Abstract
The direct and indirect pathways of the basal ganglia have been proposed to oppositely regulate locomotion and differentially contribute to pathological behaviors. Analysis of the distinct contributions of each pathway to behavior has been a challenge, however, due to the difficulty of selectively investigating the neurons comprising the two pathways using conventional techniques. Here we present two mouse models in which the function of striatonigral or striatopallidal neurons is selectively disrupted due to cell type–specific deletion of the striatal signaling protein dopamine- and cAMP-regulated phosphoprotein Mr 32kDa (DARPP-32). Using these mice, we found that the loss of DARPP-32 in striatonigral neurons decreased basal and cocaine-induced locomotion and abolished dyskinetic behaviors in response to the Parkinson's disease drug L-DOPA. Conversely, the loss of DARPP-32 in striatopallidal neurons produced a robust increase in locomotor activity and a strongly reduced cataleptic response to the antipsychotic drug haloperidol. These findings provide insight into the selective contributions of the direct and indirect pathways to striatal motor behaviors.
Keywords: basal ganglia, DARPP-32, locomotor behavior, striatonigral, striatopallidal
The basal ganglia (BG) are subcortical structures that coordinate vital behaviors including movement, reward, and motivational processes (1). BG dysfunction is associated with several human diseases, including Parkinson's disease (PD), schizophrenia, and drug addiction. The striatum receives the majority of input into the BG, and projects to the output nuclei of the BG via two pathways that work together to modulate behavior: the D1 receptor (D1R)-expressing direct pathway, which projects to the substantia nigra pars reticulata (SNpr), and the D2 receptor (D2R)-expressing indirect pathway, which projects to the medial globus pallidus (GP) (2). Classical models of the BG propose that these two efferent pathways exert opposing effects on locomotor behavior (3, 4); namely, activation of the direct pathway increases locomotor activity, whereas the indirect pathway exerts a tonic inhibitory tone. Thus, an imbalance of these two pathways would disrupt coordinated movement, resulting in hypokinetic or hyperkinetic movement disorders (3). Direct confirmation of these hypotheses has been hindered by difficulty in selectively targeting direct and indirect pathway neurons with traditional surgical and pharmacological techniques.
An understanding of the differential contribution of the direct and indirect pathways to behavior is essential for generating more selective therapies for BG-related disorders. This would be especially valuable in the case of PD and schizophrenia, where motor complications can result from pharmacological treatment. For example, the positive symptoms of schizophrenia are effectively treated with typical antipsychotic drugs, such as haloperidol; however, these drugs are often associated with extrapyramidal side effects including catalepsy, manifested as extreme hypolocomotion and rigid immobility (5). Haloperidol-induced catalepsy has been proposed to result from high antagonist activity at striatal D2 receptors (6). The behavioral contribution of the direct versus indirect pathway to this behavior has not been determined, however. In addition, whereas the commonly prescribed PD drug L-DOPA is highly effective during the early stages of the disease, long-term use is associated with the development of debilitating abnormal movements known as L-DOPA–induced dyskinesia (7). Chronic L-DOPA treatment is associated with hypersensitivity of the direct pathway at the biochemical level (8, 9); however, the behavioral contributions of the two pathways to L-DOPA–induced dyskinesia have not been conclusively demonstrated.
To gain a better understanding of how the two output pathways regulate striatal motor responses, we selectively deleted the central signaling protein DARPP-32 in direct pathway striatonigral neurons or indirect pathway striatopallidal neurons. DARPP-32 is a key regulator of protein kinase and phosphatase signaling in both types of striatal neurons, and is required for the biochemical, electrophysiological, and behavioral responses to a variety of agents that target the striatum (10, 11). Thus, selective deletion of DARPP-32 in striatonigral or striatopallidal neurons is expected to impair the functional efficacy of the respective pathway. Using this functional knockdown approach, we examined the contribution of the two striatal projection neurons to several different types of striatal-based motor activities. We provide direct evidence for the classical theory of the basal ganglia showing that the direct and indirect pathway exert opposing influences on the control of locomotor activity. In addition, we demonstrate largely nonoverlapping contributions of the two pathways to several different forms of motor behaviors, including cocaine-induced locomotion, haloperidol-induced catalepsy, and L-DOPA–induced dyskinesia.
Results
Generation of Conditional DARPP-32 Knockout Mice.
