Abstract
Because of the perception that depleting hematopoietic grafts of T cells will result in poorer immune recovery and in increased risk of graft rejection, pure hematopoietic stem cells (HSC), which avoid the potentially lethal complication of graft-versus-host disease (GVHD), have not been used for allogeneic hematopoietic cell transplantation (HCT) in humans. Ideal grafts should contain HSC plus mature cells that confer only the benefits of protection from pathogens and suppression of malignancies. This goal requires better understanding of the effects of each blood cell type and its interactions during engraftment and immune regeneration. Here, we studied hematopoietic reconstitution post-HCT, comparing grafts of purified HSC with grafts supplemented with T cells in a minor histocompatibility antigen (mHA)-mismatched mouse model. Cell counts, composition, and chimerism of blood and lymphoid organs were evaluated and followed intensively through the first month, and then subsequently for up to 1 yr. Throughout this period, recipients of pure HSC demonstrated superior total cell recovery and lymphoid reconstitution compared with recipients of T cell-containing grafts. In the latter, rapid expansion of T cells occurred, and suppression of hematopoiesis derived from donor HSC was observed. Our findings demonstrate that even early post-HCT, T cells retard donor HSC engraftment and immune recovery. These observations contradict the postulation that mature donor T cells provide important transient immunity and facilitate HSC engraftment.
Keywords: immune reconstitution, graft composition, mice
Allogeneic hematopoietic cell transplantation (HCT) comprises the only curative treatment option for a spectrum of fatal malignancies and insufficiency syndromes of the blood (1). Furthermore, the replacement of the immune system by HCT has the potential to cure nonmalignant disorders, such as severe autoimmune diseases (2), and to induce immune tolerance to transplanted organs (3). However, the high treatment-related morbidity and mortality continue to limit the application of this powerful cellular therapy to a broader patient base. The main obstacles to the success of HCT are graft-versus-host disease (GVHD), infections, and relapse, all of which are critically influenced by the composition of the graft. In adults, the most commonly used sources of allogeneic hematopoietic cells are mobilized peripheral blood (MPB), or bone marrow (BM). Both graft types contain mixtures of hematopoietic stem cells (HSC), progenitors, lineage-committed cells, and mature cells. Donor T cells are the main mediators of acute GVHD but are also credited with conferring beneficial graft-versus-tumor (GVT) effects, are thought to enhance engraftment, and provide protective immunity post-HCT. Indeed, early attempts to reduce GVHD by T-cell depletion (TCD) were hampered by unacceptable rates of engraftment failure (4–7). In retrospect, these results may have been due to an insufficient stem/progenitor cell content of the manipulated BM products (8), as later trials revealed significantly improved rates of GVHD and similar engraftment in patients given TCD as compared with whole BM grafts (9–12).
Although there are serious concerns that TCD results in significantly increased infectious complications, GVHD itself is known to impair immune function post-HCT (3–5, 13). GVHD-associated lymphoid hypoplasia has been well described (6–8, 14–17). Myelosuppression, the most telling manifestation of graft-versus-host reactions (GVHR) directed against hematopoietic elements has also been reported. It appears that the hematopoietic system is the most sensitive target of donor immunity, as relatively low numbers of T cells that do not damage other target organs can nonetheless induce marrow aplasia (13, 18–20). Given the important consequences that attack of the BM and lymphoid organs have on the outcomes of clinical allogeneic HCT, surprisingly little is known about GVHR as it pertains to hematopoiesis and the establishment of donor chimerism.
In this study, we sought to better delineate the impact of graft content on hematopoiesis and immune recovery in an mHA-mismatched murine GVHD model. Transplants of grafts composed of purified HSC versus HSC plus unfractionated splenocytes or splenic T-cell subsets were compared. As early as day (d) 7 post-HCT, marked differences were noted in hematopoietic reconstitution in the blood, BM and lymphoid organs between recipients of purified HSC and HSC plus lymphocytes. Mice given HSC alone had faster recovery of blood counts, prompt production of B lymphocytes, and faster normalization of the myeloid to lymphoid ratio compared with recipients of HSC plus lymphocytes. In this latter group, donor lymphocytes mediated early infiltration of the marrow and spleen, resulting in elimination of host cells and suppression of HSC-derived hematopoiesis. In fact, retardation of engraftment rather than facilitation was observed. These studies highlight the rapidity by which de novo hematolymphoid reconstitution can occur following transplantation of purified HSC while underscoring the deleterious effects that accompanying lymphoid cells have on HSC engraftment and hematopoiesis.
Results
GVHD and Survival.
