Abstract
The neurodegenerative disorder Huntington’s Disease is caused by an expansion in the polyglutamine repeat region of the protein huntingtin. Multiple studies in cellular and animal model systems indicate that this mutation imparts a novel toxic function required for disease pathogenesis. However, huntingtin is an essential cellular protein in higher vertebrates, whose normal function is not yet well-understood. Emerging data suggest an important role for wild-type huntingtin in the intracellular transport of vesicles and organelles. Here, we discuss current progress on the role of huntingtin in vesicular trafficking, as recent work has led to the proposal that huntingtin may be a critical regulator of vesicular transport along the cellular cytoskeleton. We will discuss a model in which huntingtin acts as a scaffold to coordinate the function of molecular motors during vesicular transport. Analysis of the cellular consequences of knockout or knockdown of huntingtin expression suggests that the disease-causing mutation in HD may also induce loss of function effects. This inhibition of normal huntingtin function, in conjunction with the toxic gain-of-function induced by the expansion of the polyglutamine repeat, may affect disease progression. Therefore, further exploration of the mechanisms involved should provide insight into both the regulation of intracellular trafficking and the degeneration of neurons seen in HD.
Huntingtin: an essential multi-domain protein in health and disease
The devastating neurodegenerative disorder Huntington’s Disease (HD) is caused by a pathogenic expansion of the polyglutamine repeat region of the protein Huntingtin (huntingtin). Extensive research has focused on the defects that result from expression of mutant huntingtin (see [1] for a comprehensive recent review). Expansion of the polyglutamine repeat in huntingtin is clearly toxic to a subset of mammalian neurons; the primary cell types affected in HD are neurons in the striatum and cerebral cortex, which control cognitive and movement functions [6]. HD is an autosomal dominant disorder, and experiments in multiple cell and animal model systems support the hypothesis that the expansion of the polyglutamine repeat region in huntingtin causes a toxic gain-of-function, meaning that the mutation imparts an additional, deleterious function for the protein not shared by the wild type form [6]. Pathogenic mechanisms that have been proposed to explain this toxic gain-of-function include protein aggregation, altered gene expression, impaired autophagy and proteasome function, mitochondrial dysfunction leading to accumulation of reactive oxygen species, and increased apoptosis [1]
However, relatively little is known about the cellular function of wild-type huntingtin. Huntingtin is a primarily cytoplasmic protein, known to be both vesicle- and microtubule-associated [3] [4] [5]. Huntingtin is encoded by a single gene and is ubiquitously expressed in mammals. Knockout of the gene encoding Huntingtin in the mouse is lethal early in embryogenesis (~E8.5), prior to organogenesis {Nasir 1995} {Duyao 1995}{Zeitlin 1995}. Expression of huntingtin is also essential for normal development in zebrafish, as knockdown by antisense morpholino oligonucleotides leads to multiple developmental abnormalities including defects in cellular iron uptake (endocytosis) {Lumsden 2007}. Huntingtin is not expressed in lower eukaryotes such as yeast.
Huntingtin is a multi-domain protein which may have multiple distinct cellular roles. Proposed cellular functions include transcriptional regulation, nucleo-cytoplasmic shuttling, synaptic function, and anti-apoptotic activity; the data supporting these possibilities have recently been reviewed in [1] and will not be discussed further here. Instead, we will focus on a possible function for huntingtin in vesicular trafficking and transport in the cell.
Recent developments in our understanding of the cytoplasmic function of huntingtin have led to new insights into the role of this protein as an integrator of vesicular trafficking. Accumulating data on roles for huntingtin in endocytosis, endosomal motility, and axonal transport have led to an emerging model for huntingtin as an integrator of transport along the cellular cytoskeleton. As a growing number of mutations in trafficking proteins are causally linked to neurodegenerative diseases {ChevalierLarsen 2006}, it is reasonable to propose that defects in normal huntingtin function caused by polyglutamine repeat expansion may contribute to disease progression or severity. Improved understanding of this aspect of HD pathogenesis may therefore provide insights useful for the design of targeted therapeutic interventions in future.
Huntingtin builds a network of interactions with motor and motor-associated proteins
Huntingtin interacts with a diverse array of cellular proteins through multiple interaction domains (Fig. 1A; reviewed in [14] [15]). Several of the known binding partners for huntingtin are involved in vesicle trafficking and intracellular transport.
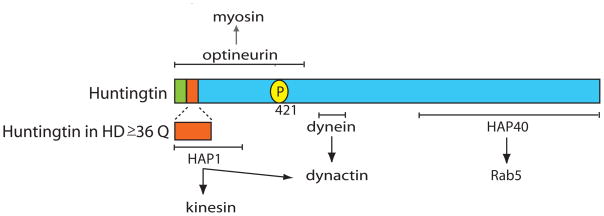
Figure 1.
