Abstract
The Rem, Rem2, Rad, and Gem/Kir (RGK) family of small GTP-binding proteins potently inhibits high voltage-activated (HVA) Ca2+ channels, providing a powerful means of modulating neural, endocrine, and muscle functions. The molecular mechanisms of this inhibition are controversial and remain largely unclear. RGK proteins associate directly with Ca2+ channel β subunits (Cavβ), and this interaction is widely thought to be essential for their inhibitory action. In this study, we investigate the molecular underpinnings of Gem inhibition of P/Q-type Ca2+ channels. We find that a purified Gem protein markedly and acutely suppresses P/Q channel activity in inside-out membrane patches, that this action requires Cavβ but not the Gem/Cavβ interaction, and that Gem coimmunoprecipitates with the P/Q channel α1 subunit (Cavα1) in a Cavβ-independent manner. By constructing chimeras between P/Q channels and Gem-insensitive low voltage-activated T-type channels, we identify a region encompassing transmembrane segments S1, S2, and S3 in the second homologous repeat of Cavα1 critical for Gem inhibition. Exchanging this region between P/Q and T channel Cavα1 abolishes Gem inhibition of P/Q channels and confers Cavβ-dependent Gem inhibition to a chimeric T channel that also carries the P/Q I-II loop (a cytoplasmic region of Cavα1 that binds Cavβ). Our results challenge the prevailing view regarding the role of Cavβ in RGK inhibition of high voltage-activated Ca2+ channels and prompt a paradigm in which Gem directly binds and inhibits Cavβ-primed Cavα1 on the plasma membrane.
Keywords: β subunit; electrophysiology; modulation; Rem, Rem2, Rad, and Gem/Kir proteins; T-type Ca2+ channels
High voltage-activated (HVA) Ca2+ channels, which include L-, N-, P/Q-, and R-type channels, are essential for diverse biological processes, ranging from gene transcription and neurotransmission to hormone secretion and heart beat. They contain a pore-forming α1 subunit (Cavα1), a membrane anchored α2δ subunit, and a cytosolic β subunit (Cavβ; review in ref. 1). Cavα1 has four homologous repeats, each consisting of six transmembrane segments (S1–S6) and a pore-forming loop. Cavα1 is the principal subunit of HVA Ca2+ channels and is the main determinant of the unique pharmacological and biophysical properties of each channel type. Cavβ is an auxiliary subunit that is indispensible for transporting Cavα1 to the plasma membrane and fine-tuning channel gating (reviews in refs. 2 and 3). Both effects depend critically on the binding of Cavβ to the α interacting domain (AID) in the cytoplasmic loop (referred to as the I–II loop) connecting the first two homologous repeats of Cavα1 (2–11). Gating regulation by Cavβ also needs a continuous α-helix between the AID and the S6 segment of the first repeat (IS6) of Cavα1 (3, 8, 9, 12).
The activity of HVA Ca2+ channels is regulated by numerous signaling pathways and interacting proteins with profound functional consequences (review in ref. 1). Recently, members of the Rem, Rem2, Rad, and Gem/Kir (RGK) family of Ras-related monomeric small GTP-binding proteins, which are known to regulate cytoskeleton remodeling through the Rho/Rho kinase signaling cascade (review in ref. 13), have emerged as the most potent protein inhibitors of HVA Ca2+ channels (14–31). RGK proteins are present in many tissues and cells where HVA Ca2+ channels are expressed, including the brain and cardiac, skeletal, and smooth muscles (review in ref. 13). Accordingly, they are emerging as strong regulators of hormone secretion and cardiac and brain physiology, both in vivo and in vitro. For example, in the heart, dominant negative suppression of endogenous Rad increases L-type Ca2+-channel currents and action-potential duration in cardiac cells and produces longer QT intervals and arrhythmias (24). Rem2 prevents glucose-stimulated insulin secretion in pancreatic β cells (19) and regulates the development of both excitatory and inhibitory synapses, presumably through a feedback loop that controls Ca2+ influx (32). Finally, alteration in Gem regulation of Cav1.2 L-type Ca2+ channels may contribute to certain neural phenotypes displayed in Timothy Syndrome, a genetic disorder characterized by cardiac and neurological defects and autism (33).
All RGK proteins associate directly with Cavβ in vitro and in cells (14–17, 19–21, 23, 25, 27–29, 34), and Cavβ is required for RGK-induced inhibition of HVA Ca2+ channels (14, 15, 22). Two modes of action have been reported: (i) RGK proteins reduce the number of HVA Ca2+ channels on the cell surface, either by interfering with channel trafficking to the plasma membrane or increasing endocytosis of surface channels (14, 16, 17, 20, 24, 31, 34, 35), and (ii) RGK proteins inhibit channels already on the plasma membrane (18, 19, 21, 25, 31). The molecular mechanisms of either mode of action are unknown, but because of the central role of Cavβ in HVA Ca2+-channel trafficking and gating, it is widely assumed that both forms of inhibition rely on the RGK/Cavβ interaction (13–29, 31). This key hypothesis, however, has not been tested.