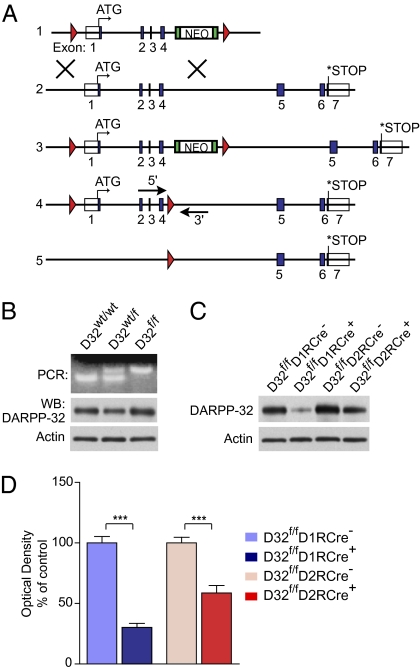
To conditionally delete DARPP-32, we generated mice carrying two loxP sites flanking the first four exons of the DARPP-32 gene (Fig. 1A). Expression of Cre recombinase results in deletion of exons 1-4 of the DARPP-32 gene including the start codon, generating a nonfunctional allele (Fig. 1A). The introduction of loxP sites into the mouse DARPP-32 gene had no effect on the expression of DARPP-32 protein in heterozygous (D32wt/f) or homozygous (D32f/f) floxed mice (Fig. 1B). D32f/f mice were bred to BAC mice expressing Cre recombinase under the control of the D1 or D2 receptor promoters. These mice were previously shown to express Cre selectively in striatonigral and striatopallidal neurons, respectively (12).
Fig. 1.
Generation of DARPP-32 conditional KO mice. (A) Schematic of the generation of the conditional DARPP-32 allele. Blue boxes represent exons; red triangles represent loxP sites. (1) Targeting construct; (2) endogenous DARPP-32 gene; (3) targeted DARPP-32 gene; (4) targeted DARPP-32 gene after in vitro removal of neomycin (NEO) cassette, with arrows showing the location of the genotyping primers; (5) DARPP-32 allele after Cre-mediated deletion of exons 1–4. (B) (Upper) Genotype PCR for the floxed DARPP-32 allele in WT (D32wt/wt), heterozygous (D32wt/f), and homozygous (D32f/f) floxed mice. (Lower) Western blots of striatal lysates from mice from the three genotypes showing equivalent DARPP-32 protein expression and β-actin loading control. (C) Homozygous floxed DARPP-32 mice were bred to D1R-Cre or D2R-Cre mice to conditionally delete DARPP-32. Western blots of striatal lysates from D32f/fD1RCre+ and D32f/fD2RCre+ conditional KO mice show a loss of DARPP-32 protein compared with Cre-negative littermates. (Lower) β-actin loading control. (D) Quantification of striatal DARPP-32 protein levels for mice of the indicated genotypes expressed as a percentage of Cre-negative littermate levels. n = 12 mice per genotype. Bar graphs show group mean ± SEM. ***P < 0.001, unpaired two-tailed t test.
We validated that Cre expression resulted in loss of striatal DARPP-32 protein by Western blot analysis. D32f/f mice expressing Cre under the control of the D1R promoter (D32f/fD1RCre+) showed a 69.8 ± 3.3% reduction in DARPP-32 compared with Cre-negative littermates (D32f/fD1RCre−), and, accordingly, mice expressing Cre from the D2R promoter (D32f/fD2RCre+) exhibited a 41.3 ± 6.1% reduction in DARPP-32 versus Cre- negative littermates (D32f/fD2RCre−) (Fig. 1 C and D). These values are consistent with previous reports demonstrating approximately equal numbers of striatonigral and striatopallidal neurons in the striatum (13–15).
Deletion of DARPP-32 in Striatonigral and Striatopallidal Neurons.
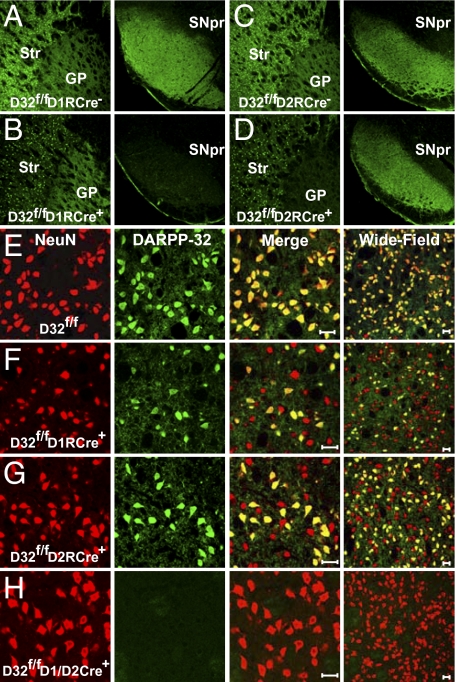
We verified that DARPP-32 was selectively deleted in striatonigral and striatopallidal projection neurons by analyzing axonal DARPP-32 expression in the SNpr, the primary target area for striatonigral neurons, and in the GP, the main target area for striatopallidal neurons. As expected, D32f/fD1RCre+ mice exhibited a selective loss of DARPP-32–positive terminals in the SNpr, but no change in immunolabeling in the GP (Fig. 2 A and B). Conversely, D32f/fD2RCre+ mice showed a selective loss of DARPP-32 in the GP, but preservation of DARPP-32–positive terminals in the SNpr (Fig. 2 C and D). Importantly, we observed no obvious morphological or anatomical changes in the BG as a result of the conditional deletion of DARPP-32.
