Abstract
Nuclear envelope breakdown (NEBD) is an essential step during the G2/M transition in higher eukaryotic cells. Increasing evidence supports the notion that both microtubules and microtubule-associated motor proteins are critical regulators of NEBD. Although it has been described that p150Glued, the major component of the dynein/dynactin complex, localizes in the nuclear envelope during prophase, the exact role of p150Glued and its regulation during NEBD are largely elusive. Polo-like kinase 1 (Plk1), the best characterized Ser/Thr kinase, is involved in mitotic entry in several systems; however, the targets of Plk1 during NEBD are unknown. Herein, we show that in mammalian cells both Plk1 and p150Glued regulate NEBD and that Plk1 interacts with and phosphoryates p150Glued during NEBD at prophase. Using various approaches, we showed that Plk1 phosphorylates p150Glued at Ser-179 and that the pS179 epitope is generated at the nuclear envelope of prophase cells. Significantly, Plk1-mediated phosphorylation of p150Glued at Ser-179 positively regulates its accumulation at the nuclear envelope during prophase. Finally, we found that cells expressing the Plk1-unphosphorylatable mutant (p150Glued-S179A) arrest at G2, as indicated by reduced NEBD, increased levels of cyclin B and phospho-H3, but a decreased level of Cdc2 kinase activity. Taking these data together, we conclude that Plk1 phosphorylation of p150Glued might be one major pathway of NEBD regulation.
Keywords: mitosis, microtubule dynamics, dynactin
Dynactin, a multisubunit protein complex, can be detected on a variety of endomembranes, such as the nuclear envelope (NE) at prophase (1). Because of its ability to bind dynein directly and enhancement of dynein processivity, p150Glued, the largest subunit of dynactin, plays a critical role in the function of the dynein/dynactin complex (2–4). During the G2/M transition, nuclear membranes that surround chromosomes in interphase are replaced by cytoplasmic spindle microtubules. By the end of prophase, the major NE structural components become distributed throughout the entire cell (5, 6). Although the mechanism of NE breakdown (NEBD) is largely elusive, the function of microtubules involved in the process has been suggested. Before NEBD, early spindle microtubules connect to the NE and cause invaginations in the nuclear lamina, followed by generation of pocket-like distortions of the NE (7). One of the minus-end-directed motor proteins, dynein, facilitates NEBD by pulling nuclear membranes poleward along astral microtubules leading to nuclear membrane detachment (1). Although a requirement for Polo-like kinase 1 (Plk1) in mitotic entry has been suggested, the detailed mechanism is still unknown. Here, we demonstrated that Plk1 phosphorylation of p150Glued at Ser-179 facilitates NEBD at prophase in cultured cells.
Results
p150Glued Interacts with Plk1 in Vivo.
In a search for Plk1-interacting proteins using a yeast two-hybrid system, we have identified p150Glued as a potential Plk1 target (Fig. S1 A and B). To determine whether such an interaction is of physiological significance, we further analyzed the association between Plk1 and p150Glued in cells. Extracts from asynchronous or mitotic HeLa cells were prepared and subjected to immunoprecipitation (IP) with p150Glued antibodies, followed by Western blotting. A complex was clearly detected between endogenous Plk1 and p150Glued in mitotic cells, but not in interphase cells (Fig. S1C), confirming that p150Glued is a Plk1 interacting-partner in mammalian cells. Moreover, we detected that p150Glued interacts with Plk1 through its C-terminal polo-box domain, which was proposed as the motif for association with its substrates (Fig. S1D).
Plk1 Phosphorylates p150Glued both in Vitro and in Vivo.
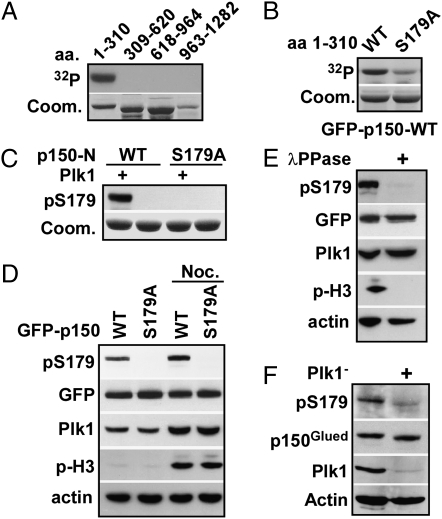
To determine whether p150Glued is a substrate of Plk1, four purified p150Glued regions were subjected to Plk1 kinase assays. As indicated in Fig. 1A, the N-terminal fragment of p150Glued containing amino acids 1 to 310 was phosphorylated by Plk1 in vitro. To map the phosphorylation sites, we generated a series of serine/threonine to alanine mutations within amino acids 1 to 310 of p150Glued. All purified mutant proteins were subjected to Plk1 kinase assays. Compared with the phosphorylation level of p150Glued-WT, the phosphorylation level of p150Glued-S179A by Plk1 was significantly reduced, indicating that Ser-179 is the major phosphorylation site for Plk1 (Fig. 1B). Next, metabolic labeling followed by phosphopeptide mapping was used to examine p150Glued phosphorylation in vivo (Fig. S1E). It is clear that p150Glued was phosphorylated at multiple sites and that introduction of the S179A mutation led to complete disappearance of one phosphopeptide, indicating that Ser-179 is one of multiple sites phosphorylated in vivo. Significantly, Plk1 depletion significantly reduced the intensity of this peptide, suggesting that Plk1 is the kinase responsible for Ser-179 phosphorylation. Moreover, Plk1 depletion also affected the phosphorylation levels of other peptides, indicating that Plk1 might also target other sites in vivo (Fig. S1C). Plk1 prefers a target sequence containing negatively charged residues nearby, particularly at the -2 position (8). The amino acid sequence around Ser-179 in p150Glued (ELS179S) fits the Plk1 target consensus sequence (Fig. S1F). Finally, alignment analysis showed that the sequence around Ser-179 is highly conserved across species, indicating that phosphorylation of Ser-179 might occur in most eukaryote organisms.