In this study, we investigated the molecular mechanism of Gem inhibition of P/Q-type Ca2+ channels expressed in Xenopus oocytes. We unambiguously show that Gem directly inhibits P/Q-type Ca2+ channels on the plasma membrane and that this inhibition requires Cavβ. Surprisingly, we discover that Gem inhibition does not require a direct Gem/Cavβ interaction or a structural element critical for Cavβ regulation of channel gating. Instead, Cavβ seems required only to prime Cavα1 for Gem inhibition. We find that Gem and P/Q channel Cavα1 coimmunoprecipitate, and we identify a region in Cavα1 crucial for Gem inhibition. Our results show an essential and hitherto unrecognized contribution from Cavα1 to RGK inhibition and lead us to propose a model for RGK inhibition of HVA Ca2+ channels.
Results
Gem Directly Inhibits P/Q-Type Ca2+ Channels on the Plasma Membrane.
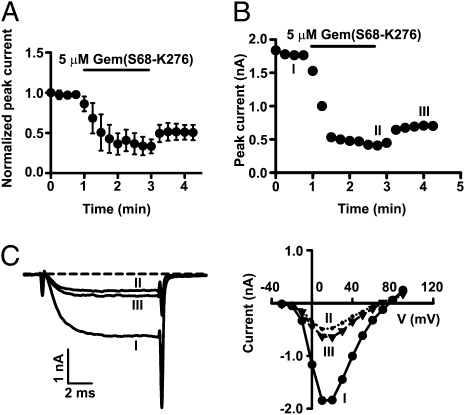
To date, there is no report of direct inhibition of surface HVA Ca2+ channels by Gem. Thus, we first investigated whether Gem inhibited P/Q-type Ca2+ channels on the plasma membrane. To accomplish this, we designed a Gem construct (S68-K276 of human Gem) that contained most of the Gem protein and could be readily purified. Gem(S68-K276), when purified and applied to the intracellular face of giant inside-out membrane patches from Xenopus oocytes expressing P/Q channels, markedly and quickly (in ~1 min) reduced the macroscopic currents (Fig. 1). This inhibition was only partially reversible (Fig. 1 A and B), partly because of the difficulty of washing out the applied protein in this recording configuration, and it was largely voltage-independent, as indicated by the current–voltage relation (Fig. 1C Right). This result shows that Gem(S68-K276) acutely inhibits P/Q channels on the plasma membrane. As expected, Gem(S68-K276) inhibited whole-oocyte P/Q-channel currents when tonically expressed (Fig. S1).
Fig. 1.
Gem acutely inhibits surface P/Q-type Ca2+ channels. (A) Time course of current inhibition (recorded at +20 mV) by 5 μM purified Gem(S68-K276) in inside-out membrane patches from oocytes expressing Cav2.1, α2δ, and β3. In this figure and subsequent similar figures, currents were normalized and then averaged (n = 5). (B) Exemplar time course of inhibition in a single patch, with the same conditions as in A. (C) Current traces recorded at +20 mV (Left) and current–voltage relationships (Right) taken at the times indicated by I, II, and III in B.
Cavβ Is Required for Gem Inhibition of Surface P/Q-Type Ca2+ Channels.
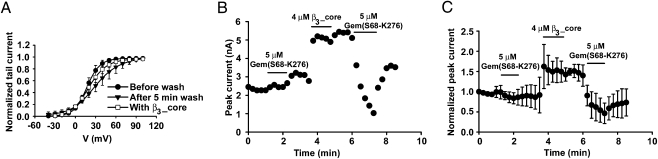
We next investigated whether Cavβ was required for the acute and direct inhibition of surface P/Q channels by Gem. To obtain large populations of surface P/Q channels that did not contain a Cavβ (β-less channels), we created, following our previous strategy (12), a mutant β3 named β3_Mut2 (bearing the M196A/L200A mutation). β3_Mut2 retained the ability to chaperone Cav2.1 to the plasma membrane but with vigorous perfusion, could quickly dissociate from the surface channels in inside-out patch recordings, leaving functional β-less channels behind. This dissociation was ascertained by monitoring the positive shift of the activation curve and its subsequent restoration (i.e., negative shift) after the application of purified β3_core (amino acids G16–G366 of β3) (Fig. 2A). Purified Gem(S68-K276) did not inhibit the β-less channels but quickly and strongly suppressed the same population of channels after they bound β3_core (Fig. 2 B and C). The inhibition developed well beyond the current level before the application of β3_core, indicating that Gem(S68-K276) did not simply reverse the gating modulation by β3_core. These results indicate that Cavβ is necessary for Gem-induced acute inhibition.