Schematic of huntingtin with binding sites for molecular motor and motor-associated proteins denoted. The N-terminal ER localization signal (aa1–18){Atwal 2007} is shown in green and the polyglutamine repeat region is shown in red. Normal huntingtin contains 35 or fewer polyglutamines and 36 or more has been associated with HD{Imarisio 2008}. The myosin VI linker protein optineurin is known to associate with the N-terminal region of huntingtin. HAP1 also interacts with the N-terminal region of huntingtin and directly interacts with the plus-end-directed microtubule motor kinesin as well as dynactin. The minus-end-directed microtubule motor dynein interacts with huntingtin (aa600–698) and with the dynein activator dynactin. Huntingtin is phosphorylated on serine 421 (yellow circle). HAP40, an effector of the small GTPase Rab5, binds to the C-terminal region of huntingtin.
Huntingtin-associated protein 1 (HAP1) is a huntingtin binding partner that has been proposed to facilitate interactions with microtubule motor proteins. HAP1 is associated with the plus-end-directed microtubule motor protein kinesin. Kinesin light chain was identified as a HAP1-binding partner in a yeast two hybrid screen; this binding was verified by GST-pull down assays from cellular extracts [16] Co-immunoprecipitation of HAP1 and kinesin from cell and tissue extracts has been reported by multiple labs [16], [19], {Colin 2008}.
An interaction between HAP1and the p150Glued subunit of dynactin was also identified in a yeast two hybrid screen [17] [18] Dynactin is an essential co-factor for the transport of membranous organelles by the minus-end-directed microtubule motor cytoplasmic dynein. The association between HAP1 and dynactin was further substantiated by in vitro binding assays and co-immunoprecipitation [17] [18]. While HAP1 has not yet been shown to bind to either kinesin or dynactin directly, co-immunoprecipitation experiments indicate the formation of an endogenous complex of HAP1, kinesin, and dynactin{Rong 2006}.
Biochemical evidence indicates that there is a direct interaction between huntingtin and cytoplasmic dynein, as demonstrated initially in a yeast two hybrid screen and verified by the direct binding of purified cytoplasmic dynein to a GST-huntingtin fusion construct [19]. Mapping experiments identify a binding site for the dynein intermediate chain to residues 600–698 of huntingtin. Immunoprecipitation experiments with an anti-dynein antibody demonstrate the co-precipitation of a complex that includes cytoplasmic dynein, dynactin, huntingtin, kinesin, and HAP1 [19], further supporting the hypothesis that huntingtin is involved in the bi-directional transport of intracellular vesicles along the microtubule cytoskeleton.
In addition to these interactions with microtubule-based motors, huntingtin is also linked to actin-based motors through optineurin [20] (Fig. 1a), a protein that links myosin VI to Golgi membranes [21]. This observation, as well as those of Pal et al [22] (discussed below) suggest that huntingtin may somehow integrate intracellular trafficking along both the microtubule and actin cytoskeletons.
Analysis of mutant Huntingtin paves the way for studies of wild type huntingtin function
The polyglutamine repeat expansion mutation in the gene encoding huntingtin causes misfolding and cellular accumulation of the protein, including aggregate formation. Typically, efficient clearance of misfolded and aggregated proteins by the cell depends on trafficking of membranous cargo along the cellular cytoskeleton. Accumulation of aggregates may indicate either an inability to degrade the mutant protein, or alternatively may indicate an overall inhibition of the cellular trafficking and degradative machinery. Increasingly, defects in components of this cellular machinery are being implicated as direct causes of neurodegenerative disease [7].
A further indication that a neuronal population that is severely affected in HD, striatal neurons, suffers from defective intracellular trafficking is that the levels of brain-derived-neurotrophic factor (BDNF) are decreased in these cells [8] [9]. BDNF is secreted by cortical neurons and endocytosed by striatal neurons [10]; striatal neurons require BDNF for survival{Baquet 2004} and perturbations in BDNF trafficking may be devastating to these cells. These observations suggest that the polyglutamine expansion in mutant huntingtin may detrimentally affect its own trafficking and degradation, but may also act more broadly to poison trafficking pathways in the cell, including the trafficking of neurotrophic factors such as BDNF.
In fact, studies have demonstrated that mutant huntingtin causes defective axonal trafficking in multiple model organisms, including squid, Drosophila, and mice. To assess the effect of mutant huntingtin on vesicle transport, truncated huntingtin constructs containing the disease-causing expanded polyglutamine repeat region were added to vesicles isolated from squid and resulted in decreased bidirectional motility of vesicles compared with controls [11]. Analysis of a Drosophila model of HD, created by expressing exon 1 of mutant huntingtin containing the expanded polyglutamine repeat region, also revealed defects in axonal transport. The soluble pool of motor proteins was decreased in these flies, leading to the suggestion that the expression of mutant huntingtin caused a depletion of active motors by sequestering dynein/dynactin and kinesin into aggregates [12].
