Abstract
The basolateral amygdala (BLA) is thought to be essential for fear learning. However, extensive training can overcome the loss of conditional fear evident following lesions and inactivation of the BLA. Such results suggest the existence of a primary BLA-dependent and a compensatory BLA-independent neural circuit. We tested the hypothesis that the bed nuclei of the stria terminalis (BST) provides this compensatory plasticity. Using extensive context-fear conditioning, we demonstrate that combined BLA and BST lesions prevented fear acquisition and expression. Additionally, protein synthesis in the BST was critical only for consolidation of BLA-independent but not BLA-dependent fear. Moreover, fear acquired after BLA lesions resulted in greater activation of BST regions that receive hippocampal efferents. These results suggest that the BST is capable of functioning as a compensatory site in the acquisition and consolidation of context-fear memories. Unlocking such neural compensation holds promise for understanding situations when brain damage impairs normal function or failure to regulate compensatory sites leads to anxiety disorders.
Keywords: amygdala, basolateral amygdala, context, plasticity, bed nucleus of the stria terminalis
A largely supported view in the neuroscience of associative memory is the existence of essential neural circuits that have the capacity to learn, retain, and retrieve specific classes of experience 1–7). For example, in reflexive motor learning or Pavlovian eyeblink-conditioning, the integration of sensory stimuli within the cerebellar interpositus nuclei are required for the acquisition, retention, and retrieval conditional responses (8). Similarly, the striatum is considered critical for habit learning (9, 10) and the hippocampus essential for spatial learning (11). In fear-conditioning, a circuit centered around the basolateral amygdala complex (BLA; consisting of the lateral amygdala, basomedial, basolateral, and posterior nuclei) is viewed essential for the acquisition and expression of fear memories (12–16). The importance of the BLA for fear memory has been supported by numerous studies showing that disruption of protein synthesis, NMDA receptor function, neuronal activity, synaptic transmission, and plasticity all prevent the establishment of fear memories (14–18).
Context fear learning normally occurs when hippocampal context information, which exits the subiculum, converges with aversive information in the BLA (15). In turn, when the contextual information activates the BLA, this information is relayed to the central nucleus of the amygdala (CEA), whose efferents to the ventral periaqueductal gray (vPAG) trigger the expression of fear as indexed by conditional freezing (conditional response) (19). Lesions or inactivations of the CEA can disrupt contextual fear (20, 21; but see 22). Of particular relevance in this study is that pretraining lesions or inactivations of the BLA have been shown to strongly impair the acquisition of fear (23–25). However, with extensive overtraining (~75 trials), the loss of conditional fear evident following lesions or inactivations of the BLA can be overcome (25–27). These findings suggest that under some circumstances, alternate neural pathways can acquire fear. Certainly, the enormous interconnectivity of the brain affords such potential (28) in that there are multiple routes from information sources to adaptive behaviors. Indeed, plasticity in multiple brain regions outside of the BLA is important for the acquisition of fear (20, 29, 30). This interconnectivity and availability of alternate pathways capable of undergoing plasticity does not protect against future damage, as posttraining lesions of the BLA eliminate fear even when previously intact rats are overtrained (26).
To address such findings, we proposed a dynamic-systems view of memory where the most efficient neural pathway between information and action dominates processing. However, if information flow is precluded in such a neural pathway, plasticity in alternate regions is capable of compensating, albeit with less efficiency (27, 31). This theory is evident, as animals that learn fear in the absence of the primary pathway require significant overtraining (>50 trials) and begin to display significant forgetting after 7 d (25, 32). Thus, although plasticity in the BLA normally supports contextual fear-conditioning, without the BLA, some alternate regions must compensate.
The alternate or compensatory regions for BLA function are currently unknown. Therefore, the goal of the following experiments was to identify this compensatory region. A likely candidate would be a group of neurons that interconnects the hippocampal formation (HPF), which processes contextual information, and vPAG, which generates several fear-related behaviors. To support fear-learning, this compensatory network would also need to be capable of undergoing long-term plasticity. The bed nuclei of the stria terminalis (BST) is such a structure composed of two main divisions: the posterior and the anterior division, with the latter composed of the lateral and medial groups. Recent studies have shown that the BST is capable of undergoing experience-dependent plasticity (33, 34). Like the BLA, the anteromedial group of the BST receives direct projections from the ventral HPF and sends information to the vPAG (35, 36). Such neural connectivity provides a route by which contextual information encoded by the HPF could bypass the BLA and activate the vPAG to generate freezing. Conversely, the BST is capable of receiving noxious information (i.e., footshock) both directly and indirectly (37, 38). Consistent with this hypothesis, lesions or inactivations of the BST result in a wide range of fear- and anxiety-related deficits (39–42). In addition, the anterolateral group of the BST—perhaps through its interconnections with the posterior BLA (BLAp), the only BLA nuclei that projects to the BST (43)—contributes to the expression of context fear under normal conditions (44).