Fig. 2.
Selective deletion of DARPP-32 in striatonigral or striatopallidal neurons. (A–D) Immunofluoresence performed on brain sections from D32f/fD1RCre− (A), D32f/fD1RCre+(B), D32f/fD2RCre− (C), and D32f/fD2RCre+ (D) mice using an antibody against DARPP-32. (Left) Somatic DARPP-32 staining in the striatum (Str) and axonal labeling in the GP. (Right) Corresponding axonal DARPP-32 labeling in the SNpr. (E–H) Immunofluoresence of striatal sections from D32f/f (E), D32f/fD1RCre+ (F), D32f/fD2RCre+ (G), and D32f/fD1/D2RCre+ mice (H) costained with antibodies against NeuN (red) and DARPP-32 (green). The third column shows a merged image, and the fourth column shows a merged image of a different brain section at lower magnification. (Scale bar: 20 μm.)
In the striatum, we found that approximately one half of the striatal neurons identified by NeuN staining exhibited a loss of DARPP-32 immunoreactivity in the D32f/fD1RCre+ and D32f/fD2RCre+ mice, confirming deletion of DARPP-32 from a subpopulation of striatal neurons (Fig. 2 E–G). To confirm that this loss of DARPP-32 occurred in separate striatal cell populations, D32f/fD1RCre+ and D32f/fD2RCre+ mice were crossed to generate double-knockout (KO) mice. Striatal sections from these mice showed a complete loss of DARPP-32 immunoreactivity indicating that Cre expression from the D1R and D2R promoters efficiently deleted DARPP-32 from all medium spiny neurons (MSNs) (Fig. 2H).
Synaptic Plasticity Is Disrupted in D32f/fD1RCre+ and D32f/fD2RCre+ Mice.
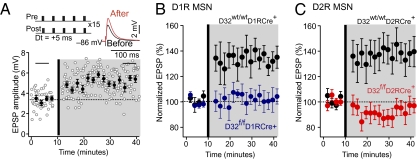
The striatum receives dense innervation from cortical glutamatergic afferents, and plasticity at these synapses is thought to underlie important striatal functions, such as sensorimotor and associative learning (16–19). To determine whether loss of DARPP-32 in striatonigral or striatopallidal neurons affected synaptic plasticity at corticostriatal synapses, we paired afferent stimulation with postsynaptic spikes in D1R and D2R MSNs to induce long-term potentiation (LTP) (Fig. 3A). To identify D1R and D2R MSNs, D32f/fD1RCre+ and D32f/fD2RCre+ mice were bred to mice expressing EGFP under the control of the D1R and D2R promoters, respectively (12). Immunostaining for DARPP-32 in these mice revealed a selective loss of DARPP-32 in the respective EGFP- labeled cell population (Fig. S1). Notably, the presence of D1R-EGPF– or D2R-EGFP–positive neurons with normal morphology and synaptic function in the conditional KO mice demonstrates that these neurons were still present after deletion of DARPP-32.
Fig. 3.
Corticostriatal LTP is disrupted in DARPP-32 conditional KO mice. (A) LTP was induced by a positive pairing timing protocol as shown at the upper left. Plots show EPSP amplitude as a function of time. The dashed line shows the average EPSP before induction. The induction was performed at the vertical bar. Filled circles show the averages of 12 trials (±SEM). The traces in the upper right show the average EPSP before induction (black trace) and after induction (red trace), from the time periods identified by the horizontal black bars on the plot. Pre, presynaptic; post, postsynaptic. (B and C) D32f/fD1RCre+ and D32f/fD2RCre+ mice were bred to D1R- and D2R-EGFP mice, respectively, to identify D1R and D2R expressing MSNs. EGFP-positive MSNs from D32wt/wtD1RCre+ (B) and D32wt/wtD2RCre+ (C) mice were used as controls and displayed significant LTP. LTP induction was disrupted in EGFP-positive MSNs from D32f/fD1RCre+ mice (B) and D32f/fD2RCre+ mice (C). (D1R MSNs: n = 5 per genotype, P < 0.05; D2R MSNs: n = 5–6 per genotype, P < 0.05, Mann–Whitney rank sum test).