Fig. 2.
Cavβ is required for Gem inhibition of surface P/Q-type Ca2+ channels. (A) Voltage-dependence of activation under the indicated conditions for channels composed of Cav2.1, α2δ, and β3_Mut2. Activation curves were obtained in inside-out patches before the wash out of β3_Mut2, after 5 min of wash, and 1 min after subsequent application of β3_core (n = 5). (B) Time course of Gem action on β-less and β-containing channels. Currents (recorded at +20 mV) were obtained from an inside-out patch from an oocyte expressing Cav2.1, α2δ, and β3_Mut2. Before time 0, the patch had been washed for 5 min such that the channels had lost β3_Mut2 and become β-less; 5 μM Gem(S68-K276) had no effect on the β-less channels. Subsequent application of 4 μM purified β3_core increased the current, which was suppressed, with partial reversibility, by the second application of 5 μM Gem(S68-K276). (C) Same plot as in B for data pooled from five patches. For each patch, the current was normalized by that at time 0.
Role of Cavβ in Gem Inhibition.
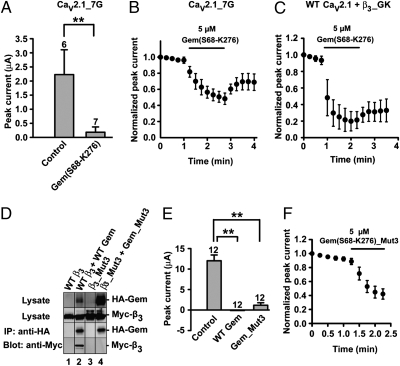
Why is Cavβ necessary for Gem inhibition of P/Q channels? Cavβ regulates Ca2+-channel gating through high-affinity binding of its guanylate kinase (GK) domain to the AID in the I–II loop of Cavα1 and through low-affinity interactions involving other regions of Cavβ and Cavα1 (2–11). Thus, it is possible that Cavβ’s mandatory role arises from Cavβ-induced gating changes and the underlying low-affinity Cavβ/Cavα1 interactions. To test this possibility, we examined the effect of Gem on channels formed by α2δ, β3, and a mutant Cav2.1 named Cav2.1_7G, in which a seven-glycine linker was inserted between the AID and IS6. The hepta-glycine linker disrupts the rigid α-helix between IS6 and the AID and renders Cavβ completely impotent in regulating channel gating without compromising its chaperone effect (8–10, 12). However, Cav2.1_7G channels were inhibited by Gem(S68-K276) in both whole oocytes (Fig. 3A) and inside-out patches (Fig. 3B). Thus, Cavβ-induced gating changes are not a prerequisite for Gem inhibition. Furthermore, in agreement with a recent study in whole oocytes (29), we found that the GK domain of β3 (β3_GK) alone was sufficient to support Gem inhibition of P/Q channels in inside-out patches (Fig. 3C), indicating that low-affinity interactions engaging Cav2.1 and other regions of β3 are not required.
Fig. 3.
The role of Cavβ in Gem inhibition of P/Q channels. (A) Inhibition of P/Q channels composed of Cav2.1_7G, α2δ,and β3 by constitutively expressed Gem(S68-K276) in whole oocytes. (B) Time course of inhibition of Cav2.1_7G channels by 5 μM purified Gem(S68-K276) in inside-out patches (n = 5). (C) Time course of inhibition of P/Q channels containing β3_GK by 5 μM purified Gem(S68-K276) in inside-out patches (n = 5). (D) Western blot showing the abolishment of Gem/β3 interaction by targeted mutations. Immunoprecipitation (IP) of Gem was carried out using an anti-HA antibody from the lysates of HEK 293T cells expressing the constructs indicated on the top of each lane. HA-Gem and Myc-β3 coimmunoprecipitated (lane 2), but HA-Gem_Mut3 and Myc-β3_Mut3 did not (lane 4). Similar results were obtained in two other experiments. (E) Inhibition of P/Q channels containing β3_Mut3 by constitutively expressed WT Gem or Gem_Mut3 in whole oocytes. In this figure and other similar figures, asterisks indicate P < 0.01, the number of recordings is indicated above the bar, all recordings were obtained from the same batch of oocytes, and similar results were obtained from at least two different batches of oocytes. (F) Time course of inhibition of β3_Mut3-containing P/Q channels by 5 μM purified Gem(S68-K276)_Mut3 in inside-out patches (n = 5).