To determine whether the BST compensates for the loss of the BLA, subjects with sham or BLA lesions were presented with context-fear overtraining with the BST lesioned or intact. Next, to examine whether the BST is an alternate site of fear learning-related plasticity, protein synthesis in the BST was inhibited in subjects overtrained with the BLA lesioned or intact. To determine if the BST regions we cannulated interconnect with fear circuitry, we infused a retrograde tracer through the chronically implanted cannula. Finally, we examined the expression of an immediate early gene within the BST following the retrieval of fear, in relation to whether the primary BLA-dependent pathway is functional or not.
Results
Role of the BST in Overtrained Fear (Experiment 1).
Our first experiment determined the contribution of the BST to BLA-independent fear. We reasoned if the BLA and BST were in a competitive/compensatory relationship, a combined BLA+BST lesion should cause a greater impairment than lesions of either alone. Therefore, we overtrained rats with excitotoxic lesions of the BLA, BST, or both. Fig. 1 shows the extent of these lesions.
Fig. 1.
Histological analysis of BLA or BST lesions for exp. 1. Reconstruction of the minimal (dark gray) and maximal (light gray) excitotoxic lesion in the region of the BLA (AP 2.6–3.3 mm) and BST (AP 0.0–1.0 mm). Coronal section images taken and adapted from Swanson (52). The largest extent of any given BLA lesion included LA, BLAa, BLAp, and BMAp; the smallest lesion only included damage to the LA. The largest extent of BST lesion included subdivisions pr, rh, al, tr, am, mg, v, and if, and the smallest lesion was limited to pr, mg, and if.
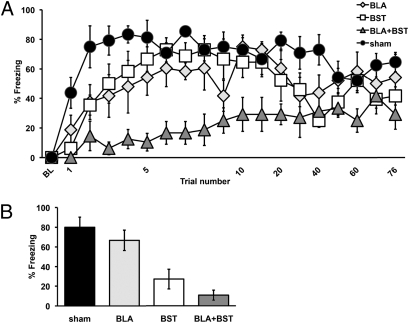
Fig. 2 (Upper) displays the mean percent-freezing from sham (n = 6), BLA (n = 6), BST (n = 6), and BLA+BST (n = 6) lesion animals during acquisition as measured by postshock freezing. Pretraining BLA and BST lesions delayed the acquisition of conditional fear relative to controls. These impressions were supported by a split-plot ANOVA indicating reliable main effects for Lesion [F(3,418) = 77.98, P < 0.0001] and Trials [F(18,418) = 10.93, P < 0.0001] and an interaction between Lesion and Trials [F(54,418) = 1.76, P < 0.005]. Newman–Keuls post hoc tests revealed that the BLA, BST, and BLA+BST lesion groups showed significantly less freezing than the sham group (all P < 0.05). Freezing in the BLA+BST lesion group was significantly lower than the BLA- and BST-only lesion groups (both P < 0.05). Although pretraining lesions of these regions retarded acquisition of conditional freezing in singly lesioned animals during the first few footshocks with overtraining, subsequent footshocks yielded freezing levels similar to those expressed in intact rats. In contrast, BLA+BST-lesioned rats failed to show significant context-fear acquisition.
Fig. 2.
Behavioral measurement. (A) Behavioral measurement of fear learning in sham, BLA, BST, or combined BLA+BST lesion animals. Acquisition of conditional fear as indexed by percent-time observed freezing during an immediate postshock period. Mean (±SEM) percentage of observations spent freezing during a 76-footshock contextual-fear conditioning session. (B) Context-fear memory in sham, BLA, BST, or combined BLA+BST lesioned animals. Total mean (±SEM) percentage of observations spent freezing during an 8-min test session for conditional fear.
