Abstract
Coping with intermittent social stress is an essential aspect of living in complex social environments. Coping tends to counteract the deleterious effects of stress and is thought to induce neuroadaptations in corticolimbic brain systems. Here we test this hypothesis in adult squirrel monkey males exposed to intermittent social separations and new pair formations. These manipulations simulate conditions that typically occur in male social associations because of competition for limited access to residency in mixed-sex groups. As evidence of coping, we previously confirmed that cortisol levels initially increase and then are restored to prestress levels within several days of each separation and new pair formation. Follow-up studies with exogenous cortisol further established that feedback regulation of the hypothalamic-pituitary-adrenal axis is not impaired. Now we report that exposure to intermittent social separations and new pair formations increased hippocampal neurogenesis in squirrel monkey males. Hippocampal neurogenesis in rodents contributes to spatial learning performance, and in monkeys we found that spatial learning was enhanced in conditions that increased hippocampal neurogenesis. Corresponding changes were discerned in the expression of genes involved in survival and integration of adult-born granule cells into hippocampal neural circuits. These findings support recent indications that stress coping stimulates hippocampal neurogenesis in adult rodents. Psychotherapies designed to promote stress coping potentially have similar effects in humans with major depression.
Keywords: gene expression, learning, neuroplasticity, resilience, hippocampus
Although stress generally inhibits proliferation of new cells (1, 2) and thereby decreases neurogenesis in the hippocampus (3–5), recent studies of rodents suggest that coping with mild intermittent stress increases adult neurogenesis in the hippocampal dentate gyrus (6). Psychotherapies designed to promote coping in humans with depressive disorders may potentially have similar effects (7, 8), but the neurogenic potential of stress coping has not been examined in human or nonhuman primates. Studies of primates are important for understanding neurogenesis in adult brain systems with established neural circuits and life spans that differ significantly from rodents (9).
For most adult human and nonhuman primates, coping with stressful psychosocial demands spontaneously occurs in the absence of therapeutic interventions or guidance (10, 11). Male squirrel monkeys, for example, travel alone, in pairs, or in all-male groups that undergo stressful changes in membership. Changing male social associations occur in free ranging naturalistic conditions because of competition for limited access to residency in mixed-sex groups (12, 13). As evidence of coping, we and other investigators determined that plasma levels of cortisol initially increase and then are restored to prestress levels during intermittent social separations and new pair formations (14–16). Follow-up studies with exogenous cortisol further established that feedback regulation of the hypothalamic-pituitary-adrenal (HPA) axis is not impaired (17). Moreover, we found that plasma levels of adrenocorticotropic hormone (ACTH) measured in undisturbed home cage conditions and after exposure to a nonsocial stressor (i.e., restraint) do not differ significantly in males exposed to prior intermittent separations and new pair formations compared with control monkeys housed continuously with a familiar male (17).
To further investigate this model of coping with intermittent social stress, 12 squirrel monkey males were randomized in adulthood to the following conditions. In one condition, six monkeys were housed continuously with a familiar adult male companion. In the other condition, six monkeys were housed for 3 wk alone and then 9 wk with an unfamiliar male. Six repeated sessions of living alone and in new pairs were conducted over the 18-mo study. Measures of learning and neurogenesis were examined for indications of adaptive changes in the adult hippocampus. Corresponding changes in gene expression were also examined to identify potential molecular mechanisms for future investigation.
Results
Learning performance was assessed during week 4 for each of the six new pair formation sessions. Each monkey was required to learn and remember the spatial orientation of a baited box to efficiently retrieve food treats by reaching toward the location that was baited on the preceding trial. Spatial learning is mediated, in part, by hippocampal neurogenesis in rodents (18–21), and learning assessed on the same test as that used here correlates with squirrel monkey hippocampal volumes measured in vivo by neuroimaging (Fig. S1).