In embryonic striatal neurons isolated from transgenic mice expressing huntingtin with an expanded polyglutamine repeat region, McMurray and colleagues observed inhibition of vesicular transport in both the anterograde (outward from the cell body) and retrograde (toward the cell body) directions. Mitochondrial transport was also significantly slower in neurons from transgenic mice relative to wild type, and was characterized by frequent pauses as well as a reduction in distance traveled in either direction [13]. To elucidate the mechanism responsible for these defects, the group analyzed extracts from human control and HD brain tissue. They demonstrated from gel filtration and fractionation experiments that components of both kinesin and dynein–dynactin associate with mutant huntingtin. Based on their observations, they suggested that poorly soluble protofibrillar complexes of mutant huntingtin found in HD brain may sequester motor proteins, thus depleting the soluble pool available to drive intracellular transport [13].
In order to study the cell-type specificity of the transport deficit caused by expression of mutant huntingtin, Her and Goldstein compared the motility of APP-YFP and BDNF-mCherry fusion proteins in striatal, hippocampal, and cortical neurons cultured from mutant mice expressing huntingtin with expanded polyglutamine repeats. They found cell type-specific differences indicating that intracellular transport in both striatal and hippocampal neurons is more sensitive to expression of mutant huntingtin, while transport in cortical neurons is not significantly affected{Her 2008}. However, cortical neurons were sensitive to loss of huntingtin, and over-expression of wild type huntingtin stimulated transport in all neuronal cell types tested, consistent with the results of Gauthier et al.{Gauthier 2004}.
Together, these observations indicate that expression of expanded polyQ-huntingtin has deleterious effects on intracellular transport. Proposed mechanisms to explain this inhibition include the depletion of soluble motor pools through an association with protofibrillar complexes or intracellular aggregates of mutant huntingtin. Alternatively, inhibition of transport might be indirect, caused for example by altered regulation of motor function, or a potentially by a generalized metabolic defect such as a depletion of cellular energy stores. Or, the observed deficits may arise because the polyglutamine expansion associated with disease causes a disruption in the normal function of huntingtin required for intracellular transport. To answer these questions, studies focused on elucidating the role of wild type huntingtin in trafficking within the cell are required.
Insight into huntingtin function from knockout and knockdown studies
Knockout of the gene encoding huntingtin is embryonic lethal in the mouse{Duyao 1995}{Nasir 1995}{Zeitlin 1995}, whereas mice with a single copy of the mouse Hdh gene are phenotypically relatively normal{Duyao 1995} (DUYAO ET AL, 1995) although one line has been reported to display minor cognitive deficits{Nasir 1995}. Tissue-specific depletion of huntingtin in the forebrain results in progressive neurodegeneration, consistent with a post-developmental role for huntingtin in neurons[DRATAGSIS 2000] In contrast, genetic ablation of HAP1 has shown that expression of this protein is not required during embryogenesis{Chan 2002}. HAP1 knockout mice display early postnatal lethality from depressed feeding behavior due to hypothalamic dysfunction, but further studies have shown that HAP1 is not required in adult neurons{Dragatsis 2004} Thus, while huntingtin plays an essential cellular role, the interacting protein HAP1 is non-essential, potentially through functional redundancy.
Several studies have examined the cellular effects due to a knockout or knockdown of huntingtin expression. A careful study of subcellular organelle structure and function in embryonic stem cells cultured from the homozygous huntingtin knockout mouse showed that these cells had defects in mitochondrial localization, transferrin receptor recycling, and Golgi organization{HilditchMaguire 2000}. These observations support a role for huntingtin in intracellular membrane trafficking. Mitochondrial motility was examined in cells with a more than 50% decrease in huntingtin expression relative to wild-type levels [13]. An increase in pausing, a decrease in velocity, and a decrease in bidirectional motility are associated with decreased huntingtin expression–similar to the defects induced by the expression of mutant huntingtin.. Similar observations were made upon ablation of the huntingtin gene in primary cortical cultures from mouse, as a depletion of huntingtin to less than 25% of normal levels results in decreased transport velocity of the reporter construct APP-YFP{Her 2008}.
Further insights come from a study that dissected the roles played by normal huntingtin and HAP1 in vesicular trafficking [9]. Saudou and coworkers developed an assay to measure the dynamics of vesicle transport, focusing on the motility of post-Golgi secretory vesicles labeled with GFP-BDNF. By co-transfecting neuroblastoma cells with BDNF-GFP and either wild-type or mutant huntingtin, they demonstrated that overexpression of wild-type huntingtin enhances BDNF transport. Furthermore, cells depleted of huntingtin by siRNA exhibited a decrease in vesicle velocity, and an increase in the number of stationary vesicles as well as the number of pauses during vesicle transport. These observations provide further evidence that wild-type huntingtin functions to enhance vesicular transport.
To examine if neuronal BDNF transport was affected by expression of mutant huntingtin, the investigators used neuronal cell lines containing an expanded polyglutamine repeat region inserted into endogenous, full-length huntingtin. They observed a decrease in intracellular BDNF transport, which was rescued by expression of exogenous wild type huntingtin, suggesting that mutant huntingtin may act as a loss of function mutation with respect to BDNF transport. In order to define the mechanism by which huntingtin might enhance transport, the investigators focused on the role of HAP1 because it is known to interact with dynactin [17] [18]. Cells were depleted of HAP1 by siRNA and again BDNF trafficking was inhibited, suggesting that huntingtin and HAP1 may function in the same pathway to mediate transport of BDNF-bearing vesicles.