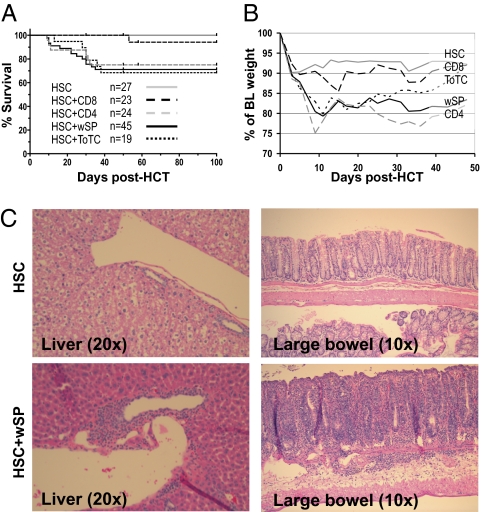
To study the association between graft composition, GVHD, and immune reconstitution in mHA-mismatched mice, lethally irradiated BALB.B mice received purified HSC or HSC plus mature lymphoid cells, including whole splenocytes (wSP), total T cells (CD4 and CD8; ToTC), CD4, or CD8 cells from C57BL/6 (B6) donors (Fig. S1). Fig. 1A shows the survival and Fig. 1B the weight curves for these groups. As expected, recipients of HSC only showed no signs of GVHD, whereas differential effects were observed depending upon the type of graft supplement. GVHD was pronounced in recipients of ToTC, wSP and CD4 cells, as 32%, 30%, and 25% died before d40, respectively. In contrast, CD8 cells caused only mild signs of GVHD, and only one of 23 mice died on d53 post-HCT (Fig. 1A). The severity of GVHD symptoms was also reflected by their weight course (Fig. 1B) and organ histology, which confirmed the clinical findings (Fig. 1C). The weight curves are censored to include only mice surviving >4 wk, as only those animals were available for chimerism analysis.
Fig. 1.
GVHD in B6 into BALB.B recipients. (A) Kaplan–Meier curves showing percent survival and (B) median percentage of baseline (BL) weight of BALB.B mice given B6 HSC alone or HSCwSP, ToTC, CD4, or CD8 cells. Data were from six independent experiments. (C) H&E staining of GVHD target tissues from representative recipients of HSC (Upper) or HSC+wSP (Lower) at 4 wk post-HCT. Liver and bowel architecture were normal in HSC recipients, but had lymphocyte infiltrates and parenchymal cell damage in mice given HSC+wSP.
Reconstitution of Blood.
Absolute cell content.
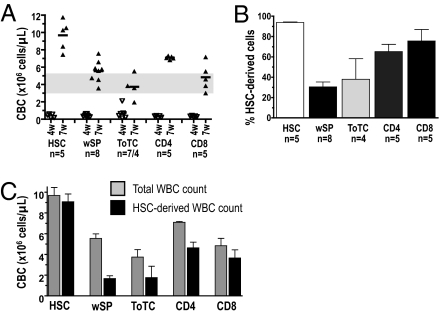
Hematopoietic reconstitution was assessed by absolute WBC counts. Immediately post-HCT, at 2–3 wk, a marked drop of the absolute WBC numbers in all groups was noted that persisted until 6 wk post-HCT and stabilized thereafter. Recipients of pure HSC recovered WBC more rapidly than all other groups and achieved significantly higher total cell counts by 7 wk (Fig. 2A), whereas WBC levels were lowest for mice that received ToTC. Evidence that mature donor lymphocytes suppressed donor HSC contribution to hematopoiesis is shown in Fig. 2 B and C, which compare spleen-derived vs. HSC-derived blood elements, respectively, as determined by the percentage and absolute number of WBC. For recipients of HSC only, most WBC were generated from the infused HSC. In contrast, only ~30% of WBC were donor HSC derived in recipients of wSP or ToTC. The addition of either CD4 or CD8 cells also reduced the HSC contribution to recipient WBC. Thus, compared with mice that received lymphocyte-replete grafts, HSC recipients had superior recovery of WBC established on the basis of true HSC-derived hematopoiesis.
Fig. 2.
White blood cell counts. (A) WBC counts of BALB.B mice given B6 HSC alone or HSC+wSP, ToTC, CD4, or CD8 measured by Coulter Counter at 4 wk and 7 wk post-HCT. Control WBC values for age adjusted (12 wk) WT BALB.B mice are shaded in gray. At 7 wk, recipients of HSC alone had higher WBC counts (median 10 × 106/μL) than mice given HSC+wSP (median 5.7 × 106/μL; P = 0.003), HSC+ToTC (median 3.7 × 106/μL; P < 0.001), HSC+CD4 (median 7 × 106/μL; P = 0.03), or HSC+CD8 T cells (median 4.6 × 106/μL; P = 0.002). (B) Proportion of HSC-derived donor cells in the blood at 7 wk post-HCT. HSC recipients had a significantly higher proportion (median 93%) compared with HSC+wSP (median 29%; P < 0.001), ToTC (median 31%; P = 0.07), or CD4 (median 61%; P = 0.02), but not CD8 (median 82%; P = 0.2) groups. (C) Absolute number of donor HSC-derived WBC 7 wk post-HCT. Recipients of HSC only had significantly higher absolute numbers of WBC derived from donor HSC (9 × 106/μL) compared with mice given HSC+wSP (median 1.6 × 106/μL; P < 0.001), HSC+ToTC (median 1.1 × 106/μL; P = 0.002), HSC+CD4 (4.2 × 106/μL; P = 0.002), or HSC+CD8 T cells (median 3.2 × 106/μL; P = 0.001). B and C display the mean and SEM for each respective group, derived from one experiment with five to eight mice per group.
Blood composition and GVHD.