All members of RGK proteins interact directly with Cavβ (14–17, 19–21, 23, 25, 27–29, 34). Thus, a prevalent hypothesis is that RGK proteins need to bind Cavβ to exert their inhibitory effect (13–29, 31). To test this hypothesis, we abolished Gem/β3 interaction by simultaneously mutating to alanine three residues on Gem and three residues on β3 that have been shown to be critical for this interaction (34). Coimmunoprecipitation confirmed that the mutant Gem (R196A/V223A/H225A named Gem_Mut3) and the mutant β3 (D194A/D270A/D272A named β3_Mut3) did not interact with each other (Fig. 3D and Fig. S2). This result is in agreement with a previous biochemical study showing that mutating any one of these residues abolished or severely weakened Gem/β3 interaction (34). Strikingly, Gem_Mut3 and Gem(S68-K276)_Mut3 were fully capable of inhibiting channels formed by Cav2.1, α2δ and β3_Mut3 when either constitutively expressed (Fig. 3E) or acutely applied (Fig. 3F). These results indicate that the Gem/β3 interaction is not required for Gem-induced acute inhibition.
Gem and Cavα1 Coimmunoprecipitate.
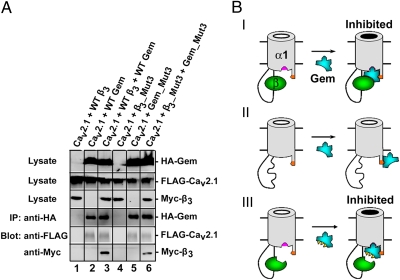
Because Gem_Mut3 did not bind β3_Mut3, the observation that Gem_Mut3 inhibited surface P/Q channels containing β3_Mut3 (Fig. 3 E and F) suggested that Gem_Mut3 might directly interact with Cav2.1. In an initial test for this possibility, we found that Cav2.1 and β3 coimmunoprecipitated with Gem (lane 3 of Fig. 4A and Fig. S3). This result is in accord with previous results showing the existence of RGK/Cavβ/Cavα1 tripartite complexes (21, 25, 27, 34). However, coimmunoprecipitation of Cav2.1, β3, and Gem could simply be a result of the Gem/β3 and β3/Cav2.1 interactions. Remarkably, however, further experiments show that Cav2.1 and β3_Mut3 coimmunoprecipitated with Gem_Mut3 (Fig. 4A, lane 6), although Gem_Mut3 and β3_Mut3 did not bind each other, as shown in Fig. 3D. Moreover, Cav2.1 coimmunoprecipitated with either Wild Type (WT) Gem or Gem_Mut3 in the absence of an exogenously expressed Cavβ (Fig. 4A, lanes 2 and 5). These results suggest that either Gem directly associates with Cav2.1 or Gem and Cav2.1 both associate with an unknown protein in the same complex. In either case, this association is Cavβ-independent.
Fig. 4.
Model of Gem inhibition of surface P/Q channels. (A) Gem coimmunoprecipitates with Cav2.1 in a Cavβ-independent manner. IP of Gem was carried out using an anti-HA antibody from the lysates of HEK 293T cells expressing the constructs indicated on the top of each lane. Cav2.1 and β3 were detected by an anti-FLAG and anti-Myc antibody, respectively. Western blot shows coimmunoprecipitation of Gem and Cav2.1 (lane 2), Gem_Mut3 and Cav2.1 (lane 5), and Gem_Mut3, Cav2.1, and β3_Mut3 (lane 6). In groups without Gem, no coimmunoprecipitation of Cav2.1 was observed (lanes 1 and 4). As a positive control, Cav2.1 and WT β3 are coimmunoprecipitated by WT Gem (lane 3). Similar results were observed in three other experiments. (B) Model of Gem inhibition of surface P/Q channels. Gem associates directly with Cav2.1 through an anchoring site on Cav2.1 (indicated by the orange patch), with (I and III) or without (II) Cavβ. Binding of WT Cavβ (I) or β3_Mut3 (III) to Cav2.1 induces an inhibitory binding site in Cav2.1 (indicated by the pink patch) where WT Gem (I) or Gem_Mut3 (II) binds to cause inhibition.
Model for Gem Inhibition of Surface P/Q-Type Ca2+ Channels.
The preceding results lead us to propose a Cavβ-priming model for Gem inhibition of P/Q-type Ca2+ channels on the plasma membrane (Fig. 4B). The most distinct feature of this model is that the interaction between Gem and Cavβ is not necessary for Gem's inhibitory effect, but a direct association between Gem and Cav2.1 is essential. In this model, Gem interacts directly with Cav2.1 through an anchoring site, with or without Cavβ present. In the presence of Cavβ and Gem, Cav2.1 forms a multimeric complex with both proteins on the plasma membrane (Fig. 4B, row I). Binding of Cavβ to Cav2.1 produces a conformational change, resulting in the formation of an inhibitory binding site in Cav2.1 where Gem contacts to produce inhibition (Fig. 4B, row I). When Cavβ dissociates or is washed off from the surface Cav2.1, the inhibitory binding site disappears, and Gem becomes unable to inhibit Cav2.1, although it can still be attached to Cav2.1 through the anchoring site (Fig. 4B, row II). With mutant forms of Cavβ and Gem that cannot bind each other (Fig. 4B, row III), inhibition can still proceed, because the ability of Cavβ and Gem to bind Cav2.1 is uncompromised. According to this model, the essential role of Cavβ is to convert Cav2.1 into a state permissive for Gem inhibition.
