Abstract
The Deepwater Horizon oil spill has led to the use of >1 M gallons of oil spill dispersants, which are mixtures of surfactants and solvents. Because of this large scale use there is a critical need to understand the potential for toxicity of the currently used dispersant and potential alternatives, especially given the limited toxicity testing information that is available. In particular, some dispersants contain nonylphenol ethoxylates (NPEs), which can degrade to nonylphenol (NP), a known endocrine disruptor. Given the urgent need to generate toxicity data, we carried out a series of in vitro high-throughput assays on eight commercial dispersants. These assays focused on the estrogen and androgen receptors (ER and AR), but also included a larger battery of assays probing other biological pathways. Cytotoxicity in mammalian cells was also quantified. No activity was seen in any AR assay. Two dispersants showed a weak ER signal in one assay (EC50 of 16 ppm for Nokomis 3-F4 and 25 ppm for ZI-400). NPs and NPEs also had a weak signal in this same ER assay. Note that Corexit 9500, the currently used product, does not contain NPEs and did not show any ER activity. Cytotoxicity values for six of the dispersants were statistically indistinguishable, with median LC50 values ∼100 ppm. Two dispersants, JD 2000, SAF-RON GOLD, were significantly less cytotoxic than the others with LC50 values approaching or exceeding 1000 ppm.
Introduction
The massive oil spill from the Deepwater Horizon oil platform in the Gulf of Mexico has led to the use of correspondingly large volumes of the oil spill dispersant Corexit 9500 (Nalco Energy Services, L.P., Sugar Land, TX). In excess of 1.5 M gallons of dispersant have been released into the Gulf as of June 26, 2010. Oil spill dispersants are complex mixtures of two basic components [1]. The first is comprised of one or more surfactants that can emulsify oil. The second is a hydrocarbon-based solvent mixture that helps break up large clumps of high molecular weight, more viscous oil. There is limited information on the potential of dispersants to cause acute or long term toxicity in aquatic species or humans.
EPA's Office of Research and Development was asked to evaluate the potential toxicity of eight oil spill dispersants, including Corexit 9500. Because of the need for rapid turnaround, it was decided to employ a series of in vitro, cell-based assays. One mode of toxicity that is of concern for dispersants is endocrine disruption [2], due to of the fact that nonylphenol ethoxylates (NPEs) are used in some of the dispersants as part of the surfactant component. NPEs can degrade to produce nonylphenol[3], which can strongly interact with the estrogen receptor[4-7]. NPEs themselves have been shown to inhibit testicular growth in rainbow trout[8]. Because of this fact, the focus of our in vitro studies was on measuring potential interaction of the dispersants with the estrogen receptor (ER) and the androgen receptor (AR).
Here we describe the results of a series of rapid in vitro tests to determine the interaction of eight oil spill dispersants with ER, AR and other receptors and transcription factors. Using several different high-throughput screening assay technologies, we were able to rapidly produce data on the dispersants and 23 reference compounds. A multiplexed reporter gene assay battery that is part of EPA's ToxCast program was used to evaluate activity against a panel of 73 transcription factors and nuclear receptors[9, 10]. Besides AR and ER, this multiplexed battery probed a wide range of targets relevant to potential toxicity pathways. Cell-based assays using beta-lactamase reporter genes under control of either the AR or the ER were also used for qHTS (quantitative high-throughput screening)[11, 12]. Cytotoxicity endpoints were measured in order to quantify the relative mammalian cell lethality of the dispersants.
When looking at this in vitro data, several limitations need to be considered. First, not all assays were run in metabolically competent cells, so that the effects of biotransformation are not fully accounted for. Second, these assays cannot account for the complex interactions between cells and organs that occur in a whole organism on the path to toxicity. Third, only short term effects can be directly studied in these assays. Nonetheless, these in vitro screening tests were able to provide a rapid comparison of each dispersant's potential for endocrine activity and relative cytotoxicity in three mammalian cell types.
Methods
Chemicals
All assays evaluated eight commercially available oil spill dispersants that were obtained directly from the respective manufacturers. EPA chose these eight dispersants from those listed on the National Contingency Plan Product Schedule[13] based on three criteria: 1) lower toxicity of the dispersant or of the dispersant when mixed with oil; 2) availability of sufficient quantities to respond to the Gulf spill; and 3) immediate availability of samples for testing. These included Corexit® 9500 (Nalco Inc., Sugarland TX), JD 2000™ (GlobeMark Resources Ltd., Atlanta, GA), DISPERSIT SPC 1000™ (U.S. Polychemical Corp., Chestnut Ridge, NY), Sea Brat #4 (Alabaster Corp., Pasadena, TX), Nokomis 3-AA (Mar-Len Supply, Inc., Hayward, CA), Nokomis 3-F4 (Mar-Len Supply, Inc., Hayward, CA), ZI-400 (Z.I. Chemicals, Los Angeles, CA) and SAF-RON GOLD (Sustainable Environmental Technologies, Inc., Mesa, AZ). All are liquid solutions. Further information on the dispersants, including the limited publicly available information on their composition is given in SI:Appendix A.1. The dispersants were diluted in water and tested in vitro at concentrations ranging from 0.01 to 1000 ppm (vol:vol) in the presence of a final concentration of 0.5% DMSO to account for reference compound solvent.
