suggesting some outdoor influence on indoor concentrations. For other compounds, indoor concentrations were similar, except at the maxima, where Richmond levels were almost always highest. A smaller number of samples in Bolinas limited opportunities to observe extreme values and power to detect significant differences.
Findings are detailed below by chemical group, with discussion of known sources, outdoor and indoor air concentrations.
Phthalates
We tested for 11 phthalates, including butyl benzyl phthalate (BBP), di-n-butyl phthalate (DBP), DEHA, DEHP, DEP, and DIBP. Phthalate concentrations in various media have been reported since the 1970s (13,14).
Outdoor Air
(Tables S1 and S2). Several phthalates were detected outdoors. DEHA and DIBP were detected >MRL in >90% of outdoor samples in each community; DBP and DEP were detected in 30−70%. The other phthalates were rarely or never detected. DEHA and DIBP were observed at significantly higher median concentrations in Richmond and DEHP was detected only in Richmond. Most outdoor DEHP findings are qualified due to a few values in elevated blank samples (see Supporting Information).
Ambient concentrations of DEP were substantially higher than those reported in suburban and urban locations in China (15) and in Paris (16) but were comparable to those measured outside homes in another suburban California community (17). Outdoor concentrations of BBP and DBP were lower than those reported outside day care centers in North Carolina (18). Outdoor concentrations of DBP were comparable to those in other studies (1).
Indoor Air
Four phthalates (DEHA, DBP, DEP, DIBP) were detected in 100% of the homes (Table S3; Figure 1). Median concentrations were significantly higher in Richmond than Bolinas for DIBP and DEHP, and DEHP was significantly more frequently detected in Richmond. DEP was the most abundant chemical, with concentrations ranging from 110 to 2500 ng/m3 (median 330 ng/m3). Indoor concentrations of DEP were lower than in Cape Cod (2) and slightly lower than a suburban California study (17). DBP was found at the second highest concentrations (median 140 ng/m3); levels were slightly lower than in Cape Cod (2) and suburban California (17). Indoor concentrations of DIBP were slightly lower and ranged from 17 to 1700 ng/m3 (median 130 ng/m3). DEHA concentrations were higher than Cape Cod; however, DEHP concentrations were similar (2). BBP, DEHP, DBP, and DEP median concentrations were lower than those measured previously in Berlin apartments (19).
Indoor−Outdoor Relationships
Indoor concentrations of BBP, DEHA, DEHP, DBP, DEP, and DIBP were greater than outdoors (Figure 1) and indoor−outdoor differences for paired samples were significantly above zero (p < 0.05) (e.g., DBP and DIBP in Figure S1). No correlation between indoor and outdoor levels was observed, suggesting dominant indoor sources (Figure 2). However, both indoor and outdoor concentrations were higher in Richmond than Bolinas for DEHP and DIBP (Tables S2 and S3), suggesting outdoor sources; it is also possible that indoor sources may influence outdoor concentrations, especially in densely populated areas (8).
Alkylphenols
Target alkylphenols were 4-t-nonylphenol (NP), 4-t-nonylphenol monoethoxylate (NP1EO), and 4-t-nonylphenol diethoxylate (NP2EO). The alkylphenol ethoxylates are commonly used as surfactants, for example in detergents, and as “inert” ingredients in pesticides, and NP also originates from plastics containing tris(nonylphenol)phosphite (1) and may have other uses. We measured the branched chain (tertiary) compounds. Other studies have analyzed only straight chain compounds such as 4-n-octylphenol and 4-n-nonylphenol, which are typically not detected (20) because they are not used commercially.
Outdoor Air
(Table S2). NP was infrequently detected outdoors in both Richmond (15%) and Bolinas (11%), and NPEOs were not detected. Outdoor NP concentrations were within the range previously reported (1) but lower than levels outside North Carolina day care centers (18).
Indoor Air
NP was detected in >95% of indoor air samples, with similar concentrations in both locations. NP1EO was also frequently detected indoors in both communities (>95%). NP2EO was infrequently detected (Table S3). Indoor concentrations of NP were similar to or slightly lower than reported previously (1).
Indoor−Outdoor Relationships
Indoor concentrations of NP ranged from <MRL to 89 ng/m3 (median 53) and were higher than outdoors (range <MRL to 40 ng/m3, 14% > MRL) (Figure 1). The difference between indoor and outdoor NP ranged from 2 to 85 ng/m3 (median 40) indicating indoor sources are important (data not shown). For NP1EO, indoor concentrations ranged from <MRL to 72 ng/m3 (median 20) but the compound was never detected outdoors, again indicating indoor sources.