Identification of a Cavα1 Region Critical for Gem Inhibition.
To further test the Cavβ-priming model, we attempted to identify the molecular determinants in Cavα1 that are critical for Gem inhibition. To this end, we took advantage of the observation that RGK proteins do not regulate the activity of low voltage-activated T-type Ca2+ channels, which do not associate with Cavβ or require Cavβ for their activity (15, 18), and we constructed chimeras between Cav2.1 and Cav3.1, a T-type channel α1 subunit.
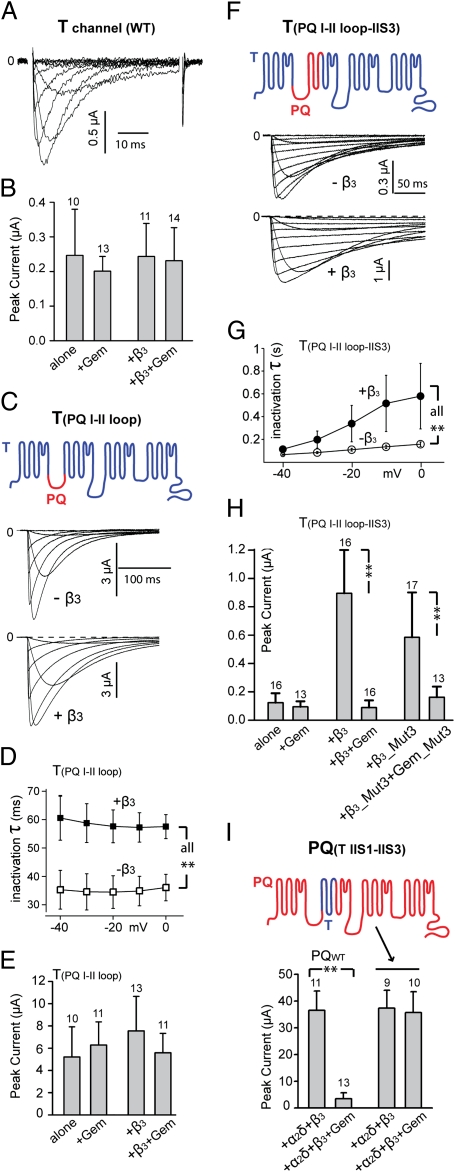
Expression of Cav3.1 in Xenopus oocytes produced typical, small, and fast inactivating T-type currents, which were unaffected by the coexpression of β3 (Fig. 5 A and B). Consistent with previous reports (15, 18), Gem did not affect T-type currents, either with or without the coexpression of β3 (Fig. 5B). Replacing the I–II loop of Cav3.1 (from I394 to I739) with that of Cav2.1 (from L359 to M483) produced a mutant named T(PQ I-II loop) that had larger currents (Fig. 5 C and E). Coexpression of β3 with T(PQ I-II loop) did not significantly increase the peak current amplitude but markedly slowed the speed of inactivation (Fig. 5 C and D), indicating that β3 was able to bind T(PQ I-II loop). This result was expected, because the I–II loop of Cav2.1 contains the Cavβ-binding AID. However, coexpression of Gem with T(PQ I-II loop) had no effect on the amplitude of the current, either in the presence or absence of β3 (Fig. 5E). These results indicate that binding of β3 to the α1 subunit alone is not sufficient to confer Gem inhibition to T-type channels.
Fig. 5.
The region encompassing IIS1–IIS3 of Cav2.1 is essential for Gem inhibition. (A) Representative family of currents from WT T-type channels. (B) Comparison of peak currents in oocytes expressing Cav3.1 and the indicated proteins. (C) Representative family of currents from channels formed by T(PQ I-II loop) (schematized in Top), with or without coexpression of β3. (D) Comparison of the time constant of inactivation of channels formed by T(PQ I-II loop) alone and by T(PQ I-II loop) + β3 (n = 5–6). (E) Comparison of peak currents in oocytes expressing T(PQ I-II loop) and the indicated proteins. (F) Exemplar family of currents from channels formed by T(PQ I-II loop—IIS3) (schematized in Top), with or without coexpression of β3. (G) Comparison of the time constant of inactivation of channels formed by T(PQ I-II loop—IIS3) alone and T(PQ I-II loop—IIS3) + β3 (n = 5–6). (H) Comparison of peak currents in oocytes expressing T(PQ I-II loop—IIS3) and the indicated proteins, showing inhibition of channels containing T(PQ I-II loop—IIS3) and β3 by WT Gem and inhibition of channels containing T(PQ I-II loop—IIS3) and β3_Mut3 by Gem_Mut3. (I) Comparison of the effect of Gem on currents produced by WT P/Q channels formed by Cav2.1, α2δ, and β3 and mutant channels formed by PQ(T IIS1—IIS3) (schematized in Upper), α2δ, and β3, showing the complete lack of inhibition of the mutant channels. Similar results were observed in at least three batches of oocytes.