All assays were also run on reference compounds recommended for validating ER /AR assays by ICCVAM (Interagency Coordination Committee on the Validation of Alternative Methods)[14] and the U.S. EPA[15]. A preliminary set of compounds was obtained from stocks at EPA facilities in RTP NC. Subsequently, we ordered fresh samples of a larger set from Sigma-Aldrich (St. Louis MO). Included in the reference chemicals are both straight chain and branched NP isomers and corresponding example NPEs. The reference chemicals are 17β-Trenbolone (10161-33-8), 17β-Estradiol (50-28-2), Atrazine (1912-24-9), Bisphenol A (80-05-7), Butylbenzyl phthalate (85-68-7), Dibutyl phthalate (84-74-2), Flutamide (13311-84-7), Linuron (330-55-2), 4–Nonylphenol (linear) (104-40-5), DDE- p,p′– (72-55-9), Methoxychlor (72-43-5), Procymidone (32809-16-8), Vinclozolin (50471-44-8), 2,4,5-T (93-76-5), Bicalutamide (90357-06-5), Cyproterone acetate (427-51-0), Genistein (446-72-0), 4-(tert-octyl)Phenol (140-66-9), 4-Hydroxytamoxifen (68392-35-8), 5α-androstan-17β-ol-3-one (521-18-6) and 4-Nonylphenol, (branched) (84852-15-3). The two nonylphenol ethoxylates are Tergitol NP-9 (127087-87-0) and Igepal CO-210 (68412-54-4). Reference chemicals (powder form) were solubilized in DMSO to a final stock concentration of 20 mM with serial dilutions performed in DMSO. Further information, including lot and batch are given in SI:Appendix A.2. Chemicals were diluted to their final testing concentration in cell culture medium resulting in a final solvent (DMSO) concentration of 0.5%.
In Vitro Assays
Assays were performed by two separate laboratories. More complete details are provided in SI:Appendix B.
Attagene Inc. (Cary, NC) performed a battery of 48 cis- and 25 trans- receptor or transcription factor activation assays[9, 10] in human liver-derived HepG2 cells, including two ER assays and one for AR. All assays were run in eight to 16-point concentration-response format, with four replicates run over two weeks. This collection of assays allows us to evaluate a broad range of pathways potentially perturbed by the dispersants and to look for non-specific assay interference effects that could lead us to discount activity in the ER assays. Attagene assays are described in more detail in SI:Appendix B.1.
The NIH Chemical Genomics Center (NCGC) performed qHTS (quantitative high-throughput screening) assays[11, 12, 16] for activity against ER and AR. These cell-based assays (AR bla and ER bla assays) use the ligand binding domains of either human AR or ER fused with yeast GAL4 DNA-binding domains to drive expression of beta-lactamase reporter genes. Each assay was run in a 24 concentration dilution series, and replicated in assays run on at least five separate days. Details of the NCGC protocols are given in SI:Appendix B.2 and B.3.
Concentration-response data for all assays were sent to EPA NCCT where curve fitting procedures were applied to determine if there was significant activity in each chemical-assay pair, and if so to extract an EC50 value (concentration at which 50% of the maximal effect was seen). The curve fitting procedure is described in SI:Appendix C. For all cell-based assays, we also assessed concentrations at which cytotoxicity occurred, calculating an LC50 value (concentration at which 50% of cells were killed). We additionally calculated LC20 values and discounted assay activities observed at concentrations above these.
Results
Cytotoxicity Data
The dispersants were tested for cytotoxicity / cell viability in three cell types: the ARHEK293 and ER-HEK293 cell lines (5 h incubation) from NCGC and HepG2 cells by Attagene (24 h incubation). All LC50 values for these cell types are plotted in Figure 1 and numerical values are listed in SI:Appendix D. For comparison, we also include LC50 values from whole animal, aquatic species lethality assays for the mysid, Americamysis bahia, in a 48-hr static acute toxicity test and an inland silverside, Menidia beryllina, 96-hr static acute toxicity test[17]. One can see that the cell-based LC50 values overall vary by about two orders of magnitude, and that the values for any given chemical typically span less than one order of magnitude. The rank order of cytotoxicity for each dispersant varied across the 3 cell types tested. There is significant overlap in the range of cytotoxicity for all of the dispersants except JD 2000 and SAF-RON GOLD, which were only cytotoxic in one cell line, and then at high concentrations. The whole animal LC50 values are almost always lower than the cell-based LC50 values. As with the cell-based assays, JD 2000 is the least toxic in the aquatic species assays.