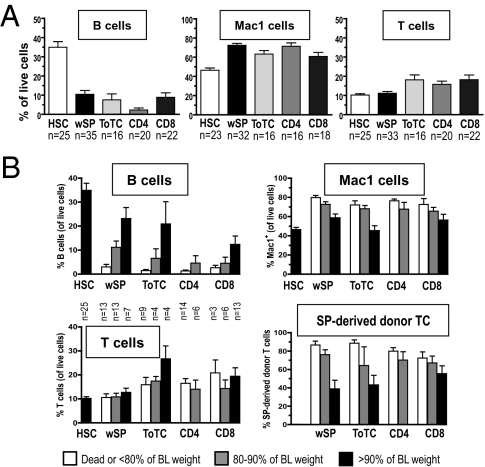
The blood of WT mice consists of 30–55% B cells (B220+), 30–50% T cells (TCRβ+), and 10–20% Mac1+ granulocytes/monocytes. Fig. 3 shows that graft content substantially affected regeneration of specific WBC lineages. This effect was most pronounced for B cells, which promptly recovered to normal levels in recipients of pure HSC (median 38%), but were severely suppressed in mice given HSC plus lymphocytes. In the latter groups, B cells comprised only a median 0.75–6% of live cells at 1 mo (p < 0.0001). B cell levels did not significantly differ with the type of lymphocyte supplement, except that B lymphopenia was most pronounced in recipients of CD4 cells. The degree of B lymphopenia correlated with GVHD severity as mice with more extreme weight loss had proportionally lower B cell levels (Fig. 3B). B cell recovery occurred slowly as GVHD symptoms resolved and reached normal levels by 3 mo (Fig. S2A). There was also a clear predominance of myeloid (Mac1+) cells in mice given lymphocyte-replete grafts. Mac1+ cells accounted for a median of 63–76% of blood cells at 1 mo in these mice, as compared with 46% for HSC recipients (Fig. 3A). The degree of acute GVHD was also associated with this shift to myelopoiesis (Fig. 3B), as greater weight loss correlated with higher Mac1+ cell levels. In contrast, in all groups, T cells were reduced for several months, with median levels ranging from 10% to 16% in the first 3 mo (Figs. 3A and S2B). These reduced T-cell levels were independent of graft content, and there was no obvious correlation with symptoms of acute GVHD. However, the proportion of donor spleen-derived T cells was higher in mice with greater weight loss (Fig. 3B).
Fig. 3.
Effects of graft composition on hematopoiesis at 1 mo post-HCT. (A) Contribution of cells by lineage determined by FACS analysis. HSC recipients had normal B cell levels (median 38%/live cells). B cell levels were significantly reduced in mice given HSC+wSP (median 6%; P < 0.0001), HSC+ToTC (median 2%; P < 0.0001), HSC+CD4 (median 0.75%; P < 0.0001), or HSC+CD8 cells (median 4%; P < 0.0001). Recovery of Mac1 cells occurred rapidly in all groups, although levels were significantly lower in recipients of HSC (median 46% of live cells) versus mice given HSC+wSP (median 73%; P < 0.0001), ToTC (median 66%; P < 0.001), CD4 (median 74%; P < 0.0001), or CD8 T cells (median 63%; P < 0.01). T-cell levels reached a median of 10%/live cells in recipients of HSC or HSC+wSP, and were slightly higher (16–17%) in recipients of HSC+ToTC, CD4 or CD8 (P < 0.01 for differences between HSC only and HSC+ToTC, CD4 or CD8). (B) Severity of GVHD was classified according to the following criteria: death from acute GVHD or weight <80%, 80–90%, or >90% of BL weight. Shown are the contributions by lineage as %/live cells for mice stratified into these groups. Weight loss correlated with B lymphopenia and predominance of Mac1 cells in the blood. There was no obvious correlation between T-cell levels and GVHD; however, higher levels of donor spleen-derived T cells corresponded with greater weight loss. Data for these figures were derived from same mice as studied in Fig. 1.
Chimerism and competitive reconstitution.