Parabens
Three parabens, butyl-, ethyl-, and methyl paraben, were analyzed. Parabens are used as preservatives and antimicrobials (21) and can be found in personal care products, pharmaceuticals, and food (21,22). Ethyl-, butyl-, and methyl paraben were not detected outdoors and ambient concentrations could not be found in the literature. Indoors, methyl paraben was detected in 33% of Richmond homes (maximum 17 ng/m3) and never in Bolinas (Table S3). Few studies have measured parabens in indoor air. Parabens were more frequently detected in Cape Cod (with similar MRLs), although concentrations were similar (2).
Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls
PBDEs and PCBs are persistent organic compounds characterized by two halogenated phenyl groups. PBDEs have been used as flame retardant additives to furniture foam (6,23). PCBs, which are banned, were used in electronic equipment and in building materials including wood floor finish and window caulking (5,24).
Outdoor Air
Neither PBDEs nor PCBs were detected outdoors (Table S2). Outdoor air concentrations have been reported elsewhere at concentrations lower than the MRL for this study (1).
Indoor Air
PCB 52, the most volatile PCB analyzed, was detected in about half of indoor air samples in Richmond and Bolinas at concentrations ranging from < MRL to 3.3 ng/m3, which is comparable to concentrations reported in other residential studies but lower than those in offices and public buildings (1). The maximum concentrations of PCBs in this study are much lower than the maximum reported in Cape Cod, where a few homes had very high concentrations, apparently due to historic use of PCB-containing Fabulon wood floor finish (2,5). Concentrations of PDBE 47 are similar to those reported previously in indoor air, although the MRL in this study is in the midrange of previous studies (1). PDBEs and PCBs appear to originate from indoor sources, as they were not detected outdoors.
Polycyclic Aromatic Hydrocarbons
Results for PAHs are described in the Supporting Information, since these have been more frequently studied by others.
Pesticides
A total of 38 pesticides were targeted including banned organochlorines (e.g., DDT, PCP), and current use products such as carbamates (e.g., propoxur), organophosphates (e.g., chlorpyrifos), and pyrethroids (cypermethrin). o-Phenylphenol, a phenolic compound registered as a microbicide and with other uses, including as a plasticizer, was also measured. Detailed results for pesticides are shown in Supporting Information, and o-phenylphenol is also described here because of its more diverse uses.
Outdoor Air
Thirteen pesticides were detected outdoors (Tables S1, S2). o-Phenylphenol was detected in Richmond at significantly higher concentrations (median 1.2 vs 0.52 ng/m3; p < 0.05).
Indoor Air
Sixteen pesticides were detected in indoor air (Table S3). o-Phenylphenol, which was detected in 100% of indoor air samples in both studies, was found at much higher concentrations in Cape Cod (range 12−970 ng/m3, median 70 ng/m3) than the present study (range 2.8−61 ng/m3, median 8.5 ng/m3). Because this compound has a wide variety of uses, and since it was identified as an EDC during the 1990s, the lower levels in California may reflect changes in product formulations or use patterns between 2000, when Cape Cod homes were sampled, and 2006 California sampling.
Indoor−Outdoor Relationships
Among tested pesticides, only the microbicide o-phenylphenol was detected frequently, and indoor concentrations were significantly higher (p < 0.05) (Figure 1) and not correlated with outdoor levels (Figure 2), indicating dominant indoor sources.
Phenols and Miscellaneous
Phenolic compounds are characterized by a hydroxyl group bonded to an aromatic hydrocarbon. In addition to the alkylphenols, a number of commercially important phenolic compounds have been identified as EDCs (11). Sources of these phenols are varied and include pesticides, dyes, sunscreens, plastics, and industrial uses. Bisphenol A, one of the most well-studied phenols, is a high production volume chemical used in polycarbonate plastics, epoxy resins, and other applications such as carbonless paper, and has been detected in over 90% of NHANES urine samples (25). 4-t-Butylphenol was frequently detected in our previous work on Cape Cod, however we have found only limited information on its commercial uses. The 19 target phenols were analyzed in a subset of 31 (indoor) and 29 (outdoor) samples.
Outdoor Air
In general, target phenolic compounds were rarely detected outdoors. 4-t-Butylphenol was detected with the greatest frequency in Richmond (60%) and Bolinas (44%) outdoor samples (Table S2). Ambient concentrations of 4-t-butylphenol for comparison could not be located in the literature. Bisphenol A was detected in 1 Bolinas and 3 Richmond samples. 2,4-Dichlorophenol, 2,4-dihydroxybenzophenone, and 4-nitrophenol were detected outdoors, to a limited extent, primarily in Richmond.