Fig. 1.
The p150Glued is a Plk1 substrate in vitro and in vivo. (A) GST-Plk1 was incubated with four purified GST-p150Glued fragments (amino acids 1–310, 309–620, 618–964, 963–1282) in the presence of [γ-32P]ATP. The reaction mixtures were resolved by SDS/PAGE, stained with Coomassie Brilliant Blue (Coom.), and detected by autoradiography. (B) Plk1 was incubated with indicated forms of GST-p150Glued-N (amino acids 1–310) as in A. (C) Purified Plk1 was incubated with purified GST- p150Glued-N (WT or S179A) in the presence of unlabeled ATP, followed by an anti-pS179 Western blot. (D) HEK293T cells were transfected with GFP-p150Glued constructs (WT or S179A), treated with or without nocodazole, and immunoblotted with antibodies, indicated on the left. (E) Mitotic lysates from 293T cells expressing GFP-p150Glued were treated with λ-phosphatase, followed by Western blotting. (F) HeLa cells were cotransfected with pBS/U6-Plk1 and pBabe-puro at a ratio of 7:1. At 1 d posttransfection, cells were selected with puromycin for additional 36 h. After floating cells were washed away, attached cells were treated with nocodazole for 12 h and immunoblotted.
To further confirm the kinase/substrate relationship as described above, a phospho-specific antibody against Ser-179 of p150Glued was generated by immunization of rabbits with a peptide containing phospho-S179 and subsequent affinity purification. We first characterized the specificity of the antibody using in vitro kinase assays in the presence of unlabeled ATP, followed by Western blotting. As indicated in Fig. 1C, an anti-pS179 signal was detected only in the sample that was preincubated with Plk1, indicating that the antibody does not recognize unphosphorylated p150Glued. Furthermore, the S179A mutation completely abolished the anti-pS179 Western signal, indicating that the antibody recognizes only p150Glued phosphorylated at Ser-179 (Fig. 1C). Next, the pS179 signal was detected in cells expressing p150Glued-WT, but not -S179A, confirming that Ser-179 is phosphorylated in vivo (Fig. 1D). Consistent with Plk1 activity peaking in mitosis, nocodazole treatment increased the phosphorylation signal of Ser-179, arguing that Ser-179 phosphorylation is cell-cycle regulated. Treatment of lysates from cells expressing GFP-p150Glued with λ-phosphatase led to loss of the pS179 epitope, further confirming that Ser-179 is phosphorylated in vivo (Fig. 1E). Finally, Plk1 depletion strongly inhibited generation of the pS179 epitope of endogenous p150Glued protein, supporting the idea that endogenous Plk1 is responsible for p150Glued-S179 phosphorylation (Fig. 1F).
For some Plk1 substrates, Cdk1-associated phosphorylation has been shown to generate a docking site to recruit Plk1 toward these substrates. We thus examined whether the p150Glued-Plk1 interaction is regulated by a similar mechanism. By in vitro Cdk1/cyclin B kinase assay, we demonstrated that Cdk1 phosphorylates p150Glued-aa1-310 (Fig. S1G). Furthermore, we mapped Ser-212 as the major phosphorylation site by Cdk1 in vitro (Fig. S1H). Next, we tested whether the phosphorylation state of Cdk1 site would affect Plk1 binding to p150Glued in vivo. For that purpose, HEK293T cells were transfected with GFP- p150Glued-WT and p150Glued-S212A constructs and then treated with nocodazole. Whole-cell extracts were prepared, subjected to anti-GFP IP, and analyzed by anti-Plk1 Western blot. Introduction of the S212A mutation does not affect the binding affinity between Plk1 and p150Glued (Fig. S1I), indicating the association between these two partners was not Cdk1-phosphorylation dependent. To support this conclusion, typical sequential kinase assay was performed. In agreement, sequential exposure of p150Glued to Cdk1 and Plk1 did not result in a significant increasing of phosphorylation level of p150Glued (Fig. S1J). Although Cdk1 serves as a priming kinase for Plk1 in many substrates, Plk1 can also function as a self-priming kinase. However, this is unlikely the case for p150Glued, as the sequence containing S179 does not match SpSP phosphomotif, recognized by the Polo-box domain. Thus, the priming kinase to recruit Plk1 toward p150Glued needs further experimentation.
Temporal and Spatial Regulation of p150Glued-S179 Phosphorylation.