During a shock-free test session 24 h later, freezing was practically abolished in the combined lesion group (Fig. 2, Lower). BST-alone lesions also produced a deficit. These impressions were confirmed by a two-way ANOVA, which revealed a main effect of BST lesion (Fig. 2, Lower) [F(1, 20) = 63.11, P < 0.0001] and BLA lesion [F(1, 20) = 4.63, P < 0.05]. We performed a planned contrast on the effects of the single lesions versus the combined lesion and found reliably less freezing in the combined group [F(1, 15) = 32.3521, P < 0.0001].
This attenuation of freezing by pretraining BST lesions complements a previous study by Sullivan et al. (44), which showed that posttraining electrolytic lesions of the BST attenuated the expression of freezing during a test of contextual-fear memory. Freezing was lowest in the combined-lesioned rats in comparison with all other groups. The low level of freezing in the combined-lesion group is consistent with the idea that the BST compensates for damage to the BLA.
Role of Protein Synthesis in the BST in Overtrained Fear (Experiment 2).
Permanent lesions of the BST do not allow us to distinguish between contributions of the BST to learning of context fear from expression of context fear. If learning-related plasticity in the BST compensates for the loss of the BLA, then protein synthesis in the BST should be especially important for the consolidation of contextual conditioning when the BLA is lesioned. Therefore, we overtrained rats with sham or pretraining lesions of the BLA and, immediately after training, infused the protein synthesis inhibitor anisomycin (ANISO) or vehicle into the BST. Because no drug is present during training or testing, this procedure isolates the drug to an influence on posttraining consolidation. Lesion and cannula placements are shown in Fig. 3.
Fig. 3.
Histological analysis of BLA lesion and BST cannula placements for exp. 2. Reconstruction of the minimal (dark gray) and maximal (light gray) excitotoxic lesion in the region of the BLA (AP 2.6–3.3 mm) and cannula tip placement within the BST (square). Coronal section images taken and adapted from Swanson (52). The largest extent of any given BLA lesion included LA, BLAa, BLAp, and BMAp; the smallest lesion only included damage to the LA.
Fig. 4 shows that during the test session 24 h later, animals overtrained with BLA lesions showed normal levels of fear retention compared with sham overtrained rats. In intact animals, ANISO infusions into BST failed to disrupt fear retention. However, BLA-lesioned animals infused with ANISO failed to show any evidence of a fear memory. These impressions were confirmed by a two-way ANOVA, which revealed a main effect of Lesion [F(1, 22) = 19.92, P < 0.001] and Drug [F(1,22) = 4.49, P < 0.05], as well as a significant interaction between Lesion and Drug (Fig. 4) [F(1, 22) = 12.79, P < 0.01]. Planned comparison indicated that vehicle-infused BLA-lesioned animals froze similarly to BLA intact rats infused with vehicle (VEH) or ANISO. The BLA+ANISO group showed the least amount of freezing compared with BLA+VEH, SHAM+VEH, or SHAM+VEH (all P values < 0.001).
Fig. 4.
Behavioral measurement of context-fear memory in rats with combined: (i) Sham lesion and BST ACSF infusion (black), (ii) sham lesion and BST ANISO infusion (light gray), (iii) BLA lesion and BST ACSF infusion (white), (iv) BLA lesion and BST ANISO infusion (medium gray), and (v) retraining (76 trials in a novel context) in previously trained and tested BLA lesion and BST ANISO-infused animals (medium gray with description). Total mean (±SEM) percentage of observations spent freezing during an 8-min test session for conditional fear.
If ANISO caused permanent damage to the BST, it would have produced similar results to combined BLA+BST lesion animals in exp. 1. Therefore, we reovertrained the BLA+ANISO group and found that these animals acquired fear-conditioning similar to intact, BLA-lesioned, and ANISO-treated alone animals. To examine whether infusions spread to regions of the BST, which receive input from the ventral HPF, the retrograde neural tracer Fluoro-Gold was infused through the cannula previously used to infuse VEH or ANISO. Fig. 5 shows that Fluoro-Gold infusions spread to a large extent of the BST, and retrogradely labeled soma within the HPF, BLA, CEA, and prefrontal cortex (PFC).
Fig. 5.
Retrograde labeling of neuronal tracer Fluoro-gold. (Right) The putative neuroanatomical circuits underlying fear conditioning. (Left) Retrograde neuronal tracer Fluoro-Gold was infused into the BST, as highlighted in the image in the red box. A representative infusion resulted in retrograde labeling of neurons in the mPFC, CEA, and ventral (HPF). A rat with the BLA intact showed substantial retrograde labeling in the BLA, whereas a rat with a BLA lesion showed substantially less labeling within the BLA complex.