Together, these results from knockdown and knockout studies indicate that huntingtin participates actively in intracellular vesicular trafficking. HAP1 likely participates in this pathway, but is unlikely to be required, as expression of HAP1 does not appear to be required in adult neurons{Dragatsis 2004}. Observations to date also suggest that expression of mutant huntingtin is sufficient to induce defects in intracellular trafficking, and that these cellular defects may contribute to the neurodegeneration characteristic of HD.
Recently, three strong models have emerged to explain the role of huntingtin in vesicular transport. We will review each of these models and then develop a consensus mechanism that may help to guide further work in this area.
Model 1: Huntingtin phosphorylation is a molecular switch for microtubule-based transport
Two recent studies have proposed that huntingtin is a molecular switch that affects the direction of transport of motile vesicles in neurons [26] [27] (Fig. 2). The motility of post-Golgi secretory vesicles labeled with BDNF-GFP was analyzed in order to examine the effects of huntingtin phosphorylation in primary neuronal cultures. Depletion of endogenous huntingtin results in a bidirectional inhibition of the transport of BDNF-labeled vesicles; transport was restored to normal levels by expression of a huntingtin construct spanning residues 1–480 of wild type huntingtin.
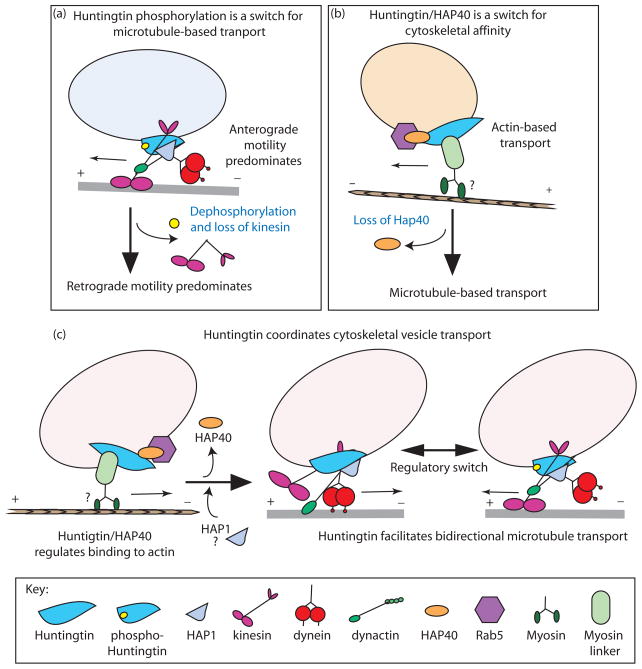
Figure 2.
Three models for how huntingtin, by acting as a scaffold to coordinate the interplay of various molecular motor and motor-associated proteins, facilitates vesicular transport along the cytoskeleton. (a) Model 1: Huntingtin phosphorylation is a molecular switch. When huntingtin is phosphorylated at serine 421, kinesin is recruited to vesicles, and anterograde vesicle motility along the microtubule towards the plus-end predominates. In the unphosphorylated state, kinesin recruitment to the vesicle is no longer stabilized, and dynein dynactin-mediated retrograde motility towards the minus-end predominates. (b) Model 2: The huntingtin HAP40 complex on Rab5-associated endosomes is a switch for cytoskeletal affinity between actin and microtubules. When there is an increase in cytoplasmic levels of HAP40, the early endosome positive for Rab5 is able to associate with actin, possibly through a linker, such as optineurin, that binds to myosin VI. When HAP40 levels are decreased, Rab5-positive early endosomes bind to the microtubule. (c) Model 3: Huntingtin coordinates cytoskeletal vesicle transport. When endocytic cargo traverses the actin cytoskeleton, huntingtin may mediate vesicle attachment to actin via a myosin adaptor protein as in (b). Upon loss of HAP40, and perhaps binding of HAP1, microtubule affinity is enhanced; additional regulatory events, such as phosphorylation of huntingtin as in (a), influence what microtubule motor will predominate, and contribute to the directionality of transport,.
Previous studies have found that activation of the IGF-1/Akt pathway is protective for neurons expressing mutant huntingtin, and proposed that this protective effect was mediated through the phosphorylation of huntingtin on serine 421 {Humbert 2002}. To evaluate the effects of huntingtin phosphorylation on transport, Colin et al utilized expression of a phosphomimetic construct (S421D) and an unphosphorylatable construct (S421A). A significant difference was noted in the parameters of vesicle motility along the microtubule: expression of S421D huntingtin resulted in faster velocity and longer runs in the anterograde direction, and expression of S421A huntingtin favored faster velocities and longer runs in the retrograde direction. Thus, it was proposed that phosphorylation of huntingtin at S421 is a directional switch, leading to a net change in direction from retrograde (minus-end directed) to anterograde (plus-end directed) motility of vesicles along microtubules. The directional change was not limited to BDNF-GFP vesicles, as similar results were observed for the net movement of synaptic vesicles (TI-VAMP-GFP) and also the kinesin-associated cargo APP (APP-YFP){Colin 2008}..