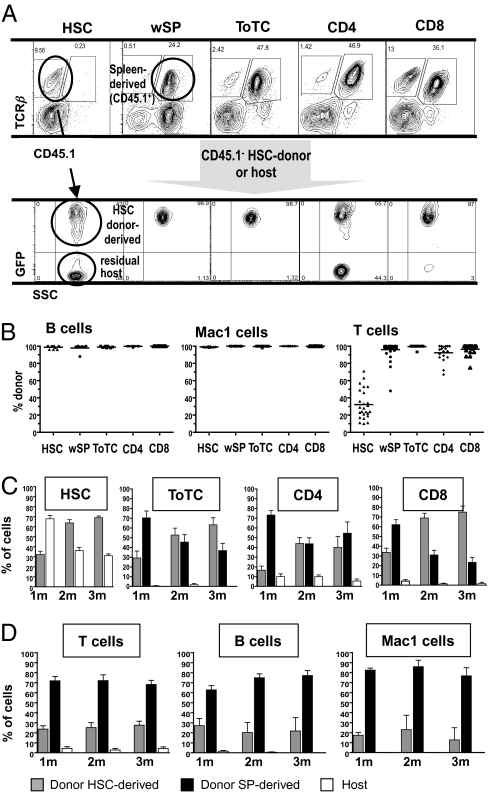
The origins of the three different hematopoietic sources (HSC- vs. spleen-derived donor vs. residual host) were determined by expression of CD45.1/2, Thy1.1/2 alleles and GFP (Fig. 4A). In this way “nascent” HSC- vs. lymphocyte-derived donor cells were identified. In all recipients, lethal irradiation eliminated host myeloid and B cells, whereas T cells were not eradicated unless mature donor T cells were given. Accordingly, in HSC recipients, myeloid and B cell lineages were promptly replaced by donor cells, whereas the T cells were derived from a mixture of donor and host (Fig. 4 B and C). Recipients of ToTC, CD4, or CD8 cells were also mixed T-cell chimeras but derived from both HSC and splenocyte donor sources (Fig. 4 A and C). As primarily T cells were infused in these groups, B and myeloid cells were mainly donor HSC derived, although minor contributions from donor spleen were detected. However, as compared with HSC recipients, the origin of hematopoiesis was distinctly different in mice given HSC+wSP. Spleen-derived donor cells not only eradicated the residual host but permanently (>1 y) dominated lymphoid and myeloid (Mac1) production (Figs. 4D and S3). The preponderance of spleen-derived myeloid cells was surprising, given the low proportion of myeloid cells and rare HSC contained in spleens (Fig. S4). Nonetheless, these data show that splenocyte-derived hematopoietic stem/progenitor cells effectively contribute and compete with the infused BM-derived purified HSC, and that the ratio between donor spleen- and HSC-derived cells, once established, remained stable.
Fig. 4.
Effects of graft composition on chimerism. (A) Delineation of T-cell source by FACS analyses of blood 4 wk post-HCT from representative mice in each transplant group. T cells from BALB.B are marked by Thy1.2, CD45.2; from B6 HSC by Thy1.1, CD45.2, GFP; and from B6 splenic cells by Thy1.1 CD45.1. Recipients of HSC remained mixed donor/host T-cell chimeras. Recipients of HSC+wSP converted to full donor type, and all T cells were derived from donor splenocytes. T cells in recipients of HSC+ToTC, CD4, or CD8 cells were derived from donor splenocytes and donor HSC with residual host in some mice. (B–D) Summary of FACS results obtained from mice studied in Figs. 1 and 3. (B) Donor/host chimerism of B, Mac1, and T cells 4 wk post-HCT. All groups converted to full donor type in the B cell and myeloid lineages. T-cell origins were mixed in recipients of HSC, whereas T cells were largely donor derived in mice given lymphocyte-replete grafts. (C) Origins of T cells as percentage of T cells from each source relative to total T cells. Gray bars indicate nascent T cells from donor HSC, black bars indicate T cells cotransferred from donor spleen cells, and white bars represent host T cells, all at 1, 2, and 3 mo post-HCT. HSC recipients remained mixed T-cell chimeras and the proportion of HSC-derived T cells increased over time. In mice given HSC+ToTC or HSC+CD8 cells, the proportion of nascent HSC-derived donor T cells increased whereas spleen-derived donor T cells decreased, and residual host cells were largely eliminated. In recipients of HSC+CD4 cells, spleen-derived T cells persistently predominated, and elimination of host T cells was incomplete. (D) Origins of cells from the different lineages at 1, 2, and 3 mo post-HCT for recipients of HSC+wSP. Residual host cells were readily cleared, and spleen-derived hematopoiesis was superior compared with lymphoid and myeloid cell production from donor HSC.
Influence of CD45 allelic differences.
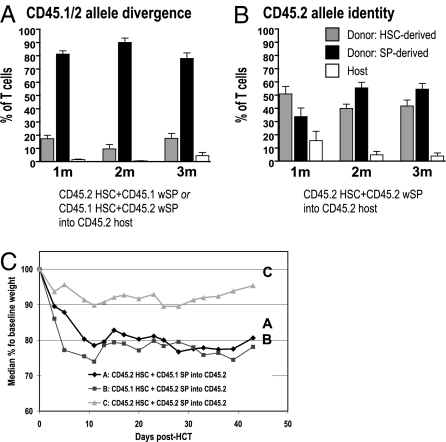
The dominance of transferred wSP over HSC in hematopoieisis led us to ask whether CD45 allelic differences permitted immune recognition of HSC and, hence, suppression of HSC blood formation. CD45.1 and CD45.2 are congenic markers commonly used in mice to distinguish the origins of hematopoiesis. For our initial studies, HSC were from B6.CD45.2 and wSP from B6.CD45.1 mice, whereas recipients were BALB.B.CD45.2. Similarly, reversal of the allele markers, such that HSC were from B6.CD45.1 and wSP from B6.CD45.2 mice, showed hematopoiesis dominated by donor splenocytes (Fig. 5A). However, when all blood-forming sources were identical at the CD45.2 allele, a markedly higher percentage of blood cells originated from donor HSC and from residual host cells (Fig. 5B). In addition, the severity of GVHD, as assessed by weight loss, was attenuated compared with transplants involving CD45 disparities (Fig. S5C). These data suggest that CD45 allelic differences between hematolymphoid sources can elicit immune responses, and the data highlight the importance of minor antigens expressed on hematopoietic cells as targets of mature lymphoid cells.