Figure 1.
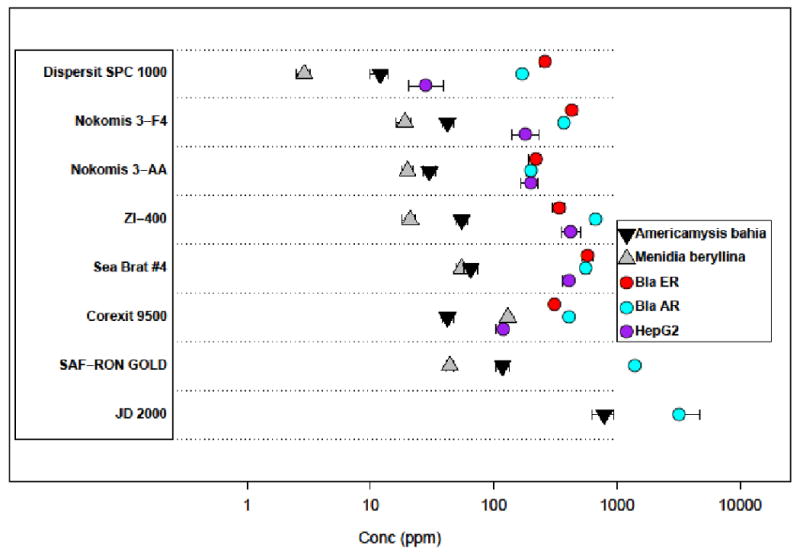
Toxicity data for the dispersants, combining data from 3 cell-based assays with data on aquatic species [12]. Each horizontal band shows the data for one dispersant. Cell-based LC50 values (concentration at which 50% lethality or effect is observed) are indicated by circles. Aquatic species LC50 values are indicated by triangles. Note that all dispersants were tested in all assays, and missing data points indicate that no toxicity was seen in that assay at the highest concentration tested (1000 ppm). 95% confidence intervals are shown for all assays.
In order to statistically assess differential cytotoxicity across the eight dispersants we performed an ANOVA to determine pairwise if either of two dispersants was more cytotoxic than the other. We performed this test with and without multiple test correction (Bonferroni). For any dispersant and assay combination that did not achieve an LC50, a default value of 3000 ppm was used; three-fold higher than the highest concentration tested. All three cell-based quantitative cytotoxicity assays were used for this analysis, but the in vivo data in Figure 1 was not. The resulting p-values, raw and corrected, are provided in Table S10. Both JD 2000 and SAF-RON GOLD are significantly less cytotoxic than the other six dispersants. DISPERSIT SPC 1000 is significantly more cytotoxic than the other dispersants in the HepG2 assay, but not the other two.
Estrogen and Androgen Receptor Activity
We observed statistically significant ER activity for two of the dispersants, Nokomis 3-F4 and ZI-400, in the Attagene trans-ERα assay (Table 1). Figure 2C and 2D shows the concentration-response curves for the two active dispersants, which have EMax (maximum fold change or efficacy) values of only 3 to 4-fold. This is in contrast to 17β-estradiol (Figure 2A), which has an EMax value of 20-fold. The corresponding reference curve for the cis-ERE assay (Figure 2B) shows that 17β-estradiol elicits a response about half of that seen in the trans assay. The performance of these ER assays was assessed for a set of 19 reference chemicals recommended by ICCVAM[14] and EPA OPPT[15] and demonstrated that these assays performed well for both positive and negative predictive value (SI:Appendix E). The trans-ERα assay correctly matched ICCVAM expectation for 15 of 17 reference chemicals, with one false positive and one false negative. A comparison of the cis and trans assays shows that the reference chemicals in the cis assay consistently produce EMax values about half of that seen in the trans assay. This would explain the absence of activity for these dispersants in the cis assay, because we do not consider curves with EMax values below 2. Additional concentration response curves in Figure 2 show data for NP and NPE compounds.
Table 1.
Summary results for the Attagene trans-ERα assay for the positive dispersants. EMax: maximal fold change.
| Chemical | EC50 (ppm) | EMax | R2 | p-value |
|---|---|---|---|---|
| Nokomis 3-F4 | 16 | 3.9 | 0.65 | 0.00017 |
| ZI-400 | 25 | 3.4 | 0.68 | 0.0041 |
Figure 2.