Expression of an Immediate Early Gene in the BST During the Retrieval of Overtrained Fear (Experiment 3).
Animals that acquired contextual-fear conditioning independent of the BLA failed to establish a long-term memory when protein synthesis was blocked within BST, suggesting protein synthesis was required for the consolidation of BLA-independent fear memories. Prior anatomical studies have shown that HPF efferents project mainly to the anteromedial group of the BST. We hypothesized that this region is activated during the retrieval of BLA-independent fear, and thus quantified the expression of the immediate early gene C-fos following tests of contextual fear. Animals were overtrained or not (training context minus any shocks) with pretraining sham or excitotoxic lesions of the BLA, and tested for fear 24 h later. Then, 1 h and 20 min following the cessation an 8-min context-fear test, animals were killed, brains were extracted, and immunohistochemistry procedures were performed (Fig. S1). Both sham and BLA-lesioned overtrained groups expressed similar levels of contextual fear (Fig. S1). Blind-counting of C-fos-positive cells were calculated within the ventral region of the anteromedial group of the BST composed of the BSTam, BSTdm, BSTmg, and BSTv (Fig. 6). As indicated in Fig. 7, animals that acquired fear in the absence of the BLA showed the highest level of C-fos activation in the ventral region of the anteromedial BST. A two-way ANOVA revealed a significant lesion by overtraining interaction [F(1,-13) = 4.54 P < 0.05]. Overtraining increased C-fos expression in BLA-lesioned animals [F(1, 13) = 8.16 P < 0.01], but overtraining had no effect on intact rats [F(1,5) = 2.161 P > 0.05].
Fig. 6.
Representative images of C-Fos–immunoreactive neurons within the BST in animals with lesions of the BLA, following an 8-min test of contextual fear. The outlined area includes BST and its subdivisions: rh, am, al, dm, mg, and v. The subdivisions in solid white text (am, dm, mg, and v) represent the anteromedial group of BST subdivisions quantified in exp. 3. (A) BLA-lesioned and not overtrained; (B) BLA-lesioned and overtrained.
Fig. 7.
Mean number of C-fos-positive cells counted in posterior ventral anteromedial region of the BST following the test of a context-fear memory (**P < 0.001).
Discussion
Over the past few decades a number of laboratories, including our own, have demonstrated the importance of the BLA in Pavlovian fear-conditioning (17, 45–47). Here we show that following damage to the BLA, with extended training, compensatory circuits within the BST are recruited and required for the acquisition, consolidation, and retrieval of conditional fear responses. Under this overtraining procedure, fear acquisition in the absence of the BLA is markedly slow, but eventually reaches normal asymptotic levels. With additional damage to the BST, fear acquisition is at a near complete loss. Disrupting BST protein synthesis immediately after training selectively prevents fear consolidation when BLA function is occluded. Conversely, the retrieval of fear in the absence of the BLA is reflected in greater levels of neural activation in BST subdivisions that receive HPF afferents. Thus, the recruitment of the BST as an alternate site of associative plasticity is contingent upon decreased BLA function and augmented conditioning procedures.
A prior study using similar overtraining procedures showed that CEA lesions or inactivations disrupt fear conditioning (21). However, it should be noted that this engagement of CEA in fear learning occurred regardless of whether the BLA was functional or not. Thus, the CEA, like other regions, such as the PAG, contribute to fear generally (i.e., BLA-dependent or BLA-independent). However, plasticity in the BST seems to be recruited when the BLA is not available. Such “compensatory” plasticity has not been reported previously. Prior findings have documented that fear conditioning normally depends on plasticity in several brain regions, such as the hippocampus, medial geniculate nucleus, auditory cortex, and CEA (20, 29, 31). Our results suggest that plasticity in the BST is not normally essential for fear conditioning but plasticity in this region is recruited when other components of the circuit (i.e., BLA) are not functioning normally. Identifying such sites of dormant plasticity may be beneficial in clinical conditions where brain pathology leads to a loss of function, such as stroke and Alzheimer's disease.