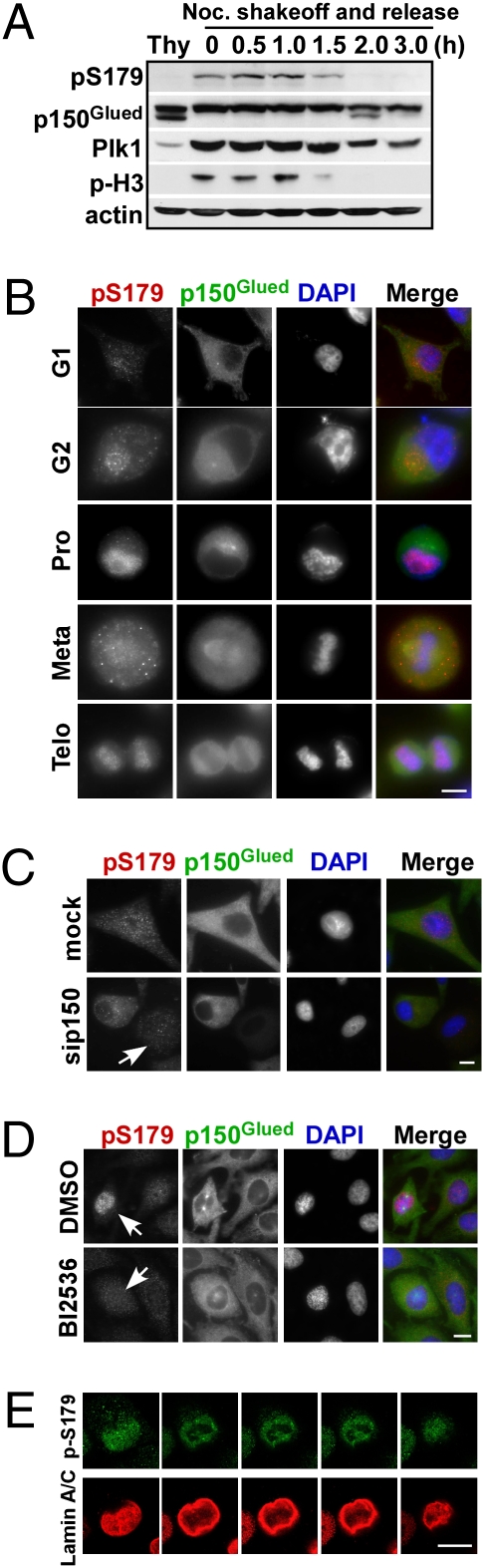
Whether p150Glued-S179 phosphorylation is regulated throughout the cell cycle was further examined in well-synchronized cultures. As expected, phosphorylation of p150Glued-S179 was detected in mitotic cells, but not in interphase cells. Moreover, the pS179 epitope was quickly lost as cells exited from mitosis, matching the Plk1 expression pattern (Fig. 2A). We next examined the subcellular localization of phospho-p150Glued during cell cycle progression by immunofluorescence (IF) staining. Consistent with the Western blotting results, the pS179 epitope is fairly low during interphase and starts to be detected surrounding centrosomes in late G2 phase. Interestingly, phospho-p150Glued localized at the NE in prophase. During metaphase and anaphase, the signal was completely diffused throughout the cell, most likely because of NEBD. Furthermore, the pS179 epitope reappeared as the NE regenerated at telophase (Fig. 2B). To confirm NE localization of the pS179 epitope, HeLa cells were costained with antibodies against pS179 and lamin A/C, a marker for the NE. As shown in Fig. S2A, phosphorylated p150Glued colocalized with lamin A/C in prophase when NEBD had not yet occurred and again in telophase when the NEs reformed. Fig. S2B is a representative image of two cells (one in interphase and one in mitosis) within one field. Again, whereas only a very weak phosphorylation signal was observed in the interphase cell, the pS179 epitope was significantly increased in the mitotic cell, confirming that phosphorylation of Ser-179 occurs at mitosis. To ensure the specificity of the subcellular localization of the pS179 epitope described above, p150Glued-depleted cells were costained with antibodies against pS179 and p150Glued. Upon p150Glued depletion (the cell indicated by the arrow in Fig. 2C), the pS179 epitope also disappeared, whereas it was still clearly visible in a nearby nondepleted cell within the same field, validating IF staining with pS179 antibodies. Moreover, incubation of cells with BI2536, a Plk1 inhibitor (9), also completely abolished the pS179 epitope, confirming that Plk1 is responsible for generation of the pS179 epitope in vivo (Fig. 2D). Finally, a series of confocal Z-scans was collected from a prophase cell costained with p-S179 and lamin A/C antibodies. The data further confirmed the concept that the pS179 epitope is enriched at the NE (Fig. 2E).
Fig. 2.
Temporal and spatial regulation of the pS179-p150Glued epitope in HeLa cells. (A) Cells were treated with nocodazole for 16 h (lanes 2–7), collected by mitotic shake-off, released for different times, and immunoblotted. In lane 1, cells were treated with thymidine for 24 h to block at G1. (B) Cells at indicated phases of cell cycle were costained with antibodies against pS179 and p150Glued. DNA was stained with DAPI. (C and D) Cells were transfected with siRNA to deplete p150Glued (C) or treated with BI2536 to inhibit Plk1 activity (D), and costained with antibodies against p150Glued and pS179. Mitotic cells are indicated by the arrows. (E) A series of Z-scans of a prophase cell stained with p-S179 and lamin A/C antibodies. (Scale bars, 5 μm.)
Both Plk1 and p150Glued Are Involved in NEBD During Prophase.