Correct test response rates increased significantly over the series of six test sessions determined by repeated measures ANOVA [F(5,25) = 3.70, P = 0.012] for monkeys intermittently housed alone and in newly formed pairs. Simple practice effects are unlikely because the performance of control monkeys housed continuously with a familiar adult male companion did not differ over the same series of repeated test sessions [F(5,25) = 0.89, P = 0.504; Fig. 1]. The experimental design was not sufficiently powered to detect a housing condition-by-test session interaction (22), but significant differences were discerned on the last two test sessions as determined by t tests for housing condition effects [t(10) = 2.35, P = 0.041 for session 5; t(10) = 2.33, P = 0.042 for session 6]. Correct test response rates were, on average, 12% greater in monkeys housed alone and in new pairs compared with control monkeys during the final two sessions (Fig. 1).
Fig. 1.
Coping with intermittent social stress improves learning over repeated test sessions administered at 3-mo intervals. Correct test response rates are presented for monkeys intermittently housed alone and in new pairs (●) compared with control monkeys (○) housed continuously with a familiar companion (mean± SEM, n = 6 per condition, *P < 0.05).
To identify adult-born cells, 50 mg/kg BrdU was i.v. administered every other day for the first 10 d of the last new pair formation and at matched time points for control monkeys housed with a familiar companion. This schedule was selected to coincide with the time period when social stimulation in songbirds and environmental enrichment in rodents rescues adult-born neurons from apoptotic cell death (23–26). Twelve weeks later, cells double-labeled with BrdU and the mature neuron marker NeuN were counted in the granule cell layer of the right hippocampal dentate gyrus (Fig. 2A).
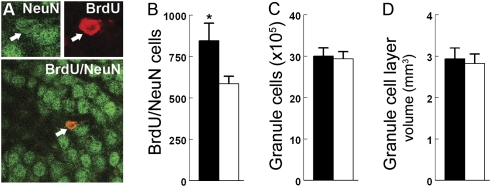
Fig. 2.
Coping with intermittent social stress enhances hippocampal neurogenesis with no net gain in granule cell counts or granule cell layer volumes. (A) Representative granule cell double-labeled with NeuN (green) and BrdU (red). Total number of (B) BrdU/NeuN-labeled granule cells, (C) thionin-stained granule cells, and (D) granule cell layer volumes from monkeys intermittently housed alone and in new pairs (solid bars) compared with control monkeys (open bars) housed continuously with a familiar companion (mean ± SEM, n = 6 per condition, *P = 0.047). All measures were collected 12 wk after the last of five i.v. injections of BrdU to provide ample time for maturation of adult-born neurons.
The total number of BrdU-labeled cells in the dentate gyrus was 45% greater in monkeys housed alone and in new pairs (911 ± 113; mean ± SEM) compared with control monkeys (629 ± 48) housed with a familiar companion [t(10) = 2.29, P = 0.045]. Most of the BrdU-labeled cells (89–96%) were NeuN double-labeled 12 wk after BrdU administration, and the percentage of BrdU-labeled cells that coexpressed NeuN did not differ between housing conditions (P = 0.704). Consequently, neurogenesis assessed in terms of the number of cells double-labeled with BrdU and NeuN in the hippocampal dentate gyrus was 44% greater in monkeys housed alone and in new pairs compared with control monkeys [t(10) = 2.27, P = 0.047] as depicted in Fig. 2B. Similar housing condition effects were not discerned for total granule cell (thionin-stained) counts or granule cell layer volumes (Fig. 2 C and D).
Microarray analysis of gene expression in the left hippocampus identified five genes that correlated with correct test response rates for learning on the last two sessions (IGF1, r = 0.66, P = 0.028; GRM7, r = 0.74, P = 0.010; ARL6IP5, r = 0.71, P = 0.013; PHLPP, r = −0.66, P = 0.028; PRG-3, r = −0.79, P = 0.004) and with BrdU/NeuN cell counts in the right hippocampus (IGF1, r = 0.64, P = 0.035; GRM7, r = 0.63, P = 0.038; ARL6IP5, r = 0.69, P = 0.020; PHLPP, r = −0.71, P = 0.014; PRG-3, r = −0.63, P = 0.037). In situ hybridization histochemistry confirmed that all five genes are expressed in the neurogenic region of squirrel monkey hippocampus, i.e., subgranular zone and granule cell layer of the dentate gyrus (Fig. 3). An additional 176 genes correlated with learning (P < 0.05) but not neurogenesis (Dataset S1), and 46 genes correlated with neurogenesis (P < 0.05) but not learning performance (Dataset S2). The microarray data for all genes detected in the squirrel monkey hippocampus are freely available for public use at http://www.pritzkerneuropsych.org/data/data.htm.