Fig. 5.
Influence of CD45.1/2 allele differences on engraftment and GVHD. Blood T-cell chimerism is shown as the median percentage of T cells derived from donor HSC (gray) and spleen (black) vs. host (white) at 1, 2, and 3 mo post-HCT. (A) CD45 allele disparity of HSC and wSP B6 donors. Regenerating blood T cells were mainly donor spleen-derived (median 84% vs. 15% donor HSC derived at 1 mo post-HCT), and host T cells were eliminated (<1%). (B) CD45.2 allele identity of HSC and wSP B6 donors. HSC were from B6.Thy1.1 and wSP were from B6.Thy-1.1.GFP+ mice. At 1 mo post-HCT, a median 33% and 58% of T cells were donor spleen vs. HSC derived, respectively. Residual host T cells (median 12.5%) persisted. A and B show means with SEM. (C) Median weights of BALB.B (CD45.2) that received HSC+wSP grafts from CD45 disparate donors (A and B) vs. HSC+wSP from CD45.2 identical donors that also shared the CD45.2 allele with recipients (C, all CD45.2). Weight loss was more pronounced for CD45 allele disparity vs. identity between donor and host. Data for these figures were derived from same mice as studied in Fig. 1.
GVHR in BM and Lymphoid Organs.
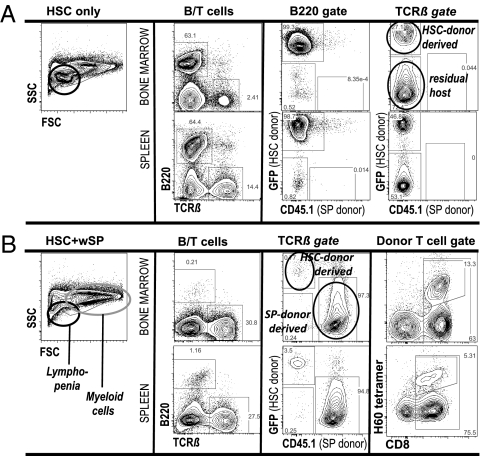
The skewing of WBC away from normal values caused by mature donor T cells involved not only blood but all hematolymphoid organs. Similar to the blood, B lymphopenia was the most notable perturbation in the BM, spleen, and lymph nodes of mice with GVHD, whereas recipients of purified HSC had unimpaired and rapid recovery of B cells. Shown in Fig. 5 are FACS plots of BM and spleen from representative recipients of HSC alone (Fig. 6A) or HSC+wSP (Fig. 6B). Two weeks after transplantation, mice given pure HSC had 30–65% B cells in BM and spleen that were largely donor type; and as in the blood, T cells originated from both donor and host (Fig. 5A). In contrast, B lymphopoiesis was markedly suppressed in recipients of HSC+wSP, and all B cells were donor spleen derived. At the same time, these tissues were heavily infiltrated with donor splenic T cells, the majority of which were CD8 T cells. Antihost alloreactivity of these donor CD8 T cells was verified by staining with a tetramer against H60, a known immunodominant minor antigen, expressed by hematopoietic cells of BALB.B host but not B6 donor mice. Of the CD8 T cells in the BM of HSC+wSP recipients, 10–25% were H60-tetramer positive (Fig. 6B), which suggested that recognition of H60 contributed importantly to the eradication of residual host BM cells. FACS analyses of the hematolymphoid organs were repeated on d28 and d50, and revealed that donor T-cell infiltration peaked at 2 wk post-HCT and decreased thereafter. The results for lymph nodes resembled those of the spleens. Of note, lymph nodes from mice that received HSC+wSP were smaller and had markedly reduced cellularity as compared with organs from mice that received HSC only, again implying that the mature donor cells retarded rather than augmented immune recovery.
Fig. 6.
Lymphoid lineage and chimerism as determined by FACS analyses of BM and spleen 2 wk post-HCT. (A) Recipients of HSC only showed prompt reconstitution of the lymphoid cells (SSC/FSC plot), which were predominantly B cells (B220) derived from the GFP+ HSC donor. T cells in BM originated mainly from the host, whereas splenic T cells were derived equally from donor HSC and the host. (B) Recipients of HSC+wSP displayed marked lymphopenia (SSC/FSC plot) secondary to marked reduction of B cells. BM and spleen were heavily infiltrated by donor T cells (spleen derived). Little to no hematopoiesis originated from donor HSC or residual host cells. The majority of donor T cells were CD8+, a substantial proportion of which were tetramer reactive against H60. Representative FACS plots from mice 2 wk post-HCT are shown. Data were confirmed in three independent experiments, with three to four animals per group.
Hematopoietic Reconstitution Early Post-HCT.