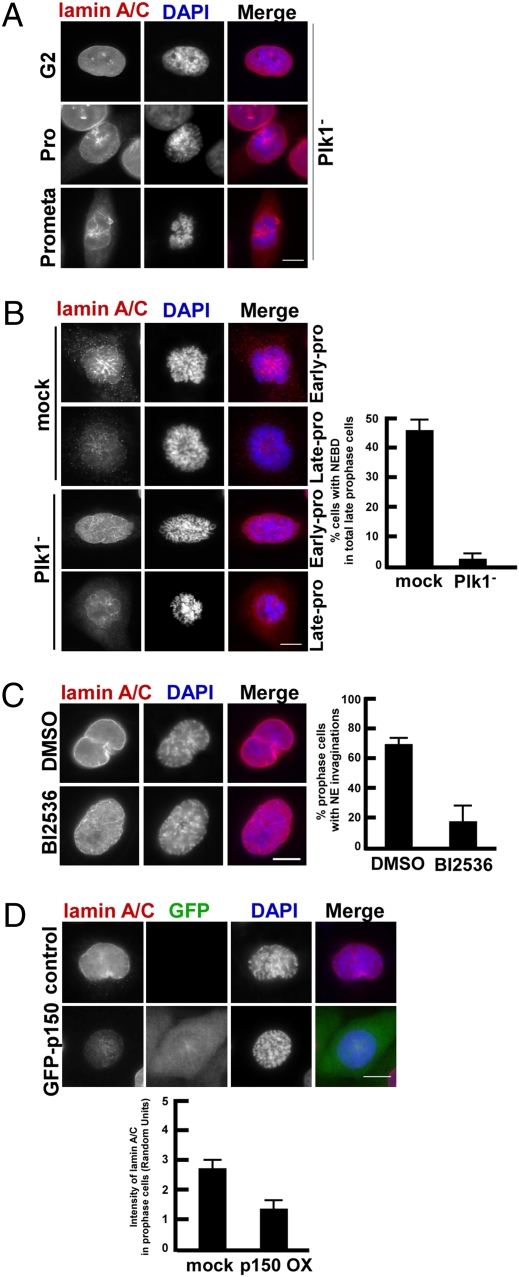
Requirement of Plk1 for NEBD has been previously described in Caenorhabditis elegans (10); however, whether this important function of Plk1 is conserved in mammalian cells is unknown. To monitor the NEBD process in detail, HeLa cells were stained with lamin A/C antibodies and examined at various cell-cycle stages. As shown in Fig. S3A, a strong lamin A/C signal was detected at the NE during interphase, whereas the signal intensity was significantly reduced in prophase cells. As the cells entered prometaphase, the lamin A/C signal was completely diffused throughout the entire cells, suggesting that NEBD is completed before prometaphase. To explore the role of Plk1 in NEBD in mammalian systems, siRNA was used to deplete Plk1 in HeLa cells (Fig. S3B). In striking contrast to control cells, no dramatic reduction of the lamin A/C signal at the NE was detected as the Plk1-depleted cells entered prophase. Moreover, lamin A/C signals surrounding chromosomes were still clearly detected in prometaphase after Plk1 depletion (Fig. 3A). Such images were rarely seen in control cells. These observations suggest that Plk1 depletion delays NEBD in mammalian cells.
Fig. 3.
Both Plk1 and p150Glued are involved in NEBD during prophase in HeLa cells. (A and B) Cells were synchronized with a double thymidine block (DTB, thymidine block for 16 h, release for 8 h, and a second thymidine block for 16 h) at the G1/S boundary with Plk1 being depleted during the 8-h interval. Upon release for 11 h, the cells were stained with lamin A/C antibodies. (A) Representative images of Plk1-depleted cells in G2, prophase, and prometaphase. (B) Plk1 depletion retards NEBD in prophase. (C) Plk1 inhibition prevents the formation of NE invaginations in prophase cells. Cells were synchronized with the DTB, released for 11 h in the presence of BI2536, and stained as in A. Prophase cells with clear NE invaginations were quantified. (D) Overexpression of p150Glued led to a premature NEBD. Cells expressing GFP-p150Glued were processed and stained as in A. (Scale bars, 5 μm.)
As described above, NEBD starts from prophase and is completed before prometaphase. Accordingly, cells with chromatin morphology characteristic of late prophase were scored for NEBD, defined as the presence of visible gaps in lamin A/C labeling at the NE. Lamin A/C staining in control cells at this stage clearly exhibited two patterns. In 50% of the cells, a complete disappearance of lamin A/C staining was detected, whereas a few weak remaining signals surrounding chromosomes were observed in the other 50% (Fig. 3B, Left), confirming that this stage is the boundary for completion of NEBD. We thus analyzed NEBD in both control and Plk1-depleted cells at this specific stage. As indicated in Fig. 3B (Right), about 45% of control cells exhibited complete NEBD, whereas the percentage in Plk1-depleted cells was significantly lower at 3%, indicating that Plk1 is required for completion of NEBD at late prophase. Shortly after the start of prophase, the NE develops invaginations as it folds inwards (1). This is the case in about 70% of control cells at prophase. In striking contrast, BI2536-mediated Plk1 inhibition resulted in the formation of net-like structures at the NE and only 18% of BI2536-treated cells showed NE invaginations (Fig. 3C). We propose that defects in the generation of NE invaginations might be a mechanism to cause NEBD delay in Plk1-depleted cells.
It has been described that overexpression of p50/dynamitin, a second dynactin subunit, eliminated NE invaginations of prophase cells (1). This result suggests that p150Glued might be also involved in NEBD, as overexpression of p50/dynamitin is a standard approach to inhibit the function of p150Glued in a dominant-negative fashion (11). Unfortunately, we could not directly analyze the effect of depletion of p150Glued on NEBD, as p150Glued-depleted cells failed to enter mitosis (Fig. S3 C and D). Nevertheless, we did show that overexpression of p150Glued facilitates the removal of lamin A/C from the NE, supporting the idea that p150Glued is a positive regulator of NEBD in mammalian cells (Fig. 3D).