Indoor Air
4-t-Butylphenol was the only target phenol commonly detected indoors. It was detected in 100% of air samples, with similar concentrations in Richmond and Bolinas (Table S3). Indoor concentrations ranged from 2.5 to 32 ng/m3 (median 12 ng/m3). Comparisons to concentrations reported in the literature are limited to the Cape Cod study where 4-t- butylphenol was detected in 100% of samples with concentrations ranging from 3.4 to 290 ng/m3 (median 16 ng/m3) (2).
Indoor−Outdoor Relationships
Concentrations of 4-t-butylphenol were significantly higher in indoor than outdoor air (Figure 1). Indoor−outdoor differences were significantly greater than 0 and indoor and outdoor levels were not correlated (Figure 2), indicating primarily indoor sources. The range of concentrations of 4-nitrophenol were similar indoors and outdoors, and other target phenols were sporadically detected indoors and not outdoors.
Mixtures
Identifying common mixtures is valuable because it suggests likely sources, identifies mixtures that should be priorities for toxicological and epidemiological studies, and alerts researchers to the possibility that unmeasured compounds that co-occur with chemicals measured in an epidemiological study may be responsible for observed health effects. To identify common mixtures, we used correlation analyses and exploratory factor analysis. This exercise is useful as a first step in identifying patterns within data and for data reduction.
Kendall’s tau correlation coefficients were calculated for all pairs of compounds in outdoor and indoor air with at least 30% estimated or >MRL values (n = 22 in outdoor air, n = 29 in indoor air). Results are shown in Figure S2 in Supporting Information. Exploratory factor analyses, conducted to identify potential structures within the data, tended to confirm the observations of the correlation analysis (Supporting Information).
Outdoor Air
Correlations of chemicals in outdoor air revealed that the alkylphenol NP was not significantly correlated with any other chemical outdoors. Correlations among the phthalates were limited and inconsistent. For example DEHA was significantly correlated with many other outdoor concentrations, including two phthalates, o-phenylphenol, pentachlorophenol (PCP), and the majority of the PAHs, while DEP was significantly negatively correlated with many of the same PAHs and not positively correlated with any compound except DBP. All PAH outdoor air concentrations were significantly and positively correlated with each other (0.45 ≤ τ ≤ 0.93). 4-t-Butylphenol was correlated with methyl phenanthrenes, o-phenylphenol, and PCP, while 4-nitrophenol correlated with virtually all PAHs (including methyl phenanthrenes), o-phenylphenol, and DEHP. These observations suggest that (1) NP is not correlated with other alkylphenols; (2) not all phthalates share common outdoor sources; and (3) individual phenols like 4-t-butylphenol and 4-nitrophenol associate with different components of the PAHs.
Indoor Air
Phthalates were generally correlated with each other, as well as with o-phenylphenol, 4-t-butylphenol, and NP—all ubiquitous commercial chemicals. They were also correlated with methyl phenanthrenes and some PAHs. NP was not correlated with other alkylphenols, suggesting that the predominant source of NP in indoor air is not alkylphenol surfactants but rather other uses of nonylphenol, such as the plasticizer tris(nonylphenol)phosphite. Nonylphenol was, however, significantly correlated with many common chemicals indoors including o-phenylphenol, 4-t-butylphenol, and some phthalates and PAHs. In general, correlations between chemicals indoors were different from those observed outdoors.
Indoor air concentrations of PAHs were significantly and positively correlated with each other, with the exception of anthracene, but correlation coefficients were typically smaller for indoor levels than outdoors. PAHs indoors were also correlated with other compounds, including phthalates, NP, o-phenylphenol, PCP, PCBs, and 4-t-butylphenol, whereas outdoor PAHs were not correlated with phthalates (except DEHA) or NP. Methyl phenanthrenes were significantly correlated with many other chemicals, especially other PAHs, DBP, NP, and 4-t-butylphenol. PCB 52 and pentachlorophenol concentrations were correlated indoors, perhaps indicating older buildings, since both chemicals have been restricted in recent years. Chlordane, also banned for home use years ago, was correlated with PCB 52.
o-Phenylphenol was significantly correlated with many compounds, including all of the phthalates. The strongest correlations were with 4-t-butylphenol, DBP, methyl phenanthrenes, and PCB 52. Despite the fact that 4-t-butylphenol and o-phenylphenol are commonly detected indoors, their sources are poorly characterized and 4-t-butylphenol is not identified as an ingredient of commercial products. These findings suggest that they may be common co-ingredients with commercial chemicals such as phthalates and NP. Unlike in outdoor air, 4-nitrophenol was not correlated with other compounds, except for a significant negative correlation with o-phenylphenol.