This demonstration of the BST as a site of fear-related mnemonic processing is unprecedented; however, its capacity to undergo other forms of experience-dependent plasticity has been previously reported (33, 34). For example, chronic immobilization significantly increases dendritic branching in the BST (33). Additionally, reward-seeking behaviors (to cocaine and palatable food) increases excitatory synaptic transmission as revealed by increases in AMPA to NMDA receptor ratios in BST neural slices (34). Several behavioral consequences of uncontrollable stress, potentiated freezing, increased escape latency (48), and enhanced trace eyeblink-conditioning (49) are disrupted by BST lesions. Perhaps the most well-studied functional role of the BST is in relation to models of anxiety. Walker and Davis have shown elegantly that disrupting BST function can attenuate the potentiated startle triggered during exposure to bright light or corticotrophin releasing hormone, whereas the same response to discrete cues previously paired with footshock remained unaffected (41). In Pavlovian fear-conditioning as measured by freezing and corticosteroid release, posttraining BST lesions selectively abolish the expression of context, but not cued conditional-fear responses (44). This finding has led to models of fear-conditioning that now incorporate the anterolateral group of the BST as part of the conditional-response expression pathway for context fear (44, 50). The results in exp. 1 that rats with only BST lesions showed less of a deficit than rats with combined lesions suggests that in intact rats, context-fear expression is only partly mediated by the BST. Normally, a BLA→CEA→PAG pathway likely mediates some fear expression. More importantly, the present results indicate that posttraining protein synthesis is necessary under conditions where fear-learning occurs without the BLA. When the BLA is functioning normally, BST plasticity is down-regulated but this plasticity is revealed when BLA function is lost. Although the mechanisms of this down-regulation of BST plasticity are not known, it likely plays a role in keeping fear appropriate to the degree of threat (31). Loss of this down-regulation could lead to excessive fear, resulting from additional plasticity (or memory) in parallel pathways. Such excessive fear may contribute to anxiety disorders.
The diversity of BST function as described above is likely reflected in the numerous subdivisions that comprise the BST, which receive a diverse set of afferents, such as those related to cognition (PFC), spatial information (HPF), and fear (BLAp and CEA), and in turn project to heterogeneous neural regions responsible for the generation of stress responses (paraventricular nucleus) and the expression of defensive behaviors (vPAG) (35).
The collective literature (described earlier) indicates that either, HPF→BLA→CEA→BST→vPAG (39, 41, 46, 47) or HPF→BLAp→BST→vPAG (22) is the primary route by which context fear is normally acquired, retained, and expressed (Fig. S2). However, like the BLA, the anteromedial group of the BST also receives direct projections from the HPF and information from nociceptive-related input (37, 51). These nuclei are well-situated anatomically to provide a compensatory site of fear-related plasticity.
In a prior study, we found that learning normally down-regulates learning in other potential yet unidentified circuits (27). Here we found that protein synthesis-dependent plasticity in the BST is not necessary for fear conditioning when the BLA is intact, but is sufficient to compensate for loss of the BLA. This theory suggests that this plasticity in the BST is not driven when the BLA learns. Therefore, when the BLA is undergoing fear-related learning, it down-regulates plasticity in the BST. Thus, with the BLA intact, no significant learning occurs in the proposed alternate pathway (27). The present study provides strong evidence that competition between faster (BLA-dependent) and slower learning circuits exist, and that under specific circumstances, the balance between these systems can be tilted to reveal a compensatory learning circuit within the brain.
Materials and Methods
Exp. 1: Single and Combined Lesions of the BLA and BST and Extensive Overtraining.
Subjects were male Long-Evans hooded rats weighing 300 to 340 g, obtained from a commercial supplier (Harlan). Rats were housed individually in standard stainless-steel cages on a 12-h light/12-h dark cycle and were provided ad libitum access to food and water. After being housed, the rats were handled daily (~60 s per rat) for 5 d to acclimate them to the experimenter. All procedures were approved by the University of California Los Angeles Animal Research Committee.
Surgery.
A detailed description of lesion surgeries can be found in SI Materials and Methods. Coordinates for excitotoxic lesions were as follows: BLA: AP, 2.6 and 3.3 mm posterior; ML, 5.1 mm lateral; and DV, 8.2 and 8.4 mm ventral to bregma. BST: AP, 0.8 mm posterior; ML, 1.1 and 2.0 mm; and DV, 6.2 and 7.0 mm.
Conditioning apparatus.