To better characterize early hematolymphoid regeneration, BM and spleens of recipients of HSC vs. HSC+wSP were examined for total cell numbers, composition, and chimerism on d4, 7, 11, 14, 17, and 21 post-HCT. During this period, extreme hypocellularity was noted, and cell numbers in BM and spleen did not differ between the HSC and HSC+wSP groups (Fig. S5A), suggesting that environmental factors may limit cell expansion. For both groups, by d14, the donor cell levels were uniformly high (Fig. S5B). However, substantial differences in the donor source and cell composition were noted. At d4, donor cells were already present in the spleens of mice given HSC+wSP, whereas the spleens of HSC recipients were mainly of host origin (Fig. S5B). The wSP-derived cells dominated total spleen cellularity in HSC+wSP recipients and persisted in the BM. Cell lineage representation also varied depending upon the graft type (Fig. S5C). Recipients of HSC alone recovered normal lineage proportions in the BM by d14, and spleens recovered by d21, albeit with persistent T lymphopenia. In contrast, the BM and spleen of mice infused with HSC+wSP contained high numbers of donor wSP-derived T cells, relatively few B cells, and granulocytes/monocytes (Mac1+) dominated the cellularity.
Analyses of the relative contribution of cells derived from the three different blood forming sources by lineage (Fig. S6A) showed that for HSC recipients donor B cells arose early (d7) and replaced the host B cells by d21. In contrast, the small numbers of B cells present in HSC+wSP recipients originated largely from wSP (Figs. S5C and S6B). The dynamics of T-cell regeneration also differed markedly between HSC and HSC+wSP recipients. For HSC recipients, a high proportion of T cells were of host type, as T cells are known to be more radiation resistant than B and Mac1 cells. In these mice, the earliest TCRβ+ cells originating from HSC were detected by d11. In mice given HSC+wSP, early expansion of wSP-derived donor T cells occurred, which resulted in both rapid eradication of residual host T cells and suppression of T lymphopoiesis from donor HSC (Fig. S6B). For both graft types, by d7, the radiation-sensitive host myeloid cells were eliminated and replaced by donor cells (Fig. S6 A and B). Recipients of HSC+wSP demonstrated increasing myelopoiesis originating from the donor splenocytes, which again shows that splenocytes contain HSC and progenitors that efficiently compete with HSC from the BM.
Discussion
An ultimate goal for the field of HCT is the engineering of grafts tailored to individual disease requirements. Purified HSC provide the platform upon which other defined mature populations (i.e., antigen-specific T cells) can be added. However, the implementation of this strategy requires better understanding of the biology of engraftment and hematopoiesis post-HCT and, in particular, of how graft composition affects these processes.
Although methods to purify HSC from human sources were developed more than a decade ago (21), concerns remain that grafts depleted of T cells will engraft poorly and result in deficient immunity, discouraging translation of this technology to patient trials. However, the earlier clinical studies from the 1980s of TCD versus nonmanipulated BM grafts that reported increased rates of engraftment failure in the TCD group (4–7) were subsequently countered by trials showing equivalence in engraftment and overall survival with TCD, and variability regarding loss of GVT effects (9–12). Similarly, the direct correlation of TCD with infectious complications is questionable. In the largest randomized trial, in which patients on the TCD arm had a 1-log10 reduction of T cells, TCD was associated with an increased risk of serious infections, specifically CMV and Aspergillus (11). However, a more recent prospective trial using rigorous TCD (5-log10) and infusion of grafts with doses of CD34+ cells at levels comparable to MPB demonstrated engraftment in all evaluable patients and death from opportunistic infection in only 2% (12). Taken in aggregate, it is clear that heterogenous factors, such as the method of host conditioning, method of TCD, and post-HCT immune suppression, influence the outcomes. Yet, the perception that donor T cells improve hematopoietic and immune recovery persists and is used to justify the continued use of grafts that carry significant risk of GVHD (cumulative risk for grade 2–4 acute GVHD 30–50%) (22, 23) and a consequent transplant-related mortality of 10–25% (24).
The data presented here and elsewhere (25) contradict the conventional view that grafts of rigorously purified stem/progenitor cells will produce inferior immunity, and suggest that such conclusions should be re-examined. The body of data demonstrating the deleterious effects of GVHR on BM and lymphoid function (13–20, 26–28) is often overlooked or underestimated. We previously reported that even low amounts of T cells cause subclinical, yet lympho-depleting, GVHR (25). Here, we focused on an mHA-antigen–mismatched strain combination and used grafts that allowed us to distinguish between “new” HSC-derived hematopoiesis versus expansion of donor lymphocytes. Any expectation that posttransplantation hematopoiesis and immune recovery, even in the earliest days after graft infusion, is enhanced by addition of donor lymphoid cells (in the absence of pharmacologic immune suppression) was refuted in our model system. Blood production, as measured by absolute cell numbers and restoration of normalized ratios of myeloid to lymphoid elements in hematolymphoid tissues, was consistently superior for recipients of HSC alone as compared with HSC plus lymphocytes. Rather than promote HSC proliferation and differentiation, mature donor T cells suppressed their activities. The heavy infiltration by T and Mac1+ cells observed in the BM and lymphoid organs led us to conclude that suppression of HSC-derived hematopoiesis was mediated by alloreactive T cells that homeostatically expanded and induced a proinflammatory environment. It has been shown that cytokines, such as IL-6, TGFβ, TNFα, and IFNγ, suppress hematopoiesis by blocking lineage commitment (29), impairing cell division (30), and inducing programmed cell death (31). Inflammation can also damage the host cellular components of HSC niches (13), making the marrow inhospitable for blood cell production. Thus, the way that donor lymphocytes exert negative effects on hematopoiesis likely involves both direct and indirect perturbation of HSC function, survival, and engraftment.