Fig. 3.
Localization of mRNA for gene correlates of learning and hippocampal neurogenesis. Radiographs of mRNA from the left cerebral hemisphere (Upper) and corresponding hippocampus (Lower) are presented for five genes identified by microarray. Abbreviations: DG, dentate gyrus; H, hilus; CA1, hippocampal CA1 field; CA2/3, hippocampal CA2 and CA3 fields; ARL6IP5, ADP ribosylation-like factor 6 interacting protein 5; IGF1, insulin-like growth factor 1; PRG-3, plasticity-related gene 3; PHLPP, PH domain and leucine-rich repeat protein phosphatase; GRM7, metabotropic glutamate receptor 7.
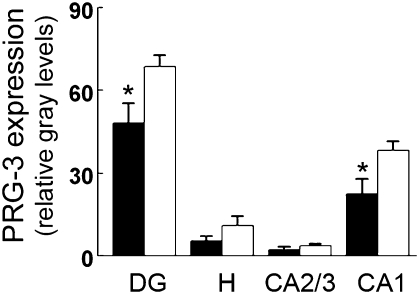
Each of the five gene correlates of learning and neurogenesis were differentially expressed in monkeys housed alone and in new pairs compared with control monkeys based on univariate (P < 0.05) and multivariate analysis of variance (Wilks’ lambda = 0.057, F(5,5)=16.55, P = 0.004). Three genes were up-regulated (IGF1, GRM7, ARL6IP5) and two genes were down-regulated (PHLPP, PRG-3) in monkeys housed alone and new pairs compared with control monkeys housed continuously with a familiar companion. Based on observed subregional differences in PRG-3 gene expression (Fig. 3), we proceeded to verify the microarray finding of PRG-3 down-regulation using in situ hybridization histochemistry to test for housing condition effects within hippocampal subregions. As depicted in Fig. 4, stress coping down-regulated PRG-3 in the dentate gyrus (t(9)=2.33, P = 0.044) and hippocampal CA1 (t(9)=2.38, P = 0.041) but not hippocampal CA2/3 or the hilus. Adequate amounts of hippocampal tissue were not available for further verification of PRG-3 down-regulation using quantitative PCR (qPCR) nor to validate the other four genes identified by microarray. Nevertheless, all five genes have been implicated in learning and hippocampal neurogenesis in rodents as described below.
Fig. 4.
Coping with intermittent social stress down-regulates PRG-3 expression in subregions of hippocampus. Gray level measures of PRG-3 gene expression in monkeys intermittently housed alone and in new pairs (solid bars) compared with control monkeys (open bars) housed continuously with a familiar companion (mean ± SEM, n = 5–6 per condition, *P < 0.05). Abbreviations: DG, dentate gyrus; H, hilus; CA1, hippocampal CA1 field; CA2/3, hippocampal CA2 and CA3 fields.
Discussion
This study tested the hypothesis that coping with intermittent social separations and new pair formations induces neuroadaptations in adult squirrel monkey hippocampus. Previously, we confirmed that cortisol levels initially increase and then are restored to prestress levels within several days of each intermittent social separation and new pair formation (14). Follow-up studies further established that HPA axis reactivity and regulation are not impaired (17). Here we showed that coping with intermittent social separations and new pair formations increased adult hippocampal neurogenesis in squirrel monkey males. Spatial learning was also enhanced and corresponding changes were discerned in the expression of genes involved in hippocampal neurogenesis and learned aspects of behavior. These results and recent studies of rodents (6) support the suggestion that psychotherapies designed to promote stress coping may stimulate hippocampal neurogenesis in humans with major depression (7).