To explain the mechanism behind this molecular switch, Colin et al performed subcellular fractionation studies, which suggest that phosphorylation of huntingtin recruits more kinesin to vesicles. They also examined the association of proteins with phosphorylated huntingtin (p-huntingtin). It is known, for instance, that HAP1 is associated with kinesin light chain [16], so one hypothesis might be that phosphorylation of huntingtin would lead to an enhanced interaction with HAP1, which in turn would lead to enhanced recruitment of kinesin, leading to a change in the directionality of transport.
However, Colin et al show that expression of the S421D fragment of huntingtin does not in fact increase the amount of associated HAP1. Instead, they found that expression of this phosphomimetic construct led to an increased association between kinesin and dynactin, suggesting that p-huntingtin stabilizes an interaction between these proteins.
Future experiments are needed to provide a detailed explanation of the molecular switch observed in these experiments. For example, while kinesin-1 (conventional kinesin) and dynein interact directly [28], and an association between kinesin-2, a distinct member of the kinesin superfamily, and dynactin has also been characterized [29], biochemical studies to date have not provided evidence for a direct interaction between kinesin-1 and dynactin. One hypothesis is that p-huntingtin enhances the association of kinesin and dynein, and this is detected indirectly as an increased recruitment of dynactin in the work by Colin et al. Alternatively, phosphorylation of huntingtin may recruit kinesin to the vesicle through an as yet unidentified effector molecule. Whatever the mechanism, the prediction is that an increased ratio of kinesin to dynein motors on the vesicle will allow kinesin to win a tug-of-war, resulting in the net movement of the vesicle towards the plus end of the microtubule. In the absence of huntingtin phosphorylation, dynein may dominate, resulting in net movement towards the microtubule minus end at the cell center.
In order to assess the role of this regulatory mechanism in the context of the mutant form of huntingtin, Zala and colleagues compared the effects of overexpression of either wild type or mutant huntingtin on vesicular transport [27]. They found that only wild type huntingtin was capable of stimulating transport of BDNF-labeled vesicles; Her and Goldstein report similar results for the transport of APP-associated vesicles {Her 2008}. However, if the mutant huntingtin construct also included the phosphomimetic S421D mutation, then normal bidirectional vesicle transport was restored. Treatment of neurons expressing mutant huntingtin with IGF-1, or co-transfection with constitutively active Akt also rescued transport defects [27], suggesting that mis-regulation of transport may be a critical aspect of the defects induced by expansion of the polyglutamine repeats found in HD.
Model 2: Huntingtin HAP40 is a switch for cytoskeletal affinity
Endosomal membrane fusion and targeting is regulated by Rab GTPases. Rab5 specifically associates with early endosomes and regulates the motility of early endosomes along microtubules [30]. Pal et al have recently shown that huntingtin forms a complex with a Rab5 effector that controls a change in the cytoskeletal affinity of early endosomes [22] (Fig. 1c). The ability of vesicles to switch from actin, most often used for short-range transport, to microtubules, which are utilized for long-distance transport, and vice versa, is a key feature of vesicle transport that is not yet fully understood mechanistically. Using both in vitro and in vivo assays, the authors showed that huntingtin effects a change in endosome association from microtubules to actin through an interaction with an additional huntingtin-binding protein, HAP40.
Using in vitro reconstitution assays, addition of recombinant HAP40 or of an eluate from a Rab5 affinity column that contained both huntingtin and HAP40 decreased endosomal motility along microtubules. Microtubule and actin binding assays revealed that HAP40 or the huntingtin/HAP40 complex decreased binding of endosomes to microtubules while enhancing binding to actin filaments.
In transfected HeLa cells overexpressing HAP40, Rab5-GFP-positive early endosomes showed a decreased rate of mobility compared with endosomes in cells transfected with Rab5-GFP alone. In addition, primary fibroblasts from HD patients had an ~10-fold increase in the level of HAP40 expression, and displayed impaired mobility of Rab5-GFP endosomes in comparison with controls. Immunocytochemistry demonstrated that the HD cells had less Rab5-GFP endosomes associated with microtubules compared with control cells. Instead the Rab5-GFP endosomes were observed in a linear array along actin filaments. These results support a role for huntingtin in modulating the affinity of early endosomes for the cytoskeleton through its interaction with the effector HAP40, and suggest a mechanism by which vesicle trafficking could be perturbed in patients with HD, where the up-regulation of HAP40 may lead to the re-direction of intracellular vesicular traffic from microtubules to actin. Since actin-based transport generally results in shorter, more dispersive motility in the cell, a relative increase in actin-bound vesicles in HD could result in a defect in long-range transport, driven by microtubule-associated motors. Thus a mis-regulation of HAP40 expression could lead to general dysfunction of endosomal trafficking pathways.