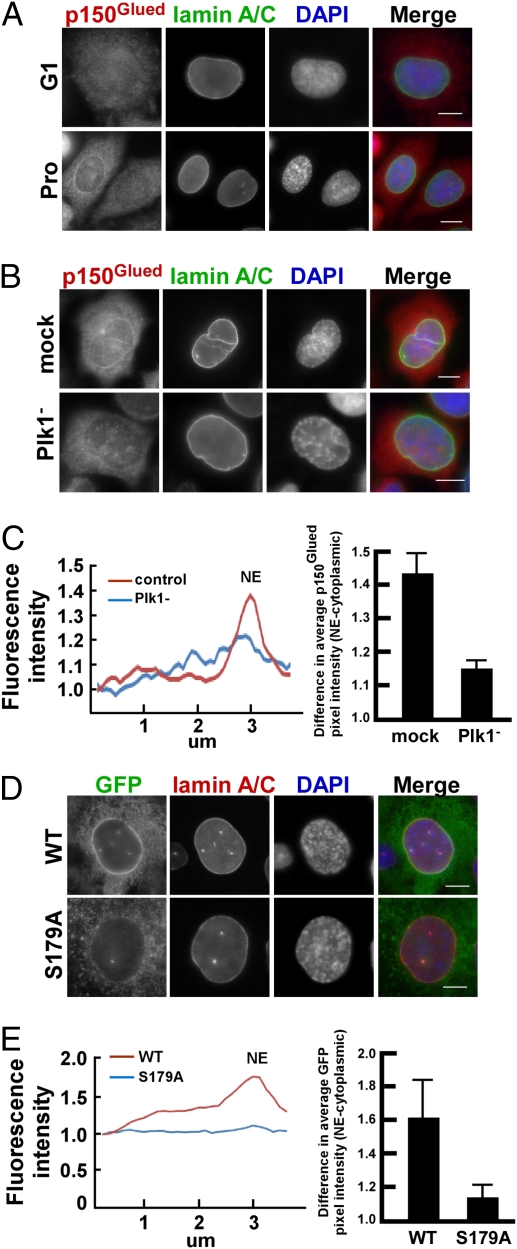
Accumulation of p150Glued at the NE in Prophase Is Positively Regulated by Plk1-Mediated Phosphorylation.
It has been reported that p150Glued colocalized with Lis1 at the NE in nocodazole-treated cells (12). Here, we further show that p150Glued accumulated at the NE in prophase cells under normal growth conditions (Fig. 4A). Considering that Plk1 phosphorylates p150Glued at Ser-179 and the pS179 epitope was detected at the NE during prophase, we next examined whether Plk1 phosphorylation of p150Glued regulates its NE accumulation at prophase. HeLa cells were depleted of Plk1, synchronized with thymidine, released into mitosis, and stained with p150Glued antibodies. As indicated in Fig. 4B, Plk1 depletion prevented the accumulation of p150Glued at the NE. Comparison of mean pixel intensities along lines across the NE further confirmed this observation, as did comparison of pixel intensities around the NE and in the proximal cytoplasm (Fig. 4C).
Fig. 4.
Plk1-mediated phosphorylation of p150Glued is essential for its NE accumulation in prophase. (A) p150Glued localizes to the NE in prophase, but not in interphase. Randomly growing U2OS cells were costained with antibodies against p150Glued and lamin A/C. (B and C) Accumulation of p150Glued at the NE in prophase is Plk1-dependent. (B) U2OS cells growing on coverslips were transfected with siRNA to deplete Plk1, blocked with thymidine for 20 h, released for 11 h, and immunostained with indicated antibodies. (C) Quantification of the results of NE accumulation of p150Glued. (D and E) Localization of p150Glued at the NE was reduced by the S179A mutation. (D) U2OS cells stably expressing RNAi-resistant GFP-p150Glued (WT or S179A) were transfected with siRNA to deplete endogenous p150Glued, synchronized, and processed as in B. (E) NE accumulation of ectopically expressed GFP-p150Glued was quantified as in C. (Scale bars, 5 μm.)
Furthermore, U2OS cell lines stably expressing RNAi-resistant GFP-p150Glued constructs (WT or S179A) were generated and those cells expressing GFP-p150Glued at levels close to the endogenous protein were selected for subsequent phenotypic analysis (Fig. S4A). Next, cells stably expressing GFP-p150Glued (WT or S179A) were depleted of endogenous protein with RNAi, and NE accumulation of GFP-p150Glued was determined. Although ectopically expressed GFP-p150Glued-WT was clearly detected in the NE of prophase cells, the S179A mutation almost completely abolished NE accumulation of GFP-p150Glued under the same condition, indicating that Plk1 phosphorylation of p150Glued is essential for this process (Fig. 4 D and E).
Plk1 Phosphorylation of p150Glued Improves Its Dissociation from Microtubules During Early Mitosis.