Whether stress coping specifically increases cell proliferation and/or new cell survival is not yet known, but the increase we found in hippocampal neurogenesis occurred with no net gain in total granule cell counts or granule cell layer volumes. These findings are consistent with evidence that in songbirds adult-born neurons replace preexisting cells (25). This possibility must be considered with caution, however, because only a small proportion of neurons in squirrel monkey dentate gyrus was labeled with BrdU. In rodents, genetic cell fating mapping studies that follow the majority of adult-born neurons (19, 27) support observations that neurogenesis adds to a net increase in granule cell numbers and granule cell layer volumes (28, 29). Further studies are needed to determine whether adult neurogenesis results in replacement or addition of neurons in primate dentate gyrus.
Mouse models designed to selectively diminish hippocampal neurogenesis have established a casual role for adult-born neurons in spatial maze learning (18–21) but not novelty exploration (20) nor contextual fear conditioning (18, 20, 21). Our findings suggest that hippocampal neurogenesis may also play role in cognitive processes that rely on positional information and not maze learning per se. In humans, hippocampal lesions have greater effects on visuospatial tests of positional information compared with spatial maze learning (30). Although improvements in learning emerged in monkeys before we administered BrdU to label adult-born cells, this does not preclude the possibility that neurogenesis was initially enhanced earlier during our study. Whether neurogenesis facilitates learning or learning promotes hippocampal neurogenesis cannot be determined from the correlative evidence that we present here for monkeys.
In addition to learning, hippocampal neurogenesis has been associated with emotion regulation in studies of major depression (7, 31, 32). Antidepressant-induced hippocampal neurogenesis occurs in bonnet monkeys (33) and antidepressant treatments in rodents appear to enhance emotion regulation by increasing neurogenesis in the ventral hippocampal dentate gyrus (1, 7). The neurogenic effect we observed in squirrel monkeys likewise tended to be greater in the anterior (ventral in rodents) compared with posterior (dorsal in rodents) hippocampal dentate gyrus, but this difference in our sample of six subjects in each housing condition was not statistically significant.
The number of BrdU/NeuN-labeled cells in squirrel monkey dentate gyrus was, on average, twofold greater than that reported for individually-housed cynomolgus monkeys examined 12 wk after a single i.v. injection of BrdU (34). This disparity may reflect discrepancies between the BrdU treatment protocols (i.e., five 50-mg/kg injections versus one 100-mg/kg injection), environmental enrichment effects (e.g., social vs. nonsocial housing) or interspecies differences in cell proliferation and survival. The percentage of BrdU-labeled cells that coexpressed NeuN in squirrel monkey dentate gyrus 12 wk after administration of BrdU was likewise greater than that reported in previous studies of nonhuman primates (33–35) but was similar to findings from rodents (36, 37).
In songbirds and rodents, adult neurogenesis is mediated, in part, by the expression of genes involved in proliferation, survival, and integration of neurons into functional circuits (38–40). Several genes identified in earlier studies are supported to various degrees by our microarray results for squirrel monkeys. Brain-derived neurotrophic factor (BDNF) gene expression correlated with learning but not neurogenesis, whereas growth factor receptor-bound protein 2 (GRB2) gene expression correlated with neurogenesis but not learning performance. Previously, these genes have been associated with learning and hippocampal neurogenesis in rodents (39–41). Of the five genes in monkeys that correlated both with learning and hippocampal neurogenesis, the most well-known encodes IGF1, which acts locally to increase neurogenesis through PI3K/Akt signaling (42). Two other genes that we identified regulate glutamate homeostasis and glutamate modulates hippocampal neurogenesis in rodents (43). Up-regulation of ARL6IP5 increases extracellular glutamate levels by reducing glutamate transport (44), whereas up-regulation of GRM7 prevents excitotoxic accumulation by feedback inhibition of glutamate release (45). The fourth gene correlate of learning and neurogenesis in monkeys encodes PHLPP, a negative modulator of PI3K/Akt signaling (46) and CREB-mediated transcription (47). Down-regulation of PHLPP enhances new cell survival (46) and mediates memory formation (47). The fifth gene encodes PRG-3, a new member of the brain-specific family of lysophospholipid-modifying proteins (LLPs) (48). Down-regulation of LLPs increases lysophosphatidic acid signaling (48), which, in turn, promotes hippocampal neurogenesis in adult mice (49).