Next, we created a chimeric Cav3.1 in which the I–II loop and the immediately adjacent downstream S1, S2, and S3 transmembrane segments of the second homologous repeat (from I394 to G828) were replaced by those of Cav2.1 (from L359 to F577); this mutant is named T(PQ I-II loop—IIS3) (Fig. 5F). In the absence of an exogenous Cavβ, the current produced by T(PQ I-II loop—IIS3) was small and unaffected by Gem (Fig. 5 F and H). Coexpression of β3 with T(PQ I-II loop—IIS3) greatly increased the peak current (Fig. 5 F and H) and slowed the inactivation speed (Fig. 5 F and G). Remarkably, in the presence of β3, coexpression of Gem drastically suppressed the peak current (Fig. 5H). The magnitude of this inhibition is nearly as large as Gem inhibition of WT P/Q channels (Fig. 5I). Thus, by grafting the I–II loop, IIS1, IIS2, and IIS3 of Cav2.1 to Cav3.1, we were able to confer full-fledged Gem inhibition to an otherwise completely resistant T-type channel. These results indicate that IIS1, IIS2, and IIS3 are critical for Gem inhibition. They also reiterate the critical role of Cavβ in Gem inhibition.
We then examined whether the Gem/Cavβ interaction is necessary for Gem inhibition of T(PQ I-II loop—IIS3) by testing the effect of Gem_Mut3. As with P/Q channels (Fig. 3 E and F), Gem_Mut3 strongly suppressed the channels formed by T(PQ I-II loop—IIS3) and β3_Mut3 (Fig. 5H), indicating that a direct physical interaction between Gem and Cavβ is not required for Gem inhibition.
In a complimentary experiment, we replaced a region harboring IIS1, IIS2, and IIS3 of Cav2.1 (from R482 to F577) with its counterpart from Cav3.1 (from K738 to G828); this mutant is referred to as PQ(T IIS1—IIS3) (Fig. 5I). Expression of this mutant Cav2.1 alone in oocytes produced little or no current, much like WT P/Q channels (Fig. S4). Coexpression of β3 greatly increased the current (Fig. S4), indicating that PQ(T IIS1—IIS3) binds β3. Strikingly, Gem had little or no effect on this current while strongly suppressing WT P/Q channel currents in the same batch of oocytes (Fig. 5I). These results identify a region in Cavα1 (IIS1–IIS3) that is essential for Gem inhibition.
Discussion
In this study, we show the following key features of Gem inhibition of P/Q-type Ca2+ channels: (i) Gem directly inhibits channels on the plasma membrane (Fig. 1), (ii) this inhibition requires Cavβ (Fig. 2), (iii) this inhibition does not require a direct physical interaction between Gem and Cavβ or Cavβ-induced gating changes (Fig. 3), (iv) Gem coimmunoprecipitates with Cav2.1 (Fig. 4A), and (v) the region encompassing IIS1–IIS3 in Cav2.1 is essential for Gem inhibition (Fig. 5 H and I). Several of these key features (including ii, iii, and v) are reinforced by the results obtained from various Cav2.1/Cav3.1 chimeras (Fig. 5). These results, in aggregate, strongly support the Cavβ-priming model proposed in Fig. 4B.
In the first study of RGK inhibition of HVA Ca2+ channels, Gem was reported to suppress the activity of L-, N-, and P/Q-type channels expressed in Xenopus oocytes and baby hamster kidney cells by decreasing their expression at the cell surface (14). By directly applying purified Gem proteins to the cytoplasmic face of inside-out membrane patches containing expressed P/Q channels, we show that the surface channels are rapidly and directly inhibited by Gem. Inhibition of surface HVA Ca2+ channels has also been observed for two other RGK proteins. Thus, Rem2 inhibits endogenous surface N-type channels in sympathetic and dorsal-root ganglion neurons (18), Rem inhibits surface L-type channels expressed in pancreatic β-cells (19, 21) and HEK 293 cells (31), and rapid translocation of a recombinant Rem derivative acutely inhibits L- and N-type channels expressed in tsA201 cells (25).
Previous studies show that RGK proteins do not affect Ca2+ channels in heterologous systems expressing only Cavα1 (14, 15, 22), suggesting that Cavβ is necessary for RGK inhibition of Ca2+ channels. Consistent with this notion, it has been shown and is reproduced in this study that T-type Ca2+ channels, which do not contain an associated Cavβ, are not modulated by RGK proteins (Fig. 5A) (15, 18). However, until now, it was unclear whether Cavβ is required for RGK inhibition of surface Ca2+ channels. By successfully producing large populations of β-less Ca2+ channels on the plasma membrane, we show unequivocally that Cavβ is essential for this direct inhibitory effect (Fig. 2).