In summary, this is the first report we are aware of with paired indoor and outdoor air concentrations for a wide range of commercially important chemicals that have been identified as EDCs. Findings support previous observations that indoor concentrations are higher than those outdoors and demonstrate that the two are generally uncorrelated, confirming the expectation that the indoor sources of the consumer product chemicals predominate. Concentrations of many of these chemicals are correlated with each other, indicating the importance of addressing mixtures in health studies and regulation. The most consistent correlations were for o-phenylphenol, NP, 4-t-butylphenol, and phthalates—all of which have been identified as potential EDCs, the phthalates as anti-androgens and the three phenols as weak estrogens (11). Interestingly, NP is not correlated with other alkylphenols, suggesting a different source, possibly the plasticizer tris(nonylphenol)phosphite. Methyl phenanthrenes indoors appear to be most correlated with ubiquitous commercial chemicals such as phthalates and o-phenylphenol, while other PAHs have important outdoor sources, as indicated by indoor−outdoor correlations and as demonstrated in other studies.
Comparisons across communities provide information about sources and potential health implications. Indoor air concentrations in Cape Cod were higher than those in this California study for banned organochlorine pesticides (but not contemporary pesticides) and PCBs, and for the commercial chemicals nonylphenol and o-phenylphenol. Differences between the Cape Cod and California studies may be due to geographic differences in use patterns or changes in product formulations between 2000, when Cape Cod homes were sampled, and 2006, when California homes were sampled. Although the phthalates DEHP, DEHA, and DIBP are typically considered indoor contaminants from plastics and consumer goods, the concentration difference between outdoor air in urban/industrial and rural communities suggests some industrial or transportation sources as well. It is interesting that aside from PAHs and three of the phthalates, which appear to have outdoor sources, few indoor concentration differences between Richmond and Bolinas were observed at the median despite differences in demographics and housing, suggesting sources are ubiquitously common across socioeconomic groups.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (grant R25 ES013258) and New York Community Trust. We thank Carla Perez, Jessica Tovar, Wanna Wright, Marleen Quint, Andrea Samulon, and Sarah Dunagan for contributions to study planning, community involvement, and data collection. We thank advisory council members for significant contributions to study design and communications: Asa Bradman of the University of California Berkeley, Barbara Brenner of Breast Cancer Action, Raymond Neutra (then at California Department of Public Health), Sharyle Patton of Commonweal, Jeanne Rizzo of Breast Cancer Fund, and community members Quint and Wright who also participated in conducting the study.
Supporting Information Available
Additional detail about chemical analytical methods, QA/QC data, PAH and pesticide results, summary statistics for combined (across both communities) outdoor and indoor air, summary statistics by community, indoor−outdoor differences, Kendall’s tau correlation estimates for indoor and outdoor air, and factor analysis. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Rudel R. A.; Perovich L. J. Endocrine disrupting chemicals in indoor and outdoor air. Atmos. Environ. 2009, 43, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel R. A.; Camann D. E.; Spengler J. D.; Korn L. R.; Brody J. G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [DOI] [PubMed] [Google Scholar]
- U.S. General Accounting Office. Indoor Pollution: Status of Federal Research Activities, Washington, DC, 1999. Available at http://www.gao.gov/archive/1999/rc99254.pdf.