In summary, results from this study of monkeys link adult hippocampal neurogenesis, learning, and the expression of genes that appear to be regulated by coping with stress. These findings are consistent with indications that stress coping stimulates hippocampal neurogenesis in adult rats (6). Follow-up studies aimed at understanding the molecular and cellular neurobiology of coping in animal models may provide mechanistic insights for the development of therapeutics that mimic or enhance adaptive stress coping in humans with major depression.
Materials and Methods
Experimental Design.
Twelve laboratory-born male squirrel monkeys (Saimiri sciureus) were randomized in adulthood (i.e., 7.2–10.6 y) to living continuously with a familiar adult male companion (n = 6) or living alone and in new pairs (n = 6) as described above. In both conditions, monkeys were housed in wire mesh cages (1.8 × 1.2 × 1.8 m) in climate controlled rooms at approximately 26 °C on 12:12 h light/dark cycles with lights on at 0700 hours. Cages were cleaned daily and monkeys were provisioned with fresh drinking water, commercial monkey chow, and fresh fruit and vegetable supplements. Various toys and swinging perches were provided to all monkeys for environmental enrichment. All procedures were conducted in accordance with the National Institutes of Health Guide and were approved by the Institutional Animal Care and Use Committee.
Spatial Learning.
Initially, each monkey was trained to retrieve marshmallow food treats from a clear Plexiglas box (8 × 8 × 8 cm) constructed with one open side. Thereafter, each monkey was individually tested at 3-mo intervals for a total of six repeated sessions. Test sessions occurred during week 4 of each new pair formation and at simultaneous time points for control monkeys housed continuously with a familiar companion. During each test session, four five-trial blocks were administered daily over 4 consecutive d. Each trial was terminated when the marshmallow was retrieved, with a 20-s delay between trials in each block, and a 30-min delay between each of the four daily five-trial blocks.
During the first two daily five-trial blocks, the box opening was oriented in the same direction, i.e., toward the left on even-numbered days and toward the right on odd-numbered days. Each monkey was required to learn and remember the initial box opening orientation. During the second two daily five-trial blocks, the box opening was oriented opposite to that presented for the first two blocks. Each monkey was now required to inhibit the previously rewarded reaching response and learn the new orientation.
After the completion of testing each day, all monkeys were returned to the home cage and fed unrestricted amounts of monkey chow with fresh fruit and vegetable supplements. The following morning, 1 h before testing, all uneaten food was removed. Each monkey was tested at the same time of day between 0900 and 1200 hours.
The marshmallow treat was always retrieved (latencies = 2 ± 2 s, mean ± SD) but retrievals were often preceded by a reach attempt error toward the incorrect side. The number of correct first reach responses performed during each of the two test types (pre- vs. postreversal) was analyzed for improvements across test sessions (1–6) using repeated measures ANOVA for each housing condition. For control monkeys housed continuously with a familiar companion, neither the test session main effect nor the session × test type interaction was statistically significant. On the other hand, a significant test session main effect was discerned for monkeys housed alone and in new pairs [F(5,25) = 3.70, P = 0.012], but the session × test type interaction was not significant. These results indicate that improvements in performance did not differ between test types. The data were therefore reexamined without test type included in the analysis and the findings are presented in Results. Housing condition differences depicted in Fig. 1 were assessed using Student t tests.
Brain Tissue Collection.
Brain tissue for BrdU immunohistochemistry, microarray analysis, and in situ hybridization histochemistry were collected between 0800 and 1000 hours while all monkeys were housed in pairs. Monkeys were anesthetized with an intramuscular injection of 10 mg/kg ketamine hydrochloride, followed by euthanasia with an i.v. overdose of 120 mg/kg pentobarbital. Craniotomies were performed, brains were removed, and the left and right cerebral hemispheres were separated by a midsagittal incision.
Left hemispheres were cut coronally into blocks using a custom-designed acrylic brain matrix. Tissue blocks were frozen in isopentane on dry ice at −40 °C for storage at −80 °C. Blocks were then cut coronally into 20-μm sections, thaw-mounted on Superfrost Plus glass slides, and stored at −80 °C for subsequent microarray analysis and in situ hybridization histochemistry.