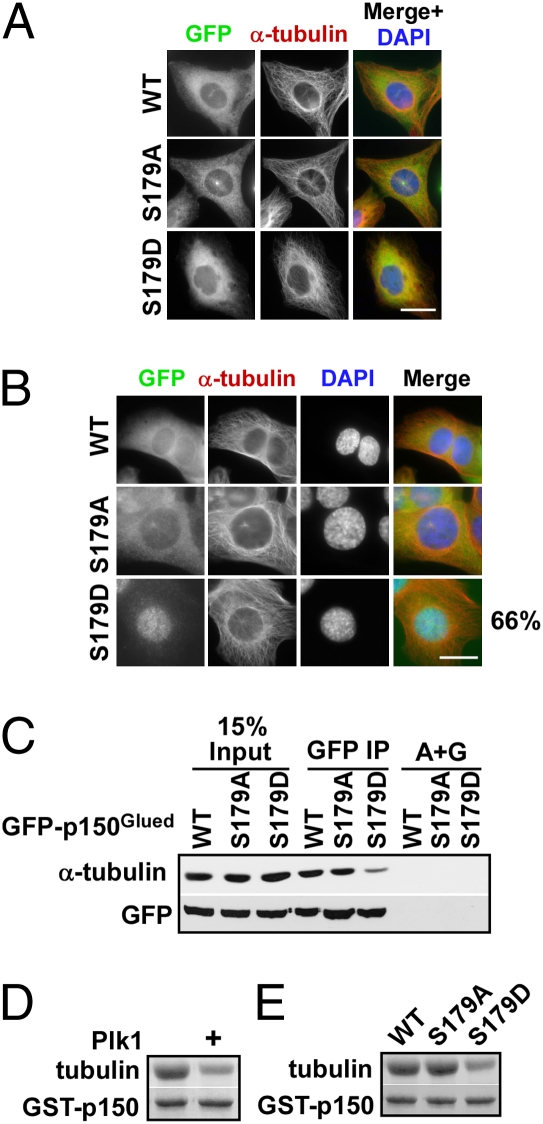
Two studies showed that phosphorylation in CAP-Gly domain of p150Glued would inhibit its interaction with microtubules (13, 14). Considering that S179 also locates very close to the CAP-Gly domain, we therefore examined the affects of Plk1 phosphorylation of p150Glued on microtubule association. By immunostaining of cells expressing different forms of GFP-p150Glued, all proteins were able to bind microtubules during interphase when Plk1 is not active (Fig. 5A). During early prophase, both GFP-p150Glued-WT and GFP-p150Glued-S179A were strongly associated with microtubules; up to 66% of GFP-p150Glued-S179D-expressing cells showed almost no microtubule association, but clear NE localization of p150Glued-S179D (Fig. 5B and Fig. S4B). This observation is consistent with the fact that the pS179 epitope was mainly detected at prophase NE. To further confirm this observation, co-IP experiments were performed to examine in vivo association between GFP-p150Glued and microtubules. As indicated in Fig. 5C, GFP-p150Glued-S179D showed a decreased microtubule binding affinity, in contrast to the finding that both GFP-p150Glued-WT and GFP-p150Glued-S179A bound to microtubules with high affinity. Finally, we assessed the binding of different forms of GST-p150Glued-N to taxol-polymerized microtubules in vitro. The GST-p150Glued-N showed a much weaker microtubule binding affinity after incubation with Plk1 kinase and ATP (Fig. 5D). In agreement, GST-p150Glued-N-S179D exhibited less association with microtubules, in comparison with GST-p150Glued-N-WT and GST-p150Glued-N-S179A (Fig. 5E). Thus, Plk1 phosphorylation of p150Glued appears to negatively regulate its interaction with the microtubules during mitosis. Based on these observations, we propose that Plk1 phosphorylation of p150Glued at least partially promotes its dissociation from microtubules at late G2 and that the released p150Glued would go to NE to initiate NEBD.
Fig. 5.
Plk1 phosphorylation of p150Glued promotes its dissociation from microtubules during early mitosis. (A and B) U2OS cells stably expressing GFP-p150Glued constructs (WT, S179A, or S179D) were stained with α-tubulin antibodies. (C) Extracts from U2OS cells stably expressing GFP-p150Glued constructs (WT, S179A, or S179D) were prepared for anti-GFP IP, followed by Western blotting with indicated antibodies. (D) After GST-p150Glued-aa-1–310 protein bound on glutathione-agarose beads was incubated with or without purified Plk1 in the presence of ATP and microtubules were added for an additional 0.5 h. Microtubules associated with the GST-fusion proteins were isolated by low-speed centrifugation, separated by SDS/PAGE, and stained by Coomassie Brilliant Blue. (E) GST-p150Glued-aa-1–310 (WT, S179A, or S179D) proteins bound on glutathione-agarose beads were incubated with microtubules and analyzed as in D. (Scale bars, 10 μm.)
We also analyzed whether phosphorylation of p150Glued at S179 affects its interaction with CLIP-170, which is a major microtubule plus-end-associated protein and supposed to be a regulator of p150Glued behavior. As indicated, we did not detect any significant difference among different p150Glued constructs (WT, S179A, or S179D), in terms of their interactions with CLIP-170 (Fig. S4E).
Phosphorylation of p150Glued at Ser-179 Facilitates NEBD at Prophase.