A striking finding of this work is that the direct association between Gem and Cavβ is not necessary for Gem inhibition (Fig. 3). This finding contradicts the general belief that RGK proteins exert their inhibitory action on HVA Ca2+ channels through Cavβ (13–29, 31). However, a direct physical interaction between RGK proteins and Cavβ has been well-documented (14–17, 19–21, 23, 25, 27–29, 34). Therefore, what is the functional importance of this interaction? One possibility is that in native cells, with physiological levels of RGK proteins, the RGK/Cavβ interaction serves to facilitate RGK inhibition by bringing RGK proteins close to surface Ca2+ channels, hence increasing the effective local concentration of RGK proteins near the channels. Another completely disparate function of this interaction could be to translocate full-length Cavβ into the nucleus (20), where Cavβ could engage in transcriptional regulation (36).
The Cavβ-priming model proposed here for Gem may also be applicable to Rem and Rem2, because they both have been shown to inhibit surface Ca2+ channels (18, 19, 21, 25, 31). This model remains speculative, and many questions remain to be answered, including the locations of the proposed anchoring site and inhibitory binding site for Gem in Cavα1. These binding sites are likely located in the cytoplasmic regions of Cavα1, given that Gem is a cytosolic protein. Indeed, a new study reports that a proximal C-terminal region of Cav1.2 can directly bind Rem, Rem2, and Rad in vitro (30). Because this binding occurs in the absence of a Cavβ, it could serve as the anchoring interaction proposed in our model. This hypothesis needs further testing. A mutant Cav1.2 containing the S1928A mutation was reported to be resistant to inhibition by Rem2 (37), which would suggest that this C-terminal region and protein kinase A (PKA) phosphorylation of Cavα1 regulate Rem2 inhibition of L-type channels. However, deleting the distal C terminus of Cav1.2 (from K1906 to L2171) has no effect on Rem inhibition of Cav1.2, although this is a region that also binds Rem in vitro (30). Thus, the role of Cavα1 C terminus in RGK inhibition remains largely unclear. The molecular mechanism of the involvement of the IIS1–IIS3 region, which is highly conserved among the Cavα1 of HVA Ca2+ channels (Fig. S5), in Gem inhibition is also unclear. One possible role of this region is mediating the conformational changes of Cavα1 that likely occur after Cavβ and/or Gem binding.
Although many of the molecular details remain to be elucidated, our findings and the Cavβ-priming model shift the focus from the RGK/Cavβ interaction to RGK/Cavα1 interactions. This shift may prompt new investigations into and expand our understanding of the molecular mechanisms of RGK regulation of HVA Ca2+ channels.
Materials and Methods
WT and mutant P/Q- and T-type Ca2+ channels were expressed in Xenopus oocytes with or without WT or mutant Gem. Ba2+ currents were recorded with inside-out patch clamp or two-electrode voltage clamp. Purified Gem protein fragments were applied to membrane patches in inside-out patch-clamp recordings. Coimmunoprecipitation was carried out in HEK 293T cells.
Details for the methods described above and construct cloning, cell culture and transfection, SDS/PAGE and Western blot, oocyte preparation and injection, protein expression and purification, coimmunoprecipitation, and electrophysiology are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Y. Mori (Kyoto University, Kyoto, Japan) for rabbit brain Cav2.1 cDNA, T. Tanabe (Tokyo Medical and Dental University, Tokyo, Japan) for α2δ cDNA, E. Perez-Reyes (University of Virginia, Charlottesville, VA) for β3 cDNA, and H. Matsunami (Duke University, Durham, NC) for HEK 293T cells. We also thank our colleagues in the laboratory for comments on the manuscript. This work was supported by National Institutes of Health Grants NS045819 and NS053494 (to J.Y.) and an Established Investigator Award from the American Heart Association (to J.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007543107/-/DCSupplemental.