The conditioning apparatus consisted of aluminum (side walls) and plexiglas (front, back, and top) chambers (28 × 21 × 22 cm; Lafayette Instruments). The floor of each chamber had 18 stainless-steel rods (4-mm diameter, 1.5 cm apart) connected to a shock scrambler and generator (which, along with internal ventilation fans, supplied background noise of 70 dB, A scale). The chambers were cleaned with 5% ammonium hydroxide solution and scented with 0.1% benzaldehyde in 100% ethanol. These computer-controlled (Med-Associates) chambers were in a well-lit room separate from the experimenter.
Behavioral procedures.
The overtraining procedure was as follows: After a 2-min exposure to the chamber, rats were given 76 unsignaled shocks (1 mA, 2 s) with a 64-s ITI. Two minutes after the final shock, rats were removed from the chamber and returned to the home cage. The day after training, the rats were tested in the original training context.
Freezing behavior was defined as the absence of any visible movement (including the vibrissae), except that required for respiration. Freezing behavior was scored by a blind observer according to an instantaneous time-sampling procedure in which each animal was observed every 8 s. For each minute (defined as eight samples or 64 s), the number of observations scored as freezing were summed and converted to a percentage (number of freezing observations/total number of observations × 100). Previous study indicates that this measure conforms to the requirements of parametric analysis. During overtraining, freezing was measured after the 1st, 10th, 15th, 20th, 30th, 40th, 50th, 60th, 70th, and 76th shocks. During the test, freezing was measured for the entire 8 min.
Histology.
At the completion of behavioral testing (for exp. 1, 2, and 3), all rats were overdosed with sodium pentobarbital and perfused intracardially with 0.9% saline, followed by 4% buffered formalin. The brains were removed and stored in 10% formalin for at least 2 wk before slicing. These brains were cryoprotected in 30% sucrose-PBS mixture for 48 h and were sectioned in a cryostat. Coronal sections (50 μm) were taken through the extent of the BLA and BST, mounted on gelatinized slides, and stained with thionin or prepared for NeuN immunohistochemistry.
Neu-N immunohistochemistry.
For complete details of Neu-N immunohistochemistry, see SI Materials and Methods. Briefly, tissue sections were washed and rinsed before being transferred to blocking solution and incubated overnight in Neu-N antibody (1:4,000) solution. Next, tissue sections were incubated in mouse antibody IgG (1.25 h), followed by Avidin-Biotin-Peroxidase complex (ABC), then 3 to 3′ diaminobenzidine tetrahydrochloride (DAB, Sigma). Lesions were verified by reconstructing the damage on stereotaxic atlas templates (52) (for complete details, see SI Materials and Methods).
Statistical analyses.
All experiments were analyzed by appropriate ANOVA's. Thereafter, Fisher's planned comparisons were computed with significance set at P value < 0.05.
Exp. 2: BLA Lesion, BST Cannulation, Infusion, and Overtraining.
Rats received sham or bilateral lesions of the BLA (see Exp. 1, Surgery) and bilateral cannulae implantation within the BST (see SI Materials and Methods). Overtraining and memory tests were performed exactly as described in exp. 1 (Behavioral procedures). Immediately following overtraining, ACSF or ANISO were infused into the BST (for infusion procedures, see SI Materials and Methods). Following memory tests, ANISO-infused rats were once again overtrained (same context) and retested for fear memory. After the cessation of behavioral testing, a small number of ANISO-infused rats were infused with the retrograde neural-tracer Fluoro-Gold (conjugated) to estimate the spread of ANISO and identify BST afferents that were likely affected by the protein synthesis inhibitor.
Histology.
Following the final drug infusion, Fluoro-Gold–infused rats remained in their home cage for an additional 2 wk before being perfused, whereas non-Fluoro-Gold rats were perfused 1 to 2 d later (see exp. 1,Histology). Brains of non-Fluoro-Gold–infused rats were prepared for thionin staining (see Exp. 1, Histology). Brains sections of Fluoro-Gold–infused rats were mounted on slides, coverslipped, and allowed to dry. The next day, Fluoro-Gold slides were photographed with a light microscope (Zeiss) with fluorescent capabilities to determine if labeling was present in BST, BLA, CEA, HPF, and medial prefrontal cortex.
Exp. 3: BLA Lesion, Overtraining, and Fear-Retrieval Related C-fos Expression.