While these observations have led to an intriguing model, many questions remain. It remains unclear how the huntingtin–HAP40 complex enhances the affinity of Rab5-positive vesicles for actin filaments. One possible mechanism may involve optineurin, an adaptor protein that links huntingtin to the actin-based motor myosin VI [21]. Could the binding of HAP40 to the huntingtin complex on an early endosome favor recruitment of a myosin adaptor similar to optineurin, resulting in motility along the actin cytoskeleton? Furthermore, dissociation of HAP40 from the huntingtin complex might function as a regulatory switch during endocytosis or post-Golgi trafficking. This in turn would promote a switch from motility along actin to transport along microtubules, promoting a change from short-range to long-range vesicular transport.
Another important question that remains to be answered is how mutant huntingtin, the cause of HD, might cause an up-regulation of HAP40: Pal and colleagues suggest transcriptional abnormalities that arise from expression of huntingtin with expanded glutamine repeats as the most likely answer. An in-depth analysis of gene expression data from HD cells might provide confirmation of this hypothesis, although changes in HAP40 levels were not seen in a recent microarray analysis{Zhang 2008}, which examined changes in gene expression in huntingtin-null mouse embryonic fibroblasts.
Model 3: huntingtin facilitates dynein-mediated vesicle motility
We recently demonstrated that huntingtin binds directly to dynein to enhance dynein-mediated vesicle motility along microtubules [19] (Fig 2c, middle panel). HeLa cells depleted of huntingtin by RNAi exhibited partial disruption of Golgi stacks, a hallmark for defective dynein function. Additionally, interfering with huntingtin function through the addition of function-blocking antibodies to a cell-free assay for vesicular transport caused vesicles isolated from mouse brain to detach from microtubules, while those that remained bound exhibited defective bidirectional transport.
Accumulating evidence suggests that depletion of huntingtin may affect both early endosomes and downstream recycling compartments. A recent study in zebrafish demonstrated that knockdown of huntingtin resulted in defective iron utilization. Embryos were able to endocytose iron, but subsequent defects in iron trafficking led to decreased hemoglobin production [32]. An earlier study of cultured embryonic cells from the huntingtin-knockout mouse demonstrated a defect in transferrin recycling [25], again suggesting that huntingtin may play a role in the trafficking of the recycling endosome. A recent finding that huntingtin is a Rab11-associated protein that affects Rab11 GDP–GTP exchange factor (GEF) activity [33] supports a role for huntingtin in regulating recycling compartments, but more work is clearly required.
There is ample evidence that the role of huntingtin in facilitating membrane transport is not limited to the transport of a specific organelle, such as the endosome. For example, huntingtin phosphorylation effected a change in direction of both post-Golgi secretory vesicles and synaptic vesicles [26]. Also, Golgi organization was perturbed when huntingtin was depleted from HeLa cells by RNAi, suggesting a role for huntingtin in dynein-dependent Golgi membrane transport [19]. Furthermore, as mentioned above, huntingtin binds to optineurin, a linker for myosin VI on Golgi vesicles [21], which supports a role for huntingtin in Golgi membrane trafficking. Along this line, a recent study has shown that expression of mutant huntingtin results in an impairment of optineurin-dependent post-Golgi trafficking to lysosomes, and a resulting deficit in lysosomal activity {DelToro 2009}. Together, these observations suggest that huntingtin is involved in the transport of multiple types of membranous cargo throughout the cell.
A global coordinator of cytoskeletal vesicular transport
We propose that huntingtin facilitates global vesicular trafficking throughout the cell’s multiple cytoskeletal superhighways. Huntingtin is able to coordinate the binding of multiple types of motor proteins to vesicular cargo most likely by acting as a scaffold that can differentially bind to many proteins. By integrating various signals, huntingtin may play a key role in modulating vesicle transport between the actin and microtubule cytoskeletons (Fig. 2c).
Following internalization from the plasma membrane, endocytic vesicles initially traverse the actin-rich cell cortex. Huntingtin/HAP40 may regulate vesicle association with actin [22]. Through associations with a myosin VI linker protein, such as optineurin [21], huntingtin may play a role in actin-based transport of endocytic vesicles early in endocytosis. Myosin VI is the only myosin identified as of yet to move towards the minus-end of the actin filament, and it has been shown to play a role in trafficking of clathrin-coated vesicles [34].
After undergoing short-range transport along the actin cytoskeleton, endosomes are delivered to the microtubules for long-distance transport. Huntingtin may mediate this cytoskeletal switching through a dissociation of HAP40, perhaps followed by a concomitant increase in HAP1 binding, which would favor microtubule-association over actin-association. Huntingtin then facilitates dynein-mediated vesicular transport along microtubules through direct binding to dynein and indirect binding to dynactin via HAP1, possibly leading to the formation of a quarternary complex that enhances interactions of various effectors [19].
Continuing with the example of endosomal cargo, once an internalized receptor that is fated to be recycled back to the plasma membrane has been sorted to a recycling compartment, it requires a change in microtubule motors to effect a switch in the direction of vesicle motility. Huntingtin could serve as this switch through differential binding to downstream effectors. Huntingtin-associated vesicles might also be able to switch direction if increased levels of HAP1 cause an enhanced recruitment of kinesin through the HAP1–kinesin interaction [16]. Alternatively, phosphorylation of huntingtin may cause a recruitment of kinesin to vesicles and a switch in the preferred direction of vesicular motility [26].