In MSNs from EGFP-positive D32wt/wtD1RCre+ and D32wt/wtD2RCre+ mice, the spike timing protocol resulted in LTP of the synaptic response, as reported previously (16) (Fig. 3 B and C). Strikingly, LTP was absent in D1R MSNs from D32f/fD1RCre+ mice and D2R MSNs from D32f/fD2RCre+ mice (Fig. 3 B and C). These results demonstrate that cell type-specific deletion of DARPP-32 results in a functional deficit in striatonigral and striatopallidal neurons.
Basal and Cocaine-Induced Locomotor Activity Is Reduced in D32f/fD1RCre+ Mice and Enhanced in D32f/fD2RCre+ Mice.
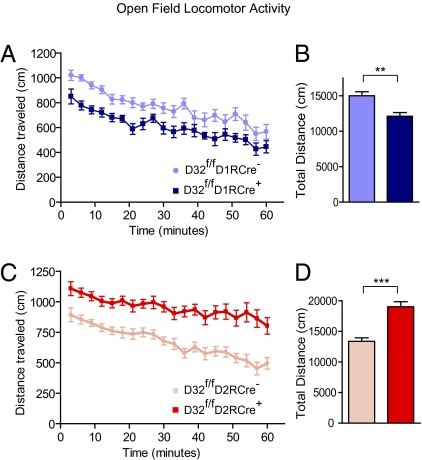
The classical model of the BG predicts that activation of the direct pathway will increase locomotion, whereas stimulation of the indirect pathway will inhibit locomotion (3). We examined the locomotor activity of the D32f/fD1RCre+ and D32f/fD2RCre+ mice in an open field to assess whether loss of DARPP-32 in striatonigral or striatopallidal neurons affects basal locomotion (Fig. 4 A–D). D32f/fD1RCre+ mice exhibited significantly reduced activity throughout the testing period compared with D32f/fD1RCre− littermates (total distance, 12,127 ± 514 cm vs. 15,006 ± 577 cm; P < 0.01) (Fig. 4B). Conversely, D32f/fD2RCre+ mice exhibited strongly increased locomotor activity compared with D32f/fD2RCre− littermates (19,028 ± 822 cm vs. 13,378 ± 557 cm; P < 0.001) (Fig. 4D). These changes in locomotor activity were not likely due to alterations in overall health of the mice, because there was no difference in the survival rate or average body weight of the conditional KO mice compared with littermates at 8 wk of age (Table S1).
Fig. 4.
Basal locomotor activity is altered in DARPP-32 conditional KO mice. Locomotor activity was measured by placing mice individually into an open-field chamber and recording the distance traveled. (A and C) Line graphs showing the distance traveled (in centimeters) in 3-min bins over a 60-min period by mice of the indicated genotypes. (B and D) Bar graphs showing the average total distance traveled (in centimeters) over the 60-min test period. n = 16–25 mice per genotype. Data are expressed as mean ± SEM. **P < 0.01; ***P < 0.001, unpaired two-tailed t test.
As an additional control, we tested the open-field activity of D1R-Cre– and D2R-Cre–expressing mice that were wild-type for the DARPP-32 gene. Neither the D1R-Cre–positive mice nor the D2R-Cre–positive mice showed significant changes in basal locomotor activity compared with Cre-negative littermates (Fig. S2), confirming that deletion of DARPP-32 was responsible for the opposing effects on locomotor activity. Taken together, these findings suggest that the loss of DARPP-32 in striatonigral neurons leads to reduced locomotor drive and decreased activity, while, the loss of DARPP-32 in striatopallidal neurons results in disinhibition of locomotor behavior and increased activity.