From the right hemispheres, the hippocampus was isolated and immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB) (pH 7.4) at 4 °C for 30 h. Hippocampal tissue was then equilibrated in 30% sucrose in 0.1 M PB at 4 °C and sectioned perpendicular to the septotemporal axis with a sliding microtome equipped with a freezing stage and set at 30 μm. Starting at a random section from the septal pole, a 1-in-12 series of sections was processed for thionin staining. All other sections were stored in 30% ethylene glycol and 25% glycerol in 50 mM PB at −20 °C for subsequent BrdU immunohistochemistry.
BrdU Immunohistochemistry.
Starting at a random section from the septal pole, a 1-in-6 series of fixed tissue sections was processed from each monkey for BrdU and NeuN fluorescent double-labeling. Sections were rinsed in 0.1 M PB and exposed to 50% formamide in 2× SSC for 2 h at 65 °C. After rinsing in 2× SSC, sections were exposed to 2 N HCl for 30 min at 37 °C and then 0.1 M boric acid (pH 8.5) for 10 min. After rinsing in Tris buffered saline (TBS) (pH 7.4), sections were exposed to 1% H2O2 in 0.1 M TBS for 2 h. After several additional rinses, sections were placed in a blocking solution consisting of 3% goat serum, 2% BSA, and 0.3% triton in 0.05 M TBS for 2 h. Sections were then incubated in rat anti-BrdU serum (1:1,000; Accurate Chemical) and mouse anti-NeuN serum (1:1,000; Chemicon) in 1% goat serum, 0.2% BSA, and 0.3% triton in 0.05 M TBS at 4 °C for 14 h. After rinsing in 0.1 M TBS, sections were incubated in goat anti-mouse serum conjugated to Alexa 488 (10 μg/mL; Molecular Probes) and goat anti-rat serum conjugated to Alexa 594 (10 μg/mL; Molecular Probes) in 2% BSA and 0.3% triton in 0.05 M TBS for 3 h. After rinses in 0.1 M TBS, sections were mounted on slides, coverslipped with Vectashield (Vector Laboratories), and coded for counting without knowledge of the treatment conditions.
Cell Counts.
All BrdU- and BrdU/NeuN-labeled cells in the granule cell layer of the dentate gyrus were counted using a 63× oil objective on a Zeiss LSM5 Pascal confocal microscope. Every BrdU/NeuN-labeled cell was visualized in serial 1-μm optical sections in the z-plane to confirm BrdU and NeuN colocalization. To ensure that NeuN staining was not due to autofluorescence, all BrdU-labeled cells were also evaluated using 330-nm excitation. The few cells showing blue (450 nm) autofluorescence were not scored as NeuN positive. Only cell caps not cut at the upper surface of the section were counted to control for oversampling.
To determine the total number of granule cells and the granule cell layer volume for each dentate gyrus, the 1-in-12 series of thionin-stained sections were analyzed using a microscope equipped with a Lucivid and StereoInvestigator software (MicroBrightField). Contours were drawn around the granule cell layer and the volume for each dentate gyrus was estimated using the Cavalieri method. A modified optical dissector method was then used to estimate the total number of granule cells in each dentate gyrus using a 100× objective. The counting frame was set at 10 × 10 μm and the counting grid was 200 × 200 μm. The dissector height was the total section thickness, and only cell caps not cut at the upper surface of the section were counted to control for over-sampling. Housing condition differences were evaluated using t tests.
Microarray Analysis.
Starting at a random section from the septal pole, a 1-in-10 series of fresh frozen tissue sections from each monkey was selected for hippocampal dissections at 0 °C under a stereo zoom microscope. All hippocampal tissue on each section was collected into RNase-free tubes and stored at −80 °C. Tissue was homogenized with a motorized pellet pestle and total RNA from each sample was extracted using TRIzol Reagent (Invitrogen). Quantification was carried out by spectrophotometric analysis and gel electrophoresis was used to verify the integrity of each RNA sample. Samples were then amplified with the two-cycle method, reverse transcribed into cDNA, labeled with biotinylated nucleotides, and hybridized to Affymetrix HG U133A 2.0 microarrays.