- Weschler C. J.; Nazaroff W. W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar]
- Rudel R. A.; Seryak L. M.; Brody J. G. PCB-containing wood floor finish is a likely source of elevated PCBs in residents’ blood, household air and dust: a case study of exposure. Environ. Health 2008, 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota A. R.; Rudel R. A.; Morello-Frosch R. A.; Brody J. G. Elevated house dust and serum concentrations of PBDEs in California: Unintended consequences of furniture flammability standards. Environ. Sci. Technol. 2008, 42, 8158–8164. [DOI] [PubMed] [Google Scholar]
- Whitmore R. W.; Immerman F. W.; Camman D. E.; Bond A. E.; Lewis R. G.; Schaum J. L. Non-occupational exposures to pesticides for residents of two US cities. Arch. Environ. Contam. Toxicol. 1994, 26, 47–59. [DOI] [PubMed] [Google Scholar]
- Jamshidi A.; Hunter S.; Hazrati S.; Harrad S. Concentrations and chiral signatures of polychlorinated biphenyls in outdoor and indoor air and soil in a major UK conurbation. Environ. Sci. Technol. 2007, 41, 2153–2158. [DOI] [PubMed] [Google Scholar]
- Brody J. G.; Morello-Frosch R. A.; Zota A. R.; Brown P.; Perez C.; Rudel R. Linking exposure assessment science with policy objectives for environmental justice and breast cancer advocacy: The Northern California Household Exposure Study. Am. J. Public Health 2009, 99 (3), S600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission DG ENV. Towards the establishment of a priority list of substances for further evaluation of their role in endocrine disruption: preparation of a candidate list of substances as a basis for priority setting; 2000. Available at http://ec.europa.eu/environment/docum/pdf/bkh_main.pdf
- Rudel R. A.; Brody J. G.; Spengler J. D.; Vallarino J.; Geno P. W.; Sun G.; Yau A. Identification of selected hormonally active agents and animal mammary carcinogens in commercial and residential air and dust samples. J. Air Waste Manage. Assoc. 2001, 51, 499–513. [DOI] [PubMed] [Google Scholar]
- Newton E.; Rudel R. Estimating correlation with multiply censored data arising from the adjustment of singly censored data. Environ. Sci. Technol. 2007, 41, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z.; Ebinghaus R.; Temme C.; Lohmann R.; Caba A.; Ruck W. Occurrence and air−sea exchange of phthalates in the Arctic. Environ. Sci. Technol. 2007, 41, 4555–4560. [DOI] [PubMed] [Google Scholar]
- Giam C. S.; Chan H. S.; Neff G. S.; Atlas E. L. Phthalate ester plasticizers: a new class of marine pollutant. Science (New York, N.Y.) 1978, 199, 419–421. [PubMed] [Google Scholar]
- Wang P.; Wang S. L.; Fan C. Q. Atmospheric distribution of particulate- and gas-phase phthalic esters (PAEs) in a Metropolitan City, Nanjing, East China. Chemosphere 2008, 72, 1567–1572. [DOI] [PubMed] [Google Scholar]
- Teil M. J.; Blanchard M.; Chevreuil M. Atmospheric fate of phthalate esters in an urban area (Paris-France). Sci. Total Environ. 2006, 354, 212–223. [DOI] [PubMed] [Google Scholar]
- Sheldon L.; Clayton A.; Keever J.; Perritt R.; Whitaker D.. PTEAM: Monitoring of Phthalates and PAHS in Indoor and Outdoor Air Samples in Riverside, CA; California Environmental Protection Agency, Air Resources Board Research Division, 1992. (Available at http://www.arb.ca.gov/research/abstracts/a933-144.htm#Section). [Google Scholar]
- Wilson N. K.; Chuang J. C.; Lyu C. Levels of persistent organic pollutants in several child day care centers. J. Exposure Anal. Environ. Epidemiol. 2001, 11, 449–458. [DOI] [PubMed] [Google Scholar]
- Fromme H.; Lahrz T.; Piloty M.; Gebhart H.; Oddoy A.; Ruden H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air 2004, 14, 188–195. [DOI] [PubMed] [Google Scholar]
- Wilson N. K.; Chuang J. C.; Morgan M. K.; Lordo R. A.; Sheldon L. S. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ. Res. 2007, 103, 9–20. [DOI] [PubMed] [Google Scholar]
- Soni M. G.; Burdock G. A.; Taylor S. L.; Greenberg N. A. Safety assessment of propyl paraben: a review of the published literature. Food Chem. Toxicol. 2001, 39, 513–532. [DOI] [PubMed] [Google Scholar]
- Shen H. Y.; Jiang H. L.; Mao H. L.; Pan G.; Zhou L.; Cao Y. F. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. J. Sep. Sci. 2007, 30, 48–54. [DOI] [PubMed] [Google Scholar]
- Hale R. C.; Alaee M.; Manchester-Neesvig J. B.; Stapleton H. M.; Ikonomou M. G. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003, 29, 771–779. [DOI] [PubMed] [Google Scholar]
- Herrick R. F.; McClean M. D.; Meeker J. D.; Baxter L. K.; Weymouth G. A. An unrecognized source of PCB contamination in schools and other buildings. Environ. Health Perspect. 2004, 112, 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barro R.; Regueiro J.; Llompart M.; Garcia-Jares C. Analysis of industrial contaminants in indoor air: Part 1. Volatile organic compounds, carbonyl compounds, polycyclic aromatic hydrocarbons and polychlorinated biphenyls. J. Chromatogr. A 2009, 1216, 540–566. [DOI] [PubMed] [Google Scholar]
- Calafat A. M.; Ye X. Y.; Wong L. Y.; Reidy J. A.; Needham L. L. Exposure of the US population to bisphenol A and 4-tertiary-octylphenol: 2003−2004. Environ. Health Perspect. 2008, 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.