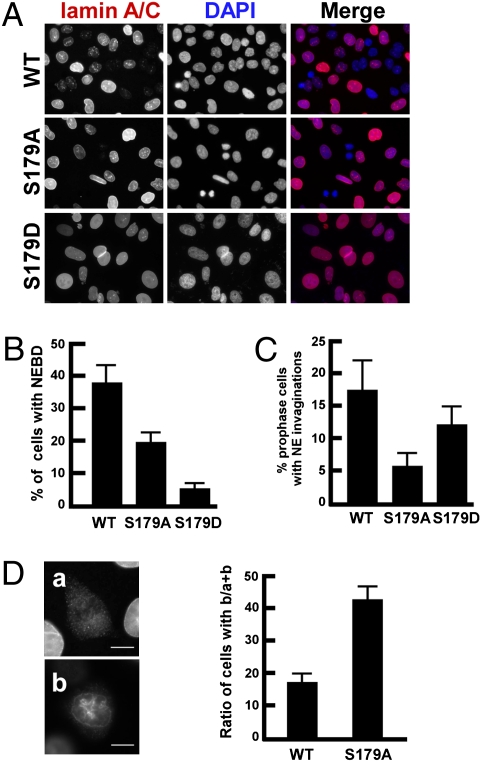
Dynein accumulates at the NE in prophase and facilitates NEBD by pulling nuclear membranes poleward along astral microtubules, leading to nuclear membrane detachment (1). Considering the partnership between p150Glued and dynein, we tested whether Plk1-mediated phosphorylation of p150Glued was involved in NEBD. U2OS cells stably expressing GFP-p150Glued constructs were depleted of endogenous p150Glued, synchronized by the DTB, released for 11 h to enrich at prophase, and stained with lamin A/C antibodies to monitor NEBD (Fig. 6A). Quantification indicated that about 37% of GFP-p150Glued-WT expressing cells showed clear NEBD, whereas only 19% of GFP-p150Glued-S179A expressing cells exhibited NEBD, suggesting that expression of the Plk1-unphosphorylatable p150Glued mutant caused a significant delay of NEBD during prophase (Fig. 6B). Consistent with the results obtained after Plk1 inhibition, expression of GFP-p150Glued-S179A also led to obvious defects in the formation of NE invaginations (Fig. 6C). As we described above, two types of lamin A/C staining patterns were detected during NEBD (Fig. 6D, Left). Therefore, U2OS cells stably expressing GFP-p150Glued constructs (WT or S179A) undergoing NEBD were selected to calculate the ratio of cells with these two different staining patterns (Fig. 6D, Right). As indicated, more than 80% of GFP-p150Glued-expressing cells undergoing NEBD showed a complete disappearance of the lamin A/C signal, whereas almost half of GFP-p150Glued-S179A-expressing cells undergoing NEBD still had remaining lamin A/C signals surrounding chromosomes, confirming that ectopic expression of the unphosphorylatable p150Glued mutant caused NEBD delay at prophase. Taking these data together, we conclude that Plk1-dependent phosphorylation of p150Glued at Ser-179 positively regulates NEBD in mammalian cells.
Fig. 6.
Expression of the unphosphorylatable p150Glued at Ser-179 retards NEBD in prophase. (A–D) U2OS cells stably expressing GFP-p150Glued (WT, S179A, or S179D) were depleted of endogenous p150Glued by RNAi, synchronized with the DTB, released for 11 h, and immunostained with lamin A/C antibodies. (B) Quantification of cells with NEBD. (C) Quantification of prophase cells with NE invaginations as in Fig. 3C. (D) Representative images of cells with two types of lamin A/C staining during NEBD. Although lamin A/C staining is completely undectable in “a,” a remaining lamin A/C signal is still clearly visible surrounding chromosomes in “b.” (Right) The ratio of b/(a+b) is shown. (Scale bars, 5 μm.)
We also examined the phenotypes of cells after they were transfected with GFP-p150Glued-S179D construct. Analysis indicated that expression of p150Glued-S179D promotes NE invagination (Fig. 6C), but not NEBD (Fig. 6 A and B and Fig. S4C), suggesting that the activating mutation does not function exactly the same as the phosphorylated p150Glued. Finally, we found that overexpression of the constitutively active Plk1 (T210D) promotes NE invagination of cells expressing p150Glued-WT, but not -S179A mutant (Fig. S4D), confirming the significance of Plk1-associated kinase activity in the process.
We further examined the phenotypes of cells expressing different forms of GFP-p150Glued (WT or S179A). FACS analysis showed that the GFP-p150Glued-S179A-expressing cells exhibited a higher G2/M content than that of cells expressing GFP-p150Glued-WT, indicating a possible G2/M arrest in cells expressing p150Glued-S179A (Fig. S5A). Consistent with the FACS profiles, immunostaining analysis with antibodies against both phospho-H3 and cyclin B revealed that expression of GFP-p150Glued-S179A, but not its WT counterpart, led to a G2/M arrest (Fig. S5 B and C). To further distinguish whether cells expressing GFP-p150Glued-S179A are arrested at G2 or M phase, we examined chromatin morphology based on DAPI staining. We conclude that ectopic expression of GFP-p150Glued-S179A led to a G2 block, as mitotic indices were almost identical for cells expressing either p150Glued-WT or -S179A (Fig. S5D). IF staining results were further confirmed by Western blotting with antibodies against phospho-H3 and cyclin B (Fig. S5E). Although a significant increase of the protein level of cyclin B was observed in cells expressing GFP-p150Glued-S179A, decreased Cdc2/cyclin B activity was detected in these cells either nocodazole-treated or -untreated (Fig. S5F), supporting the notion that expression of the Plk1-unphosphorylatable mutant of p150Glued blocked cells at G2, likely because of lack of efficient NEBD during prophase in these cells. Finally, defects in mitotic entry with cells expressing GFP-p150Glued-S179A were also detected in well-synchronized cultures (Fig. S6).