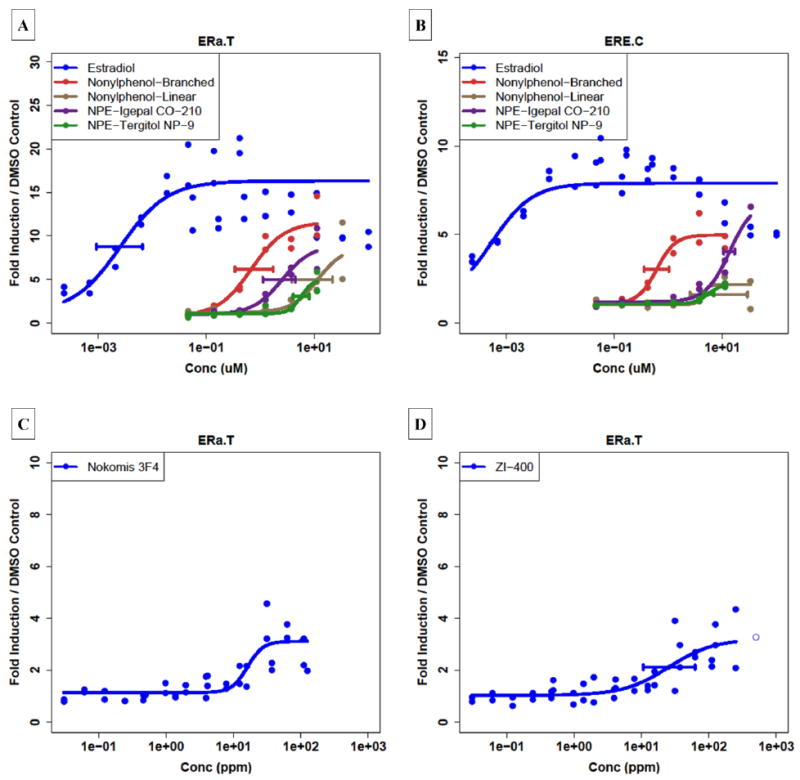
Concentration-response curves for the 17β–Estradiol, NP and NPE compounds, and the two dispersants showing activity in Attagene trans-ERα assay. (A): 17β–Estradiol and the 4 NP / NPE compounds in the Attagene trans-ERα assay; (B) same chemicals in the Attagene cis-ERE assay. (C): Concentration-response curves for ZI-400 and (D) Nokomis 3-F4 in the Attagene trans-ERα assay.
The only dispersant that showed any activity in any of the AR assays was JD 2000, which was active in both the NCGC ER and AR agonist and antagonist assays in all runs with EC50 values ranging from 100-270 ppm (AR) and 82-120 ppm (ER). From Figure 1, one can see that there was no cytotoxicity in two of the three cell lines for JD 2000. The EMax values for JD 2000 in all of these assays was significantly greater than control values, and in the antagonist assays, this dispersant looked like a “super-activator” rather than an antagonist. All of these data taken together indicates strongly that some non-specific activation is occurring that is independent of ER or AR. We have found previously that compounds identified as promiscuous “super-activators” in multiple beta-lactamase reporter gene assays with a narrow potency range (a <3-fold difference in potency is within the experimental variations of these assays) are usually auto-fluorescent or non-specific activators (unpublished data). JD 2000 was tested and found not to be auto-fluorescent. To test this as a possible artifact of the beta-lactamase assay format, JD 2000 was tested in three other beta-lactamase reporter gene assays not regulated by nuclear receptors (HIF1a, CRE and NFκB) and showed similar activation of all three (unpublished data). Note that JD 2000 was inactive in all the Attagene AR and ER assays. Thus, the activity observed for JD 2000 is likely an artifact of the beta-lactamase assay format and not due to specific AR- or ER-ligand interactions. Considering the totality of the data, we conclude that JD 2000 does not exhibit ER or AR specific activity.
Nonylphenol-Related ER Activity
It is known that some of the dispersants contain NPEs. Our initial hypothesis was that any estrogenic activity detected for the complex mixtures could be due to the NPEs or to NP itself generated by in situ degradation of the NPE, or residual contamination from synthesis of the NPE. Consequently, we tested two nonylphenols (one linear and one branched) and two commercial NPEs in the Attagene assays. Table 2 shows the results of this analysis, and Figure 2A and 2B shows the corresponding dose-response curves for the Attagene ER assays. From these data, one can see that these cell-based assays show ER activity for both the NPs and the NPEs. The branched NP is the most potent, as expected, but the second most potent is the NPE Igepal CO-210. These data indicates that the presence of an NP or NPE in a mixture could give rise to ER activity such as was seen for the dispersants Nokomis 3-F4 and ZI-400. Public information (given in SI:Appendix A) indicates that ZI-400 does in fact contain an NPE. Determining the actual source of the estrogenic activity of the commercial NPEs and the dispersants will require further experiments that are being planned.