Our data further highlight the differences in the origins of T cells that repopulate recipients of HSC as compared with HSC plus lymphoid cells. Recipients of pure HSC retained a large percentage of host T cells, and the levels of HSC-derived T cells rose gradually over time. In contrast, addition of splenocytes resulted in rapid elimination of host T and suppression of donor HSC-derived T lymphopoiesis. Spleen-derived CD8 T cells predominated in the BM early after transplantation, and a substantial proportion were reactive against the minor alloantigen H60, suggesting that CD8 T cells were responsible for clearance of the host lymphoid cells. CD4 T cells appeared to mediate GVHR and damage BM, as recipients of HSC plus CD4 T cells developed the full picture of B lymphopenia; however, incomplete eradication of host cells was observed more often. The pattern of host T-cell eradication by mature donor lymphocytes and expansion of the donor peripheral pool mirrors the clinical studies that have examined T-cell reconstitution posttransplantation (32, 33). For many months post-HCT, the T-cell compartment is abnormal, largely comprising activated T cells and few naive cells. This aberrant skewing is thought to occur because T cells expand homeostatically, and clones that recognize antigens present in the host in the peri-transplantation period (i.e., mismatched histocompatibility antigens or herpes viruses) can dominate whereas others are lost. Although early reconstitution of the T-cell compartment occurs by oligoclonal expansion, resulting in the loss of many antigen specifications, a complete T-cell repertoire can come only from naive T cells arising from stem/progenitors by way of the thymus.
The profound and lasting dominance of spleen- over HSC-derived lymphoid cells led us question whether CD45 allelic differences between the two donor parties contributed to this phenomenon. Historically, CD45 was thought to be nonantigenic (34), and CD45 congenic strains have been extensively used to determine engraftment and chimerism in congenic and MHC-matched HCT models. However, our data and others suggest that polymorphic CD45 expressed on hematopoietic cells is immunogenic (35–37). In our system, if mature donor lymphoid cells were CD45 disparate with the HSC-donor, complete elimination of host elements, domination of hematopoiesis by the donor lymphoid cells over the congenic HSC, and aggravated GVHD was observed. When all parties were CD45 identical, higher levels of residual host cells persisted, and the contribution of donor HSC to hematopoiesis was significantly increased.
In summary, our studies were motivated to better elucidate how donor immune cells influence HSC engraftment, hematolymphoid recovery, and establishment of donor chimerism. We expected that despite development of GVHD, grafts with mature donor T cells would augment HSC-derived hematopoiesis and we would observe quantitatively better lymphoid recovery compared with grafts composed solely of HSC, at least in the early phase post-HCT; rather, transplantations of pure HSC were uniformly superior in these regards. Achievement of doses of HSC relevant to clinical transplantation has already been proved. On a per-kilogram basis, the dose of HSC used in our studies (1.2 × 105 HSC/kg) that provided rapid and sustained hematopoietic recovery is less than the dose of homologous human CD34+Thy1+ HSC used in a clinical trial of autologous HCT (38), and is less than that contained in megadose CD34+ enriched grafts used in haplo-identical trials. Finally, although treatment of malignancies may require complete donor chimerism to achieve full GVT potency, other disease states, such as immune deficiencies and autoimmune disorders, do not require full conversion or may even benefit from mixed chimerism. Our findings on the superior effects on blood and lymphoid recovery after transplantation of pure HSC give us confidence that hematopoietic graft engineering will be possible, and that someday the problems of GVHD will be obsolete.
Methods
Mice.
Congenic C57BL/6 (B6) mice (H2b; Thy1.1; B6.CD45.1, B6.CD45.2, and B6.GFP) were used as donors of HSC and lymphocytes for BALB.B hosts (H2b, Thy1.2; CD45.2).
Hematopoietic Cell Transplantation.
BALB.B mice underwent lethal 800 cGy total body gamma irradiation before infusion of hematopoietic grafts. KTLS HSC were isolated by a modified procedure described by Spangrude et al. (39), which selects c-Kit+ Thy1.1lo-int Sca-1+ Lin− (CD3ε, CD4, CD5, CD8α, B220, Gr1, Mac1, TER-119) from cKit-enriched BM by FACS. In cotransfer experiments, 1 × 107 wSP, 8 × 106 ToTC, 4–5 × 106 CD4, or 2.5–3 × 106 CD8 T cells enriched from splenocytes were injected simultaneously with the HSC for GVHD induction.
Engraftment and Chimerism.
Donor engraftment was assessed in the blood on d30, 60, and 90 post-HCT. BM and spleens were harvested at different time points between d4 and 14 mo and analyzed by FACS.
Detailed descriptions of mice, hematopoietic stem cell isolation and transplantation, GVHD assessment, histology, WBC, chimerism, staining for flow cytometry, and statistical methods are provided in SI Methods.