References
- 1.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Dolphin AC. β subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 3.Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010 doi: 10.1152/physrev.00057.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He LL, Zhang Y, Chen YH, Yamada Y, Yang J. Functional modularity of the β-subunit of voltage-gated Ca2+ channels. Biophys J. 2007;93:834–845. doi: 10.1529/biophysj.106.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca(V)β modulatory properties are AID-independent. Nat Struct Mol Biol. 2005;12:372–377. doi: 10.1038/nsmb909. [DOI] [PubMed] [Google Scholar]
- 6.Walker D, et al. A new β subtype-specific interaction in α1A subunit controls P/Q-type Ca2+ channel activation. J Biol Chem. 1999;274:12383–12390. doi: 10.1074/jbc.274.18.12383. [DOI] [PubMed] [Google Scholar]
- 7.Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL., Jr. Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaValpha1 AID-CaVbeta interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Findeisen F, Minor DL., Jr. Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J Gen Physiol. 2009;133:327–343. doi: 10.1085/jgp.200810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitko I, et al. Orientation of the calcium channel β relative to the α(1)2.2 subunit is critical for its regulation of channel activity. PLoS ONE. 2008;3:e3560. doi: 10.1371/journal.pone.0003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias JM, Murbartián J, Vitko I, Lee JH, Perez-Reyes E. Transfer of β subunit regulation from high to low voltage-gated Ca2+ channels. FEBS Lett. 2005;579:3907–3912. doi: 10.1016/j.febslet.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α 1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, et al. Origin of the voltage dependence of G-protein regulation of P/Q-type Ca2+ channels. J Neurosci. 2008;28:14176–14188. doi: 10.1523/JNEUROSCI.1350-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: Regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Béguin P, et al. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 15.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci USA. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Béguin P, et al. Roles of 14-3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem J. 2005;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Béguin P, et al. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J Cell Sci. 2005;118:1923–1934. doi: 10.1242/jcs.02321. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finlin BS, et al. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- 20.Béguin P, et al. Nuclear sequestration of β-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol. 2006;355:34–46. doi: 10.1016/j.jmb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Finlin BS, et al. Analysis of the complex between Ca2+ channel β-subunit and the Rem GTPase. J Biol Chem. 2006;281:23557–23566. doi: 10.1074/jbc.M604867200. [DOI] [PubMed] [Google Scholar]
- 22.Seu L, Pitt GS. Dose-dependent and isoform-specific modulation of Ca2+ channels by RGK GTPases. J Gen Physiol. 2006;128:605–613. doi: 10.1085/jgp.200609631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correll RN, et al. Plasma membrane targeting is essential for Rem-mediated Ca2+ channel inhibition. J Biol Chem. 2007;282:28431–28440. doi: 10.1074/jbc.M706176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yada H, et al. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ Res. 2007;101:69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 25.Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating CaV channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- 26.Bannister RA, Colecraft HM, Beam KG. Rem inhibits skeletal muscle EC coupling by reducing the number of functional L-type Ca2+ channels. Biophys J. 2008;94:2631–2638. doi: 10.1529/biophysj.107.116467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correll RN, Botzet GJ, Satin J, Andres DA, Finlin BS. Analysis of the Rem2 voltage dependant calcium channel β subunit interaction and Rem2 interaction with phosphorylated phosphatidylinositide lipids. Cell Signal. 2008;20:400–408. doi: 10.1016/j.cellsig.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flynn R, Chen L, Hameed S, Spafford JD, Zamponi GW. Molecular determinants of Rem2 regulation of N-type calcium channels. Biochem Biophys Res Commun. 2008;368:827–831. doi: 10.1016/j.bbrc.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Leyris JP, et al. RGK GTPase-dependent CaV2.1 Ca2+ channel inhibition is independent of CaVbeta-subunit-induced current potentiation. FASEB J. 2009;23:2627–2638. doi: 10.1096/fj.08-122135. [DOI] [PubMed] [Google Scholar]
- 30.Pang C, et al. Rem GTPase interacts with the proximal CaV1.2 C-terminus and modulates calcium-dependent channel inactivation. Channels (Austin) 2010 doi: 10.4161/chan.4.3.11867. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang T, Xu X, Kernan T, Wu V, Colecraft HM. Rem, a member of the RGK GTPases, inhibits recombinant CaV1.2 channels using multiple mechanisms that require distinct conformations of the GTPase. J Physiol. 2010;588:1665–1681. doi: 10.1113/jphysiol.2010.187203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paradis S, et al. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krey JF, Dolmetsch RE. The Timothy Syndrome mutation in CaV1.2 causes dendritic retraction through calcium-independent activation of the RhoA pathway. Biophys J. 2009;96:221a–222a. [Google Scholar]
- 34.Béguin P, et al. RGK small GTP-binding proteins interact with the nucleotide kinase domain of Ca2+-channel β-subunits via an uncommon effector binding domain. J Biol Chem. 2007;282:11509–11520. doi: 10.1074/jbc.M606423200. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki T, et al. Direct inhibition of the interaction between α-interaction domain and β-interaction domain of voltage-dependent Ca2+ channels by Gem. J Biol Chem. 2005;280:9308–9312. doi: 10.1074/jbc.M413773200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, et al. The β subunit of voltage-gated Ca2+ channels interacts with and regulates the activity of a novel isoform of Pax6. J Biol Chem. 2010;285:2527–2536. doi: 10.1074/jbc.M109.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crump SM, et al. L-type calcium channel α-subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am J Physiol Heart Circ Physiol. 2006;291:H1959–H1971. doi: 10.1152/ajpheart.00956.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.