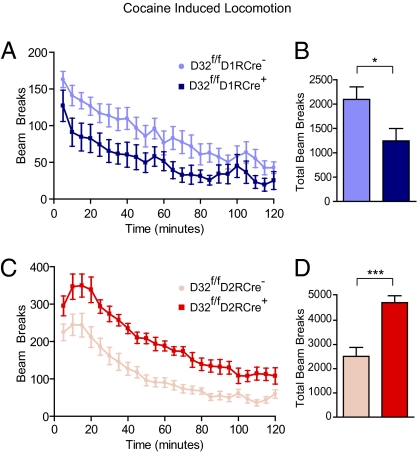
We next asked how the conditional KO mice would respond when acutely challenged with cocaine, a drug that potentiates dopamine signaling. After 3 d of habituation to the testing chambers, mice were injected with cocaine, and locomotor activity was monitored for 120 min (Fig. 5 A–D). D32f/fD1RCre+ mice exhibited a significant reduction in locomotor response to acute cocaine, as quantified by total beam breaks over the testing period (D32f/fD1RCre+: 1,243 ± 256 vs. D32f/fD1RCre−: 2,096 ± 257; P < 0.05) (Fig. 5B). In contrast, D32f/fD2RCre+ mice showed a robust locomotor response to cocaine that greatly exceeded than that of D32f/fD2RCre− littermates (4,727 ± 282 beam breaks vs. 2,515 ± 366; P < 0.001) (Fig. 5D). These findings demonstrate that DARPP-32 in striatonigral neurons, but not in striatopallidal neurons, is required for the full locomotor response to acute cocaine. These data allow us to refine earlier observations from global DARPP-32 KO mice, which lack DARPP-32 from all cell types. Such global DARPP-32 KO mice show reduced locomotor responses to acute cocaine (11), which we now can ascribe to DARPP-32 function in striatonigral neurons.
Fig. 5.
Acute locomotor response to cocaine. Mice were injected with 15 mg/kg of cocaine, and locomotor activity was monitored by photobeams for 120 min. (A and C) Line graphs showing the number of beam breaks plotted versus time for mice of the indicated genotypes. Points represent the number of beam breaks in a 5-min time interval. (B and D) Bar graphs showing the group means ± SEM of total beam breaks for the 120-min test period for mice from each genotype. n = 8–14 mice per genotype. *P < 0.05; ***P < 0.001, unpaired two-tailed t test.
Haloperidol-Induced Catalepsy Is Strongly Reduced in D32f/fD2RCre+ Mice.
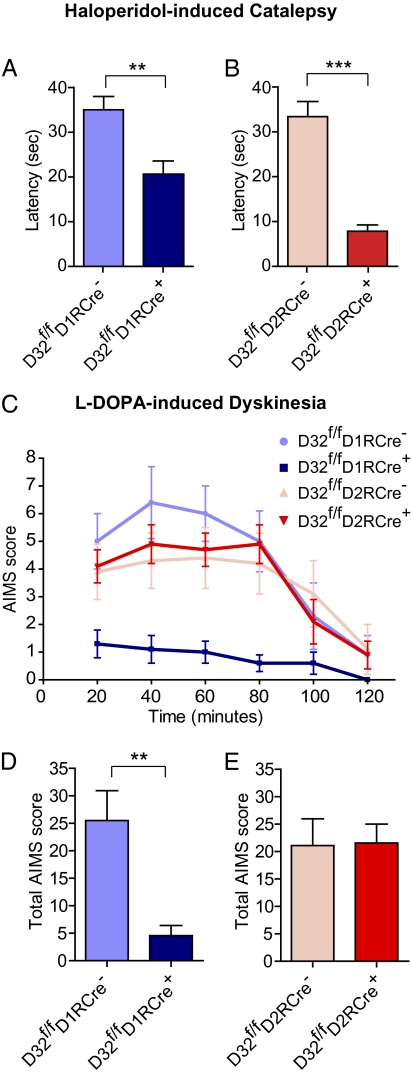
First-generation antipsychotic drugs such as haloperidol are often associated with extrapyramidal side effects, including catalepsy, which can limit their clinical utility (5). Global loss of DARPP-32 attenuates the ability of the antipsychotic drug raclopride to induce catalepsy (11); however, the cell type involved in this action is unknown. We found that selective deletion of DARPP-32 from striatonigral neurons had a small but significant effect in reducing catalepsy in response to haloperidol, measured as latency until first movement (D32f/fD1RCre+: 20.7 ± 2.9 s vs. D32f/fD1RCre−: 35.0 ± 3.0 s; P < 0.01) (Fig. 6A). In contrast, the loss of DARPP-32 from striatopallidal neurons had a more robust effect and almost completely abolished haloperidol-induced catalepsy despite the use of a relatively high concentration (1.5 mg/kg) of the drug (D32f/fD2RCre+: 7.8 ± 1.4 s vs. D32f/fD2RCre−: 33.4 ± 3.4 s; P < 0.001) (Fig. 6B). These findings suggest that DARPP-32 signaling predominantly in striatopallidal neurons is responsible for the cataleptic effect of haloperidol.
Fig. 6.