Rats received sham or bilateral lesions of the BLA (see Exp. 1, Surgery). Rats were overtrained or not (placed in the training context without receiving footshocks) and memory tests were performed exactly as described in Exp. 1 (Behavioral procedures). Ninety minutes following context memory, tests rats were overdosed and brains were prepared for thionin staining (see Exp. 1, Histology) and C-fos immunohistochemistry. Regions of the BST that have been previously shown to receive projections from the ventral HPF were analyzed for C-fos expression (35).
C-fos immunohistochemistry.
For complete details of C-fos immunohistochemistry, see SI Materials and Methods. Briefly, tissue sections were washed and rinsed before being transferred to blocking solution and incubated overnight in C-fos antibody (1:1,000) solution. Next, tissue sections were incubated in rabbit antibody IgG (1 h), followed by ABC, then DAB. After the final rinse, tissue was mounted on microscope slides, dried, dehydrated, and coverslipped to quantify C-fos expression. For details of C-fos quantification, see SI Materials and Methods.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health Grant MH 62122 (to M.S.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005754107/-/DCSupplemental.
References
- 1.Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 3.McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- 4.Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci. 1993;107:580–595. doi: 10.1037//0735-7044.107.4.580. [DOI] [PubMed] [Google Scholar]
- 5.McCormick DA, Clark GA, Lavond DG, Thompson RF. Initial localization of the memory trace for a basic form of learning. Proc Natl Acad Sci USA. 1982;79:2731–2735. doi: 10.1073/pnas.79.8.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishkin M. A memory system in the monkey. Philos Trans R Soc Lond B Biol Sci. 1982;298:83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- 7.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampus lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Packard MG. Anxiety, cognition, and habit: A multiple memory systems perspective. Brain Res. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Balleine BW. Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 11.Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 12.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 13.LeDoux JE, Farb CR, Milner TA. Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res. 1991;85:577–586. doi: 10.1007/BF00231742. [DOI] [PubMed] [Google Scholar]
- 14.Paré D. Presynaptic induction and expression of NMDA-dependent LTP. Trends Neurosci. 2004;27:440–441. doi: 10.1016/j.tins.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- 16.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 18.Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- 19.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 20.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: The central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci. 2004;24:7654–7662. doi: 10.1523/JNEUROSCI.1644-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 24.Helmstetter FJ, Bellgowan PS. Effects of muscimol applied to the basolateral amygdala on acquisition and expression of contextual fear conditioning in rats. Behav Neurosci. 1994;108:1005–1009. doi: 10.1037//0735-7044.108.5.1005. [DOI] [PubMed] [Google Scholar]
- 25.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci. 1999;19:8696–8703. doi: 10.1523/JNEUROSCI.19-19-08696.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gale GD, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponnusamy R, Poulos AM, Fanselow MS. Amygdala-dependent and amygdala-independent pathways for contextual fear conditioning. Neuroscience. 2007;147:919–927. doi: 10.1016/j.neuroscience.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young MP, et al. Non-metric multidimensional scaling in the analysis of neuroanatomical connection data and the organization of the primate cortical visual system. Philos Trans R Soc Lond B Biol Sci. 1995;348:281–308. doi: 10.1098/rstb.1995.0069. [DOI] [PubMed] [Google Scholar]
- 29.Parsons RG, Riedner BA, Gafford GM, Helmstetter FJ. The formation of auditory fear memory requires the synthesis of protein and mRNA in the auditory thalamus. Neuroscience. 2006;141:1163–1170. doi: 10.1016/j.neuroscience.2006.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn Sci. 2010;14:7–15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poulos AM, et al. Persistence of fear memory across time requires the basolateral amygdala complex. Proc Natl Acad Sci USA. 2009;106:11737–11741. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- 34.Dumont EC, Mark GP, Mader S, Williams JT. Self-administration enhances excitatory synaptic transmission in the bed nucleus of the stria terminalis. Nat Neurosci. 2005;8:413–414. doi: 10.1038/nn1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: Implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- 36.Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: Evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 37.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Deyama S, et al. Activation of the beta-adrenoceptor-protein kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J Neurosci. 2008;28:7728–7736. doi: 10.1523/JNEUROSCI.1480-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res. 1998;788:287–293. doi: 10.1016/s0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan GM, et al. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- 46.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 47.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 48.Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- 49.Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119:1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: Aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 51.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–114. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanson LS. Brain Maps: Structure of the Rat Brain. 3rd Ed. Amsterdam: Elsevier, Academic Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.