All but 1 of the 12 samples were of high and comparable quality as determined by the ratio of 28S to 18S ribosomal RNAs and signal intensity ratios from probes for the 3′ and 5′ ends of GAPDH and β-actin transcripts used as quality controls on the microarrays. The single poor-quality sample from one control monkey was excluded from all further analysis.
Scanned microarray data were initially processed by MicroArray Suite 5 (MAS5; Affymetrix) to convert raw image files (.DAT) into probe signal files (.CEL), which were subsequently analyzed using the dChip perfect match-only protocol (50). Gene expression values determined by dChip were log2 transformed, imported into R software (http://www.r-project.org), normalized using quantile normalization, median-centered to correct for technical batch-related variation, and interpreted using a custom chip description file (U133Av2_HS_UG_5) described elsewhere (51) and freely available at http://brainarray.mhri.med.umich.edu/Brainarray/Database/CustomCDF/. Of the 11,839 gene transcripts screened by microarray, 5,627 were called present by MAS5 software on one or more of the microarrays. The data for all 5,627 detected gene transcripts are freely available for public use at http://www.pritzkerneuropsych.org/data/data.htm.
In Situ Hybridization Histochemistry.
Riboprobes for in situ hybridization histochemistry were synthesized from templates cloned from squirrel monkey cDNA using primers targeted against known sequences for macaque monkeys (Macaca). IGF1 was measured using a 202-base antisense riboprobe directed against squirrel monkey IGF1 (GenBank accession no. GQ504196). PRG-3 was measured with a 575-base riboprobe directed against squirrel monkey PRG-3 (GenBank accession no. GQ845038). PHLPP was measured with a 465-base riboprobe directed against squirrel monkey PHLPP (GenBank accession no. GQ845039). GRM7 was measured with a 313-base riboprobe directed against squirrel monkey GRM7 (GenBank accession no. GQ845041). ARL6IP5 was measured with a 301-base riboprobe directed against squirrel monkey ARL6IP5 (GenBank accession no. GQ845040). Riboprobe synthesis and in situ hybridization histochemistry were carried out as previously described (52). Hybridized tissue sections were dehydrated, air-dried, and exposed to Biomax MR film for 3 d with [14C]-radiolabeled standards to assure that specific hybridization signal did not exceed the linear range of the film. No signal was generated from sense-strand controls for each of the five genes of interest. To verify PRG-3 down-regulation, we manually traced the dentate gyrus, hilus, CA1, and CA2/3 regions on digital images of each hybridized tissue section. Corresponding thionin-stained sections and the squirrel monkey brain atlas (53) were used to identify hippocampal subregions. Gray level measures were extracted from each subregion, background corrected, and averaged across four tissue sections per monkey for subsequent assessment of housing condition differences using t tests.
Supplementary Material
Acknowledgments
We thank Adrian Karssen and Jun Z. Li for invaluable assistance with the microarray analysis. This work was supported by Public Health Service Grants MH47573, NS40276, and RR17188 and the Pritzker Neuropsychiatric Disorders Research Fund.
Footnotes
Conflict of interest statement: A.F.S. consults to and has equity in BrainCells, Inc. All other authors declare no potential conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914568107/-/DCSupplemental.