Table 2.
Results of ER assays on NPs and NPEs.
| Chemical | Assay | EC50 (μM) | R2 | EMax | p-value |
|---|---|---|---|---|---|
| Nonylphenol (linear) 104–40–5 |
trans -ERα | 11 | 0.77 | 8.3 | 0.29 |
| cis-ERE | 4.3 | 0.55 | 2.7 | 0.096 | |
| Nonylphenol (branched) 84852–15–3 |
trans -ERα | 0.68 | 0.91 | 12 | 0.0049 |
| cis-ERE | 0.61 | 0.092 | 5.4 | 4.9E-5 | |
| Tergitol NP-9 127087–87–0 |
trans -ERα | 5.7 | 0.86 | 4.8 | 0.18 |
| cis-ERE | 5.6 | 0.96 | 2.1 | 0.042 | |
| Igepal CO–210 68412–54–4 |
trans -ERα | 2.5 | 0.89 | 8.5 | 0.19 |
| cis-ERE | 14 | 0.96 | 6.5 | 2.1E-11 |
Activity Against Other Biological Targets
In addition to ER and AR, we also analyzed the chemical collection (dispersants plus reference chemicals) using a multiplexed reporter gene assay battery that evaluates activity against a panel of transcription factors including nuclear receptors[9, 10]. These data also provide a measure of quality control related to the specificity of any endocrine-related activity caused by the dispersants. The description of the assay and a complete list of targets is given in SI:Appendix B.2. All of these assays were carried out twice, one week apart, and in each week, duplicate runs were performed.
Figure 3 summarize all of the results for the dispersants and helps illustrate several key points about the data. First, as the concentration of a chemical approaches the cytotoxic level, generalized cell stress occurs, accompanied by broad misregulation of transcription. When this threshold is reached, many assays in this system simultaneously activate, but this activity is assumed to be non-specific. One sentinel of this cell stress behavior is NRF2, which is an indicator of generalized oxidative stress. Therefore, if we see many assays become active at about the same concentration, especially if NRF2 is among them, we tend to discount any target specificity above that concentration. We see this behavior for Corexit 9500 (∼50 ppm), JD 2000 (∼500 ppm), Nokomis 3-AA (∼75 ppm), Nokomis 3-F4 (∼75 ppm), Sea Brat #4 (∼90 ppm) and ZI-400 (∼50 ppm).
Figure 3.
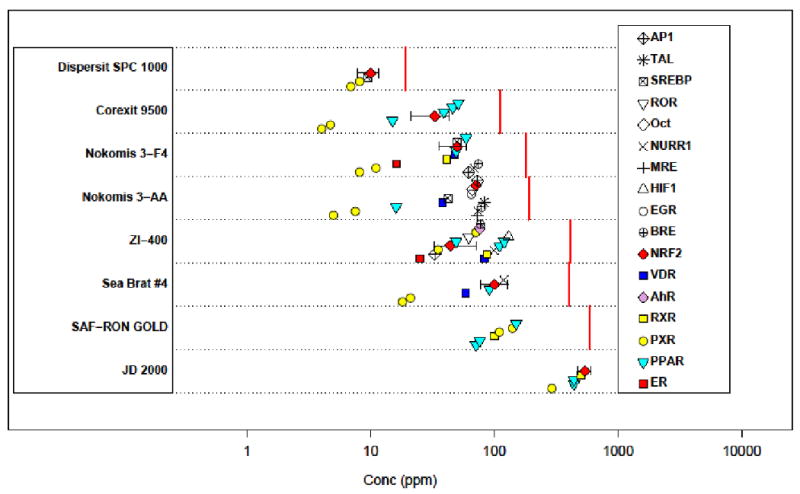
Summary plot of results for all Attagene assays and the dispersants. Each horizontal band displays EC50 values for a single dispersant. Points are staggered in the y-direction to make overlapping points visible. Multiple assays for a given gene target (e.g. PPARα, PPARδ, PPARγ) are represented by a single symbol, plotted repeatedly. 95% confidence intervals are shown on assays for the NRF2 as an example. The dispersant-specific vertical red lines indicate the LC50 for cytotoxicity in the Attagene assays (HepG2 cells).