In summary, there is a growing body of evidence that huntingtin serves as a facilitator of vesicle motility along the cellular cytoskeleton. Either depletion of endogenous huntingtin or expression of mutant huntingtin has been shown by multiple labs to affect numerous aspects of intracellular trafficking and transport. By integrating the interplay of various binding partners as well as regulatory pathways such as phosphorylation, huntingtin can coordinate vesicle binding to the appropriate molecular motor and enhance motility.
Several aspects of huntingtin function remain less clear. For instance, exactly how is huntingtin associated with vesicular membranes? Do other post-translational modifications of huntingtin, such as palmitoylation by huntingtin-interacting protein HIP14 [35] affect its ability to enhance vesicle transport? Do these modifications vary depending on the type of membranous cargo, cell type and stage of development? Huntingtin is most likely regulated spatially and temporally, and the exact mechanism by which huntingtin enhances vesicle transport will probably vary depending on these parameters. Further, as depletion of huntingtin impairs but does not completely block intracellular trafficking, compensatory mechanisms or parallel pathways may exist and must be explored.
Elucidation of the role of huntingtin in vesicular transport will potentially shape the future studies of the molecular mechanism underlying HD. Does expansion of the polyglutamine repeat region of huntingtin lead to the inhibition of normal huntingtin function, thus causing or contributing to neurodegeneration? Can altering the phosphorylation state of huntingtin or the expression levels of HAP40 rescue neurodegeneration? Certainly, characterizing the normal function of huntingtin adds to our understanding of HD pathology and will ultimately lead to more targeted therapeutic approaches.
Box 1. The Pathogenesis of Huntington’s Disease.
Huntington’s Disease (HD) is one of a group of 16 or so disorders that are caused by the expansion of CAG repeats within otherwise unrelated genes. Other trinucleotide repeat disorders affecting the nervous system include Ataxias (SCA1–3, 6, 7), Spinal Bulbar Muscular Atrophy (SBMA), and Fragile X Syndrome (FRAXA and FRAXE) (Orr and Zoghbi, 2007). HD is an autosomal dominant disease, meaning that a single copy of the mutant gene with an expanded CAG repeat region within exon 1 is sufficient to induce the disorder. Normal alleles of the gene encoding huntingtin include up to 35 tandem CAG codons, encoding glutamine residues (Fig. 1); expansion of this repeat region to encode 36 to 50 or more glutamine residues is causative for HD. The number of repeats is the key determining factor in age of onset (Wexler et al. 2004). Longer repeats are genetically unstable, and families with HD show anticipation, where disease severity increases and age of onset decreases over the generations.
HD is typically an adult-onset neurodegenerative disease, leading to striking loss of striatal medium spiny neurons. However, additional changes are also apparent in the diseased brain including pronounced cortical degeneration (Rosas et al. 2008) and hypothalamic degeneration (Kassubek et al 2004) and so the dogma that Huntington’s disease is a disease of the striatum is being challenged. Clinically, the disease is characterized by complex and variable symptoms that include chorea (spasmodic, irregular muscle contractions), dystonia (involuntary muscle contractions leading to twisting and repetitive movements and abnormal posture), as well as psychiatric (depression, apathy and irritability) and cognitive deficits. The juvenile form (caused by CAG expansions generally 70 and above) of the disease typically presents with Parkinsonian rigidity and may include eplipelptic seizures, tremor and myoclonus.
The dominant inheritance pattern seen in HD, along with numerous studies in animal and cellular model systems support the hypothesis that expression of mutant huntingtin with expanded polyglutamine repeats is toxic to the cell. There is currently little consensus as to the mechanism of toxicity, which may in fact involve multiple pathogenic pathways (Orr and Zoghbi, 2007). There is evidence for the generation of aberrant cleavage fragments, along with aberrant nuclear accumulation of the cleaved fragments. There is also evidence from multiple studies that expression of mutant huntingtin alters transcription (reviewed in Orr and Zoghbi, 2007) Additional possibilities to explain the neurodegeneration that occurs in HD include mitochondrial dysfunction, altered calcium signaling, and excitotoxicity; other mechanisms have also been proposed. Finally, there is accumulating evidence that expression of mutant huntingtin leads to altered intracellular trafficking and transport that is likely to contribute significantly to disease pathogenesis.
Box 2. Outstanding Questions.
What are the molecular cues that specify Huntingtin’s association with membranes derived from multiple subcellular organelles compartment?
Is Huntingtin capable of directly recruiting microtubule and actin motors in order to coordinate motility along the cytoskeleton?
Phosphorylation of Huntingtin on serine 421 has been carefully analyzed. To what end do other phosphorylation events, and other post-translational modifications, contribute to the regulation of Huntingtin in facilitating vesicle transport along the cytoskeleton?
Is there functional redundancy that mitigates the effect of the loss of HAP1 and is responsible for the mild phenotype of the knockout mouse?