Discussion
As we have described, p150Glued is phosphorylated at multiple sites. Two studies showed that phosphorylations in CAP-Gly domain of p150Glued would inhibit its interaction with microtubules and Aurora A, which is a mitotic kinase, was demonstrated to contribute to some of the phosphorylations (13, 14). In this communication, we demonstrated that Plk1 is a kinase responsible for phosphorylation of p150Glued at Ser-179 and Plk1-mediated phosphorylation of p150Glued at Ser-179 also improves its dissociation from microtubules, and therefore indirectly promotes its NE accumulation at prophase.
Microtubules are critical to control the rearrangements of the nuclear membranes during prophase (7). In late G2, astral microtubules develop close attachments on the NE and therefore generate essential force for the subsequent NEBD in prophase. Cytoplasmic dynein also contributes to the force generation through its interaction with nuclear membranes (1). Here, we provide evidence that p150Glued, the largest subunit of the dynein/dynactin complex, is also a facilitator of NEBD in mammalian cells. Overexpression of p150Glued significantly promoted nuclear membrane fragmentation, whereas depletion of p150Glued induced severe cell-cycle block before mitosis, likely because of the lack of NEBD at prophase.
Plk1 has various well-established roles in mitosis, including mitotic entry. Depletion or inhibition of Plk1 causes a severe delay in the G2/M transition in different systems (15, 16). In C. elegans, Plk1 was also proposed as a regulator for NEBD during meiosis (10). Our data are unique in providing direct evidence that Plk1 positively regulates NEBD through phosphorylation of p150Glued during prophase. First, Plk1 inhibition completely abolished formation of the NE folds and invaginations before NEBD. Second, depletion of Plk1 apparently delayed the removal of lamins on chromosomes at late prophase. Third, the pS179 epitope was specifically detected at prophase nuclear membranes. Fourth, the S179A mutant protein showed decreased accumulation at the prophase NE. Finally, ectopic expression of GFP-p150Glued-S179A delayed both NEBD and the G2/M transition. Overall, our findings further support the notion that microtubule-associated motor proteins play critical roles in NEBD and that Plk1-dependent phosphorylation of p150Glued provides one regulatory mechanism for cells to control the G2/M transition. We might point out that whether phosphorylation of S179 is needed not only to promote p150Glued dissociation from microtubules but also has a direct role in its association with the NE awaits further experimentation.
Materials and Methods
IF Staining.
Immunostaining was performed as previously described (12). Briefly, HeLa and U2OS cells were plated onto 12-mm glass coverslips in six-well plates. For Plk1, lamin A/C, and pS179 staining, cells were fixed with 3% paraformaldehyde followed by permeabilization with 0.1% Triton X-100 for 15 min. For detection of p150Glued on the NE, cells were permeabilized for 30 s in 0.1% Triton X-100 before fixation with 3% paraformaldehyde.
Supporting Information.
Plasmid construction, cell culture, transfections, immunoblotting, kinase assays, stable cell line generation, and IF intensity analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Raymond Erikson for his support for this work, Eleanor Erikson for critical reading of the manuscript, and Dr. Krysten J. Palmer (University of Bristol, United Kingdom) for GFP-p150Glued. This work was supported by National Cancer Institute Howard Temin Award K01 CA114401 (to X.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006615107/-/DCSupplemental.
References
- 1.Salina D, et al. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- 2.King SJ, Schroer TA. Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol. 2000;2:20–24. doi: 10.1038/71338. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karki S, Holzbaur EL. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary N, Courvalin JC. Stepwise reassembly of the nuclear envelope at the end of mitosis. J Cell Biol. 1993;122:295–306. doi: 10.1083/jcb.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Guan T, Gerace L. Integral membrane proteins of the nuclear envelope are dispersed throughout the endoplasmic reticulum during mitosis. J Cell Biol. 1997;137:1199–1210. doi: 10.1083/jcb.137.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaudouin J, Gerlich D, Daigle N, Eils R, Ellenberg J. Nuclear envelope breakdown proceeds by microtubule-induced tearing of the lamina. Cell. 2002;108:83–96. doi: 10.1016/s0092-8674(01)00627-4. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- 9.Lénárt P, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Chase D, et al. The polo-like kinase PLK-1 is required for nuclear envelope breakdown and the completion of meiosis in Caenorhabditis elegans. Genesis. 2000;26:26–41. doi: 10.1002/(sici)1526-968x(200001)26:1<26::aid-gene6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Tanenbaum ME, Galjart N, van Vugt MA, Medema RH. CLIP-170 facilitates the formation of kinetochore-microtubule attachments. EMBO J. 2006;25:45–57. doi: 10.1038/sj.emboj.7600916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebbar S, et al. Lis1 and Ndel1 influence the timing of nuclear envelope breakdown in neural stem cells. J Cell Biol. 2008;182:1063–1071. doi: 10.1083/jcb.200803071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT. A role for regulated binding of p150(Glued) to microtubule plus ends in organelle transport. J Cell Biol. 2002;158:305–319. doi: 10.1083/jcb.200201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romé P, et al. Aurora A contributes to p150(glued) phosphorylation and function during mitosis. J Cell Biol. 2010;189:651–659. doi: 10.1083/jcb.201001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, et al. CCT chaperonin complex is required for the biogenesis of functional Plk1. Mol Cell Biol. 2005;25:4993–5010. doi: 10.1128/MCB.25.12.4993-5010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian YW, Erikson E, Li C, Maller JL. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.