The ER activity for Nokomis 3-F4 occurs at a concentration well below where this nonspecific behavior is indicated. For ZI-400, the confidence intervals for ER and NRF2 overlap, indicating a possibility that the ER result is non-specific.
Starting at low concentrations, the first activity that is generally seen is for PXR (Pregnane-X-receptor), which is a xenosensor. This behavior is entirely expected, is common across many classes of organic chemicals, and is not in itself an indicator of toxicity. PXR has been reported to be a xenosensor that acts to protect against endocrine active chemicals[18]. PPAR (peroxisome proliferator activating receptor[19-23]) activity is observed for a number of the dispersants, at higher concentrations than is seen for the PXR assays. There is a significant literature on the relationship between PPAR activity and disease in rodents, although the human relevance of PPAR activity is unclear [19-22, 24-27]. However, only for Corexit 9500 and Nokomis 3-AA (and potentially for SAF-RON GOLD) is the PPAR signal well below the level of non-specific activity. Vitamin D receptor (VDR) activity is seen for Sea Brat #4 and Nokomis 3-AA below but near the concentration of non-specific behavior.
Despite occurring at the same concentration as NRF2 activity, the PXR and PPAR activity of JD 2000 cannot necessarily be dismissed as being non-specific because the many other transcription factors were not activated. It appears to be a targeted response against these two xenosensing transcription factors. A similar observation can be made about Dispersit SPC 1000. At the concentration of NRF2 activity, we only see activation of two PXR assays and one for SREBP (SREBF1 sterol regulatory element binding transcription factor 1) which is involved in fatty acid synthesis regulation.
The largest effect (in terms of EMax) of any dispersant and assay is for ZI-400 and AhR (Aryl hydrocarbon receptor), with EMax >30. The AhR is well-known for its role in mediating the adaptive metabolism of xenobiotics, and also in the toxicity that follows exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin). This indicates the potential for the presence of a relatively efficacious dioxin-like compound. In the ToxCast Phase I data set[9, 28] of 309 chemicals, we saw only three chemicals (Napropamide, Propiconazole and Tetraconazole) with AhR efficacy values higher than seen with ZI-400. It is not clear that this effect is specific and distinct from cell stress, given that it occurs in the same concentration range as activity in a number of other targets, and above the NRF2 EC50.
The activity of the dispersants in some of these assays, especially PXR and PPAR can be put into context by looking at the same type of plot for the reference chemicals (Figure S3). Besides the strong activity in AR and ER assays, which are the biological targets of these chemicals, we see many showing activity in PXR and PPAR in the 1-100 μM range. This just illustrates how ubiquitous activity in these xenobiotic receptors is.
Discussion
All of the dispersants showed cytotoxicity in at least one cell type at concentrations between 10 and 1000 ppm. JD 2000 and SAF-RON GOLD are significantly less cytotoxic than the other dispersants. DISPERSIT SPC 1000 is the most cytotoxic in the HepG2 assay and in both of the aquatic species assays. The aquatic species LC50 values tend to be lower than the cell-based LC50 values. As with the cell-based assays, JD 2000 is the least toxic in the aquatic species assay.
Androgen receptor (AR) activity was seen for only a single dispersant (JD 2000) in a single cell-based AR assay (NCGC AR). For this dispersant and assay, the AR and ER concentration-response curves were almost identical, as were the corresponding antagonist assays, and in all cases the response exceeded the positive control. Given that AR and ER have very different ligand-binding specificity, the similarity in responses between AR and ER implies a non-specific “super-activator” effect. The non-specificity of this JD 2000 result was confirmed in follow-up studies using the same assay technology three additional targets. JD 2000 was inactive in all the other (Attagene) AR and ER assays. Therefore, we do not find any evidence for biologically significant AR-specific activity for any of the dispersants.
Estrogen receptor (ER) activity was observed in two of the dispersants in the Attagene trans-ERα assay (ZI-400 and Nokomis 3-F4), although at relatively high concentrations of 10-100 ppm. No dispersant tested showed activity in more than one of the three cell-based ER assays. We have also shown that NPs and NPEs are also active in the trans-ERα assay. Therefore, the activity in ZI-400 and Nokomis 3-F4 is suggestive of the presence of an NP or NPE as part of the mixture. We know that this is the case with ZI-400. The ER effect seen for these dispersants is weak, which is consistent with only a relatively small amount of NPE being in the total mixture. In order to prove that the weak ER activity is simply due to the presence of NP or NPE, and not to some as yet unidentified component, it would be necessary to test individual ingredients in the dispersants.