Differential contribution of striatonigral and striatopallidal neurons to haloperidol-induced catalepsy and L-DOPA–induced dyskinesia. (A and B) Mice were injected with 1.5 mg/kg haloperidol, and 60 min later catalepsy was assessed by measuring the latency until first movement in seconds. Bar graphs show group means ± SEM for mice from the indicated genotypes. n = 15–23 mice per genotype. **P < 0.01; ***P < 0.001, unpaired two-tailed t test. (C and E) Mice received unilateral striatal injections of 6-OHDA and were treated for 10 d with L-DOPA. (C) Time profile of combined axial, limb, orolingual, and locomotive AIMs for mice from the indicated genotypes scored every 20 min over a 120-min period after the last drug administration. (D and E) Bar graphs showing group means ± SEM of total AIMs scored during the observation period for mice of the indicated genotypes. n = 7–9 mice per genotype. **P < 0.01, unpaired two-tailed Student t test.
L-DOPA–Induced Dyskinesia Is Selectively Disrupted in D32f/fD1RCre+ Mice.
Despite its clinical utility in early PD, long-term treatment with L-DOPA results in dyskinesia in a significant number of patients. Biochemical alterations in striatonigral neurons are correlated with the expression of L-DOPA–induced dyskinesia, suggesting that hypersensitivity of the direct pathway might be responsible for this abnormal behavioral response (20, 21). To directly test this hypothesis, we examined whether a loss of DARPP-32 signaling in striatonigral neurons could prevent the development of dyskinesia.
Following a previously validated mouse model for L-DOPA–induced dyskinesia (22, 23), DARPP-32 conditional KO mice were injected unilaterally with 6-OHDA into the striatum to induce loss of dopaminergic innervation. After a 3-wk recovery period, the mice were treated for 10 d with L-DOPA and assessed on the 10th day for the expression of abnormal involuntary movements (AIMS) (Fig. 6 C–E). We observed a robust reduction in the total AIMS score in D32f/fD1RCre+ mice compared with D32f/fD1RCre− littermates (4.6 ± 1.8 vs. 25.5 ± 5.4; P < 0.01) (Fig. 6D), such that D32f/fD1RCre+ mice exhibited little to no dyskinetic behavior. In contrast, we found no difference in the total AIMS score between D32f/fD2RCre+ and D32f/fD2RCre− mice (Fig. 6E). These results provide direct evidence that selective disruption of DARPP-32 in striatonigral neurons, but not in striatopallidal neurons, can inhibit the expression of L-DOPA–induced dyskinesia.
Discussion
Since the proposal of the classical model of the BG (3), substantial efforts have been made to uncover the selective contributions of the direct and indirect pathways to behavior. However, progress has been limited by the inability to access these neuronal populations due to the fact that they are anatomically intermixed. Here we used a genetic approach to dissect the function of these pathways by conditionally deleting the key striatal phosphoprotein, DARPP-32, in striatonigral and striatopallidal pathway neurons, using the D1R and D2R promoters to drive cell type–specific Cre recombinase expression (12). DARPP-32 plays an essential role in integrating signals from a number of behaviorally important neurotransmitters and neuromodulators that target the striatum (24). Thus, a loss of this protein would be expected to result in loss of function in each neuronal population. Supporting this, we found that deletion of DARPP-32 abolishes a key functional property of MSNs, corticostriatal LTP, in either striatonigral or striatopallidal neurons.
Using DARPP-32 conditional KO mice, we examined the contribution of striatonigral and striatopallidal neurons to different types of striatal-based motor behaviors. We found that loss of DARPP-32 in striatonigral neurons resulted in reduced basal locomotor activity, consistent with the interpretation that activation of the direct pathway normally exerts a stimulatory effect on locomotion. Conversely, selective deletion of DARPP-32 in striatopallidal neurons resulted in increased locomotor activity, suggesting that the indirect pathway exerts a basal inhibitory tone on locomotion that is disrupted by a loss of DARPP-32. This is in agreement with a recent report showing that ablation of striatopallidal neurons using a toxin resulted in increased locomotor activity (25). Interestingly, previous studies using global DARPP-32 KO mice reported no change in basal locomotor behavior (11). Given our observations of increased locomotion in the D32f/fD2RCre+ mice and decreased locomotion in the D32f/fD1RCre+ mice, the net effect of loss of DARPP-32 in all striatal neurons would be no change in activity, as was observed in the global DARPP-32 KO mice. This suggests that balanced activity in the two pathways is essential for maintaining normal locomotor function.