References
- 1.Jayatissa MN, Bisgaard C, Tingström A, Papp M, Wiborg O. Hippocampal cytogenesis correlates to escitalopram-mediated recovery in a chronic mild stress rat model of depression. Neuropsychopharmacology. 2006;31:2395–2404. doi: 10.1038/sj.npp.1301041. [DOI] [PubMed] [Google Scholar]
- 2.Czéh B, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coe CL, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 4.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 5.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 6.Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.130. 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 8.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakic P. Neurogenesis in adult primates. Prog Brain Res. 2002;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- 10.Bonanno GA. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol. 2004;59:20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- 11.Epstein S. In: Stress Reduction and Prevention. Meichenbaum D, Jaremko ME, editors. New York: Plenum Press; 1983. pp. 39–66. [Google Scholar]
- 12.Boinski S, Kauffman L, Ehmke E, Schet S, Vreedzaam A. Dispersal patterns among three species of squirrel monkeys (Saimiri oerstedii, S. boliviensis, and S. sciureus): I. Divergent costs and benefits. Behaviour. 2005;142:525–632. [Google Scholar]
- 13.Baldwin JD. In: Agression and Peacefulness in Humans and Other Primates. Silverberg J, Gray JP, editors. Oxford: Oxford University Press; 1992. pp. 72–99. [Google Scholar]
- 14.Karssen AM, et al. Stress-induced changes in primate prefrontal profiles of gene expression. Mol Psychiatry. 2007;12:1089–1102. doi: 10.1038/sj.mp.4002095. [DOI] [PubMed] [Google Scholar]
- 15.Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiol Behav. 1982;29:1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- 16.Friedman EM, Coe CL, Ershler WB. Time-dependent effects of peer separation on lymphocyte proliferation responses in juvenile squirrel monkeys. Dev Psychobiol. 1991;24:159–173. doi: 10.1002/dev.420240303. [DOI] [PubMed] [Google Scholar]
- 17.Lyons DM, Parker KJ, Zeitzer JM, Buckmaster CL, Schatzberg AF. Preliminary evidence that hippocampal volumes in monkeys predict stress levels of adrenocorticotropic hormone. Biol Psychiatry. 2007;62:1171–1174. doi: 10.1016/j.biopsych.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 20.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 22.Overall JE, Doyle SR. Estimating sample sizes for repeated measurement designs. Control Clin Trials. 1994;15:100–123. doi: 10.1016/0197-2456(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 23.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 24.Kempermann G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- 26.Shors TJ. From stem cells to grandmother cells: How neurogenesis relates to learning and memory. Cell Stem Cell. 2008;3:253–258. doi: 10.1016/j.stem.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Ninkovic J, Mori T, Götz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 29.Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- 30.Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: A meta-analysis on the effects of hippocampal lesions. Brain Res Brain Res Rev. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 32.Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ngwenya LB, Peters A, Rosene DL. Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. J Comp Neurol. 2006;498:204–216. doi: 10.1002/cne.21045. [DOI] [PubMed] [Google Scholar]
- 36.Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 37.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 38.Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci USA. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 40.Newton SS, et al. Gene profile of electroconvulsive seizures: Induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23:10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newton SS, Collier EF, Bennett AH, Russell DS, Duman RS. Regulation of growth factor receptor bound 2 by electroconvulsive seizure. Brain Res Mol Brain Res. 2004;129:185–188. doi: 10.1016/j.molbrainres.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 42.Bondy CA, Cheng CM. Signaling by insulin-like growth factor 1 in brain. Eur J Pharmacol. 2004;490:25–31. doi: 10.1016/j.ejphar.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 43.Schlett K. Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem. 2006;6:949–960. doi: 10.2174/156802606777323665. [DOI] [PubMed] [Google Scholar]
- 44.Lin CI, et al. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3-18. Nature. 2001;410:84–88. doi: 10.1038/35065084. [DOI] [PubMed] [Google Scholar]
- 45.Lavreysen H, Dautzenberg FM. Therapeutic potential of group III metabotropic glutamate receptors. Curr Med Chem. 2008;15:671–684. doi: 10.2174/092986708783885246. [DOI] [PubMed] [Google Scholar]
- 46.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bräuer AU, Nitsch R. Plasticity-related genes (PRGs/LRPs): A brain-specific class of lysophospholipid-modifying proteins. Biochim Biophys Acta. 2008;1781:595–600. doi: 10.1016/j.bbalip.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Matas-Rico E, et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci. 2008;39:342–355. doi: 10.1016/j.mcn.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karssen AM, et al. Application of microarray technology in primate behavioral neuroscience research. Methods. 2006;38:227–234. doi: 10.1016/j.ymeth.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gergen JA, MacLean PD. A Stereotaxic Atlas of the Squirrel Monkey's Brain (Saimiri sciureus) Bethesda: Public Health Service; 1962. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.