Six of the dispersants tested showed no evidence of interaction with ER in multiple in vitro test systems. Integrating over all of the ER data in this manuscript and a recently published EPA report[29] indicates that none of the eight dispersants display biologically significant endocrine activity. However, as mentioned previously, NPEs and NPs can be endocrine disruptors in fish[8], so the risk of using NPE-containing dispersants should be carefully weighed against the expected benefits. One limitation of the present study is that there are other routes by which chemicals can cause endocrine disruption, as well as other types of toxicity that have not been tested for here. Most importantly though, there were no indications of estrogenic activity for Corexit 9500, the dispersant currently being used in the Gulf of Mexico.
In the larger battery of tests run against the dispersants, we saw activity in PXR and PPAR assays for most of the dispersants. These are typical responses of cells to xenobiotics and so are not unexpected. It is worth noting the strong activity of ZI-400 relative to the AhR receptor. For some of the dispersants, other targets were activated, but typically at concentrations approaching cytotoxic levels.
The assays used in the present study are all derived from mammalian species, while the initial concern in the Gulf of Mexico is for toxicity to aquatic species. However, for many targets, including AR and ER, there is significant sequence and structural homology between mammals and fish so that chemicals active in one tend to be active in the other. The rank ordering of overall toxicity (Figure 1) shows a correlation between human in vitro cytotoxicity and fish and shrimp in vivo lethality, giving further evidence of the usefulness of the assays used here.
One concluding observation of general interest is that we were able to detect specific bioactivities in complex chemical mixtures for time-sensitive environmental issues and using high throughput screening assays. This is exciting given that one of the challenges of real world chemical toxicity testing is the fact that humans and other organisms are often exposed to complex mixtures, rather than the pure single compounds that are the subject of typical toxicity testing. The in vitro tests used in this study rapidly profiled the complex dispersant formulations without the use of animals, and screened for potential endocrine activity, other endpoints and cytotoxicity. In different circumstances, a similar rapid screening effort could be used to make time-sensitive decisions based on potential hazard and risk.
Supplementary Material
Acknowledgments
We acknowledge the contributors of data to this manuscript: Natasha Poltoratskaya, Luba Medvedeva, Matt Moeser, Elena Martsen, Ming Zeng, Alex Medvedev and Sergei Makarov (Attagene Inc., RTP, NC; EPA contract EP-W-07-049); and Thomas Dexheimer, Kristin Leister, Andrew Chen, Jennifer Fox, Omid Motabar, and Anton Simeonov (NIH Chemical Genomics Center, Rockville, MD; Tox21 partner with EPA, FDA and NTP). We also acknowledge our EPA/ORD colleagues Stephen Little, Barbara Collins and Lora Johnson for QA leadership; John Southerland for contract management; Mike Hiatt, Tammy Jones-Lepp, John Zimmerman, Andy Grange, Brian Schumacher and Ed Heithmar for analytical QC; and Doris Smith.
Footnotes
Supporting Information: Supporting information includes complete experimental and computational protocols, background on dispersants and reference chemicals, and performance criteria for ER assays.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- 1.API. A Decision-Maker's Guide to Dispersants. May 28 http://www.api.org/ehs/water/spills/index.cfm.
- 2.Eldridge JC, Stevens JT. Endocrine Toxicology. Third. Vol. 27 Informa Healthcare; New York: 2010. [Google Scholar]
- 3.Jonkers N, Knepper TP, de Voogt P. Aerobic biodegradation studies of nonylphenol ethoxylates in river water using liquid chromatography-electrospray tandem mass spectrometry. Environ Sci Technol. 2001;35(2):335–40. doi: 10.1021/es000127o. [DOI] [PubMed] [Google Scholar]
- 4.White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994;135(1):175–82. doi: 10.1210/endo.135.1.8013351. [DOI] [PubMed] [Google Scholar]
- 5.Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166(1-2):79–89. doi: 10.1016/s0300-483x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- 6.Owens W, Koeter HB. The OECD program to validate the rat uterotrophic bioassay: an overview. Environ Health Perspect. 2003;111(12):1527–9. doi: 10.1289/ehp.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folmar LC, Hemmer MJ, Denslow ND, Kroll K, Chen J, Cheek A, Richman H, Meredith H, Grau EG. A comparison of the estrogenic potencies of estradiol, ethynylestradiol, diethylstilbestrol, nonylphenol and methoxychlor in vivo and in vitro. Aquat Toxicol. 2002;60(1-2):101–10. doi: 10.1016/s0166-445x(01)00276-4. [DOI] [PubMed] [Google Scholar]
- 8.Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environmetnal Toxicology and Chemistry. 1996;15(2):194–202. [Google Scholar]
- 9.Martin MT, Dix DJ, Judson RS, Kavlock RJ, Reif DM, Richard AM, Rotroff DM, Romanov S, Medvedev A, Poltoratskaya N, Gambarian M, Moeser M, Makarov SS, Houck KA. Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA's ToxCast program. Chem Res Toxicol. 2010;23(3):578–90. doi: 10.1021/tx900325g. [DOI] [PubMed] [Google Scholar]
- 10.Romanov S, Medvedev A, Gambarian M, Poltoratskaya N, Moeser M, Medvedeva L, Diatchenko L, Makarov S. Homogeneous reporter system enables quantitative functional assessment of multiple transcription factors. Nat Methods. 2008;5(3):253–60. doi: 10.1038/nmeth.1186. [DOI] [PubMed] [Google Scholar]
- 11.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A. 2006;103(31):11473–8. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3(8):466–79. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 13.U.S. EPA. National Contingency Plan Product Schedule. June 24 http://www.epa.gov/emergencies/content/ncp/product_schedule.htm.