Psychostimulant drugs of abuse, such as cocaine, induce hyperlocomotion, which is mediated in large part via the striatum (26). Studies using constitutive D1 receptor KO mice have suggested a primary role of D1R signaling in mediating the locomotor-stimulating effects of cocaine (27, 28), but some reports also implicate D2 receptor signaling (29, 30). Here we found that the locomotor response to acute cocaine was reduced after selective deletion of DARPP-32 in striatonigral neurons, indicating an essential role for the direct pathway in this behavior. In contrast, our data indicate that D2R-expressing striatopallidal neurons normally oppose the locomotor activation induced by cocaine.
The pharmacological treatment of BG disorders is often associated with unwanted motor complications. Discovering the locus of these side effects will be essential for facilitating the development of more selective therapies. A common complication of the treatment of schizophrenia with first-generation antipsychotic drugs is the expression of extrapyramidal side effects, which can include catalepsy and drug-induced parkinsonism (5). Haloperidol-induced catalepsy is thought to result from high antagonist activity at striatal D2 receptors, resulting in overactivity of the indirect pathway and decreased movement (6). Our data are consistent with this hypothesis given the significant decrease in catalepsy observed after the loss of DARPP-32 in striatopallidal neurons. Interestingly, we observed a small attenuation in haloperidol-induced catalepsy on deletion of DARPP-32 in striatonigral neurons, perhaps revealing a previously unappreciated role of the direct pathway in this effect.
The development of dyskinesias and “on-off” effects with long-term use of L-DOPA is a major challenge in the pharmacological treatment of PD. Recent studies in rodents have uncovered L-DOPA–induced biochemical alterations, such as increased G protein expression and selective activation of ERK1/2 and mammalian target of rapamycin (mTOR) in striatonigral neurons (8, 9). Moreover, pharmacological inhibition of ERK1/2 and mTOR signaling has been shown to attenuate L-DOPA–induced dyskinesia (8, 31). These results support the hypothesis that the hypersensitivity of signaling molecules in the direct pathway that develops over the course of PD progression results in excessive uncontrolled motor stimulation in response to L-DOPA (20, 21). In agreement with this and also with a recent study using KO mice with a global deletion of the D1 or D2 receptor (32), we found that the loss of DARPP-32 in striatonigral neurons, but not in striatopallidal neurons, prevents the development of L-DOPA–induced dyskinesia. This suggests that DARPP-32 might play a primary role in the generation of signaling abnormalities implicated in dyskinesia, possibly promoting the coordinated activation of ERK1/2 and mTOR in striatonigral neurons. In support of this idea, DARPP-32 has been shown to mediate the increase in ERK1/2 phosphorylation produced by cocaine and L-DOPA (31, 33; but also see ref. 34). Further studies are needed to elucidate this point and to examine the possible involvement of DARPP-32 in mTOR-mediated signaling.
A better understanding of the specific roles of the direct and indirect pathways in normal and pathological behaviors is essential. This could facilitate the development of therapies that specifically target the disrupted pathway while leaving the other intact or, alternatively, that modulate activity in both pathways to restore proper balance to the system. Here, using DARPP-32 conditional KO mice, we provide evidence that striatonigral and striatopallidal neurons differentially modulate motor behavior under normal and pathological conditions. These mice provide unique tools for further probing the selective, and in some cases opposite, contributions of these pathways to striatal-mediated behaviors.
Materials and Methods
DARPP-32 Conditional KO Mice.
Animal protocols were performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Rockefeller University's Institutional Animal Care and Use Committee. See SI Materials and Methods for details on the generation of the conditional DARPP-32 KO mice.
Immunofluoresence.
Brain sections from perfused mice were blocked in normal serum and incubated overnight with primary antibodies against NeuN (Chemicon), DARPP-32 (Novus), and/or GFP (provided by Dr. Myriam Heiman, Rockefeller University, NY, NY) followed by incubation with secondary antibodies conjugated to Alexa Fluor 488 (Invitrogen), Cy2, and/or Cy3 (Jackson ImmunoResearch).
Electrophysiology.
Parasagittal corticostriatal slices were obtained from 19- to 30-d-old D32wt/wt D1RGFP+Cre+ and D32wt/wtD2RGFP+Cre+ mice (controls) or D32f/f D1RGFP+Cre+ and D32f/fD2RGFP+Cre+ mice (conditional KOs). Experiments were performed in the dorsal striatum, and long-lasting corticostriatal synaptic plasticity was induced using a previously described protocol (16). See SI Materials and Methods for more details.
Open-Field Testing of Basal Locomotor Activity.
Mice were placed in a 38 cm (L) × 25 cm (W) × 17 cm (H) open-field chamber, in which locomotor activity was recorded for one hr using an overhead digital camera. Mice were tracked using EthoVision software (Noldus), which recorded the distance traveled in centimeter in 3-min bins.