Does the mutant form of Huntingin have dysfunctional scaffold and/or transport activities that result in defective vesicle trafficking? Does defective vesicle trafficking contribute to the pathology of HD?
References
- 1.Imarisio S, et al. Huntington’s disease: from pathology and genetics to potential therapies. Biochem J. 2008;412:191–209. doi: 10.1042/BJ20071619. [DOI] [PubMed] [Google Scholar]
- 2.Truant R, et al. Nucleocytoplasmic trafficking and transcription effects of huntingtin in Huntington’s disease. Prog Neurobiol. 2007;83:211–227. doi: 10.1016/j.pneurobio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 3.DiFiglia M, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 4.Gutekunst CA, et al. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc Natl Acad Sci U S A. 1995;92:8710–8714. doi: 10.1073/pnas.92.19.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffner G, et al. Perinuclear localization of huntingtin as a consequence of its binding to microtubules through an interaction with beta-tubulin: relevance to Huntington’s disease. J Cell Sci. 2002;115:941–948. doi: 10.1242/jcs.115.5.941. [DOI] [PubMed] [Google Scholar]
- 6.Gil JM, Rego AC. Mechanisms of neurodegeneration in Huntington’s disease. Eur J Neurosci. 2008;27:2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 7.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Ferrer I, et al. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–261. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- 9.Gauthier LR, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Altar CA, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 11.Szebenyi G, et al. Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron. 2003;40:41–52. doi: 10.1016/s0896-6273(03)00569-5. [DOI] [PubMed] [Google Scholar]
- 12.Gunawardena S, et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 2003;40:25–40. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- 13.Trushina E, et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–8209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harjes P, Wanker EE. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem Sci. 2003;28:425–433. doi: 10.1016/S0968-0004(03)00168-3. [DOI] [PubMed] [Google Scholar]
- 15.Li SH, Li XJ. Huntingtin-protein interactions and the pathogenesis of Huntington’s disease. Trends Genet. 2004;20:146–154. doi: 10.1016/j.tig.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 16.McGuire JR, et al. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 17.Engelender S, et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet. 1997;6:2205–2212. doi: 10.1093/hmg/6.13.2205. [DOI] [PubMed] [Google Scholar]
- 18.Li SH, et al. Interaction of huntingtin-associated protein with dynactin P150Glued. J Neurosci. 1998;18:1261–1269. doi: 10.1523/JNEUROSCI.18-04-01261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caviston JP, et al. Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc Natl Acad Sci U S A. 2007;104:10045–10050. doi: 10.1073/pnas.0610628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faber PW, et al. Huntingtin interacts with a family of WW domain proteins. Hum Mol Genet. 1998;7:1463–1474. doi: 10.1093/hmg/7.9.1463. [DOI] [PubMed] [Google Scholar]
- 21.Sahlender DA, et al. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J Cell Biol. 2005;169:285–295. doi: 10.1083/jcb.200501162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal A, et al. Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington’s disease. J Cell Biol. 2006;172:605–618. doi: 10.1083/jcb.200509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner A, et al. Wild-type huntingtin plays a role in brain development and neuronal survival. Mol Neurobiol. 2003;28:259–276. doi: 10.1385/MN:28:3:259. [DOI] [PubMed] [Google Scholar]
- 24.Cattaneo E, et al. Normal huntingtin function: an alternative approach to Huntington’s disease. Nat Rev Neurosci. 2005;6:919–930. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- 25.Hilditch-Maguire P, et al. Huntingtin: an iron-regulated protein essential for normal nuclear and perinuclear organelles. Hum Mol Genet. 2000;9:2789–2797. doi: 10.1093/hmg/9.19.2789. [DOI] [PubMed] [Google Scholar]
- 26.Colin E, et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zala D, et al. Phosphorylation of mutant huntingtin at S421 restores anterograde and retrograde transport in neurons. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn281. [DOI] [PubMed] [Google Scholar]
- 28.Ligon LA, et al. A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J Biol Chem. 2004;279:19201–19208. doi: 10.1074/jbc.M313472200. [DOI] [PubMed] [Google Scholar]
- 29.Deacon SW, et al. Dynactin is required for bidirectional organelle transport. J Cell Biol. 2003;160:297–301. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen E, et al. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- 31.Driskell OJ, et al. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol. 2007;9:113–120. doi: 10.1038/ncb1525. [DOI] [PubMed] [Google Scholar]
- 32.Lumsden AL, et al. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet. 2007;16:1905–1920. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- 33.Li X, et al. A function of huntingtin in guanine nucleotide exchange on Rab11. Neuroreport. 2008;19:1643–1647. doi: 10.1097/WNR.0b013e328315cd4c. [DOI] [PubMed] [Google Scholar]
- 34.Soldati T, Schliwa M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol. 2006;7:897–908. doi: 10.1038/nrm2060. [DOI] [PubMed] [Google Scholar]
- 35.Yanai A, et al. Palmitoylation of huntingtin by HIP14 is essential for its trafficking and function. Nat Neurosci. 2006;9:824–831. doi: 10.1038/nn1702. [DOI] [PMC free article] [PubMed] [Google Scholar]