- 14.ICCVAM. ICCVAM Evaluation of In Vitro Test Methods for Detecting Potential Endocrine Disruptors: Estrogen Receptor and Androgen Receptor Binding and Transcriptional Activation Assays. April 15 http://iccvam.niehs.nih.gov/docs/endo_docs/EDAddendFinal.pdf.
- 15.U.S. EPA. OCSPP Harmonized Test Guidelines Series 890 - Endocrine Disruptor Screening Program Test Guidelines. June 11 http://www.epa.gov/ocspp/pubs/frs/publications/Test_Guidelines/series890.htm.
- 16.Auld DS, Southall NT, Jadhav A, Johnson RL, Diller DJ, Simeonov A, Austin CP, Inglese J. Characterization of chemical libraries for luciferase inhibitory activity. J Med Chem. 2008;51(8):2372–86. doi: 10.1021/jm701302v. [DOI] [PubMed] [Google Scholar]
- 17.Hemmer MJ, Barron MG, Greene RM. Comparative Toxicity of Eight Oil Dispersant Products on Standard Aquatic Species. June 30; doi: 10.1002/etc.619. http://www.epa.gov/BPSpill/reports/ComparativeToxTest.Final.6.30.10.pdf. [DOI] [PubMed]
- 18.Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact. 2005;155(3):111–28. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Guyton KZ, Chiu WA, Bateson TF, Jinot J, Scott CS, Brown RC, Caldwell JC. A reexamination of the PPAR-a activation mode of action as a basis for assessing human cancer risks of environmental contaminants. Environ Health Perspect. 2009 doi: 10.1289/ehp.0900758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, Roberts RA, Fenner-Crisp PA. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33(6):655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- 21.Lai DY. Rodent carcinogenicity of peroxisome proliferators and issues on human relevance. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2004;22(1):37–55. doi: 10.1081/GNC-120038005. [DOI] [PubMed] [Google Scholar]
- 22.Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The Toxicology of Ligands for Peroxisome Proliferator-Activated Receptors (PPAR) Toxicol Sci. 2006;90(2):269–295. doi: 10.1093/toxsci/kfj062. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi S, Matsuda T, Kobayashi S, Takahashi T, Kojima H. In vitro screening of 200 pesticides for agonistic activity via mouse peroxisome proliferator-activated receptor (PPAR)alpha and PPARgamma and quantitative analysis of in vivo induction pathway. Toxicol Appl Pharmacol. 2006;217(3):235–44. doi: 10.1016/j.taap.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Melnick RL. Is peroxisome proliferation an obligatory precursor step in the carcinogenicity of di(2-ethylhexyl)phthalate (DEHP)? Environ Health Perspect. 2001;109(5):437–42. doi: 10.1289/ehp.01109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters JM. Mechanistic Evaluation of PPARa-Mediated Hepatocarcinogenesis:Are We There Yet? Tox Sci. 2008;101(1):1–3. [Google Scholar]
- 26.Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand? J Mol Med. 2005;83(10):774–85. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 27.Poole TM, Drinkwater NR. Strain dependent effects of sex hormones on hepatocarcinogenesis in mice. Carcinogenesis. 1996;17(2):191–6. doi: 10.1093/carcin/17.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Judson RS, Houck KA, Kavlock RJ, Knudsen KB, Martin MT, Mortensen HM, Reif DM, Richard AM, Rotroff DM, Shah I, Dix DJ. Predictive In Vitro Screening of Environmental Chemicals - The ToxCast Project. Environ Health Perspect. 2010;118(4):485–492. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. EPA. Analysis of Eight Oil Spill Dispersants Using Rapid, In Vitro Tests for Endocrine and Other Biological Activity. U.S. EPA; RTP, NC: Jun 30, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