Locomotor Response to Cocaine.
Male mice were injected i.p. with saline for the first 3 testing d to habituate them to the injections, handling, and testing conditions. On the fourth day, the mice were injected with cocaine (15 mg/kg) and placed into individual clean plastic mouse cages (18 cm × 28 cm). Movement was monitored by five photobeams in one dimension (Photobeam Activity System; San Diego Instruments) for 2 h in a dark room. The number of beam breaks was recorded in 5-min bins.
Haloperidol-Induced Catalepsy.
Mice were injected with haloperidol (1.5 mg/kg i.p.) and returned to their home cage for 60 min until testing. Each animal was individually placed so that its forelimbs rested on a wire bar (0.2 cm diameter) located 4.5 cm above the countertop with its hind limbs on the countertop. Once the mouse remained immobile after release, the time until the first removal of a front paw or sustained head movement was recorded. The test was repeated three times for each animal, and the average catalepsy time was calculated.
6-Hydroxydopamine Lesion.
At age 8–12 wk, mice were stereotaxically injected with 6-OHDA as described previously (31). Each mouse received two unilateral injections (2 μL each) of 6-OHDA (3 μg/μL) into the right striatum according to the following coordinates from Bregma (in mm): AP +1, ML −2.1, DV −3.2 and AP +0.3, ML −2.3, DV −3.2. The mice were allowed to recover for 3 wk before drug treatment and behavioral evaluation.
AIMS.
Mice were treated for 10 d with a single daily injection of L-DOPA (20 mg/kg) and benserazide hydrochloride (12 mg/kg). AIMS were assessed after the last injection on day 10 using a previously established scale consisting of locomotive, axial, limb, and orolingual components (22). See SI Materials and Methods for more details.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (MH074866 to P.G., DA10044 to P.G., DA08227 to E.J.N., and NS34696 to D.J.S.), the Simons Foundation (to P.G.), the Irma L. and Abram S. Croll Charitable Trust (to P.G.), the Swedish Research Council (13482, 14862, and 20715, to G.F.), the Swedish Brain Foundation (to G.F.), and the Wenner-Gren Foundations (to G.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009874107/-/DCSupplemental.
References
- 1.Graybiel AM. The basal ganglia: Learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Gerfen CR, et al. D1 and D2 dopamine receptor–regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 3.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 4.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 6.Farde L, et al. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine: Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri NB, Bernardi G. The “magic” of L-dopa: Why is it the gold standard Parkinson's disease therapy? Trends Pharmacol Sci. 2005;26:341–344. doi: 10.1016/j.tips.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Santini E, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–633. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- 9.Corvol JC, et al. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci. 2004;24:7007–7014. doi: 10.1523/JNEUROSCI.0676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svenningsson P, et al. DARPP-32: An integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 11.Fienberg AA, et al. DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 12.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR)-signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- 13.Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor–mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds JN, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Gerfen CR. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum animal model of Parkinson's disease. Neuroscientist. 2003;9:455–462. doi: 10.1177/1073858403255839. [DOI] [PubMed] [Google Scholar]
- 21.Santini E, Valjent E, Fisone G. Parkinson's disease: Levodopa-induced dyskinesia and signal transduction. FEBS J. 2008;275:1392–1399. doi: 10.1111/j.1742-4658.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- 22.Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA–induced dyskinesia in 6-hydroxydopamine lesioned mice: Relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Lundblad M, et al. Pharmacological validation of a mouse model of l-DOPA–induced dyskinesia. Exp Neurol. 2005;194:66–75. doi: 10.1016/j.expneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Fienberg AA, Greengard P. The DARPP-32 knockout mouse. Brain Res Brain Res Rev. 2000;31:313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 25.Durieux PF, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 26.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 27.Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- 28.Xu M, et al. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 29.Chausmer AL, Katz JL. The role of D2-like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology (Berl) 2001;155:69–77. doi: 10.1007/s002130000668. [DOI] [PubMed] [Google Scholar]
- 30.Liu XY, et al. Modulation of D2R–NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal–regulated protein kinase signaling in L-DOPA–induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darmopil S, Martin AB, Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine d1 but not d2 receptors inhibits l-dopa–induced dyskinesia and histone activation. Biol Psychiatry. 2009; 66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 33.Valjent E, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerfen CR, Paletzki R, Worley P. Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal–regulated kinase. J Neurosci. 2008;28:7113–7120. doi: 10.1523/JNEUROSCI.3952-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.