Supplementary Material
Acknowledgments
We thank Kathryn Logronio for laboratory management; Joel Dollaga, Diosdado Escoto, and Ronald Mendoza for animal care; and the National Institutes of Health tetramer facility for kindly providing the customized H60 tetramers. This work was supported by a German Research Foundation (DFG) Postdoctoral Fellowship (to A.M.S.M.); a Stanford DARE Doctoral Fellowship (to J.A.L.); National Institutes of Health Grants RO1 HL087240 and PO1 CA049605; and a grant from the Snyder Foundation (to J.A.S).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009220107/-/DCSupplemental.
References
- 1.Horowitz M. Uses and growth of hematopoietic cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4th Ed. Oxford, UK: Wiley-Blackwell; 2009. pp. 15–21. [Google Scholar]
- 2.Sullivan KM, Muraro P, Tyndall A. Hematopoietic cell transplantation for autoimmune disease: Updates from Europe and the United States. Biol Blood Marrow Transplant. 2010;16(1, Suppl):S48–S56. doi: 10.1016/j.bbmt.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scandling JD, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 4.Martin PJ, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone Marrow Transplant. 1988;3:445–456. [PubMed] [Google Scholar]
- 5.Marmont AM, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 6.Kernan NA, et al. Graft failure after T-cell-depleted human leukocyte antigen identical marrow transplants for leukemia: I. Analysis of risk factors and results of secondary transplants. Blood. 1989;74:2227–2236. [PubMed] [Google Scholar]
- 7.Schmeiser T, et al. Infectious complications after allogeneic bone marrow transplantation with and without T-cell depletion of donor marrow. Infection. 1989;17:124–130. doi: 10.1007/BF01644010. [DOI] [PubMed] [Google Scholar]
- 8.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: Clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 9.Soiffer RJ, et al. Prevention of graft-versus-host disease by selective depletion of CD6-positive T lymphocytes from donor bone marrow. J Clin Oncol. 1992;10:1191–1200. doi: 10.1200/JCO.1992.10.7.1191. [DOI] [PubMed] [Google Scholar]
- 10.Keever CA, et al. Immune reconstitution following bone marrow transplantation: Comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989;73:1340–1350. [PubMed] [Google Scholar]
- 11.Wagner JE, Thompson JS, Carter SL, Kernan NA. Unrelated Donor Marrow Transplantation Trial Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): A multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 12.Jakubowski AA, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: Sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shono Y, et al. Bone marrow graft-versus-host disease: Early destruction of hematopoietic niche following MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115:5401–5411. doi: 10.1182/blood-2009-11-253559. [DOI] [PubMed] [Google Scholar]
- 14.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999;189:1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985;88:107–133. doi: 10.1111/j.1600-065x.1985.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 16.Fukushi N, et al. Thymus: A direct target tissue in graft-versus-host reaction after allogeneic bone marrow transplantation that results in abrogation of induction of self-tolerance. Proc Natl Acad Sci USA. 1990;87:6301–6305. doi: 10.1073/pnas.87.16.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holländer GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol. 1994;152:1609–1617. [PubMed] [Google Scholar]
- 18.Mori T, et al. Involvement of Fas-mediated apoptosis in the hematopoietic progenitor cells of graft-versus-host reaction-associated myelosuppression. Blood. 1998;92:101–107. [PubMed] [Google Scholar]
- 19.Piguet PF. GVHR elicited by products of class I or class II loci of the MHC: Analysis of the response of mouse T lymphocytes to products of class I and class II loci of the MHC in correlation with GVHR-induced mortality, medullary aplasia, and enteropathy. J Immunol. 1985;135:1637–1643. [PubMed] [Google Scholar]
- 20.Sprent J, et al. Profound atrophy of the bone marrow reflecting major histocompatibility complex class II-restricted destruction of stem cells by CD4+ cells. J Exp Med. 1994;180:307–317. doi: 10.1084/jem.180.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisdorf D. GVHD the nuts and bolts. Hematology (Am Soc Hematol Educ Program) 2007;2007:62–67. doi: 10.1182/asheducation-2007.1.62. [DOI] [PubMed] [Google Scholar]
- 23.Hahn T, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin MT, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 25.Tsao GJ, Allen JA, Logronio KA, Lazzeroni LC, Shizuru JA. Purified hematopoietic stem cell allografts reconstitute immunity superior to bone marrow. Proc Natl Acad Sci USA. 2009;106:3288–3293. doi: 10.1073/pnas.0813335106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker MB, Riley RL, Podack ER, Levy RB. Graft-versus-host-disease-associated lymphoid hypoplasia and B cell dysfunction is dependent upon donor T cell-mediated Fas-ligand function, but not perforin function. Proc Natl Acad Sci USA. 1997;94:1366–1371. doi: 10.1073/pnas.94.4.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakoda Y, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109:1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen VH, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945–953. doi: 10.1182/blood-2007-07-103895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda K, et al. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106:879–885. doi: 10.1182/blood-2005-02-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitnicka E, Ruscetti FW, Priestley GV, Wolf NS, Bartelmez SH. Transforming growth factor beta 1 directly and reversibly inhibits the initial cell divisions of long-term repopulating hematopoietic stem cells. Blood. 1996;88:82–88. [PubMed] [Google Scholar]
- 31.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165:538–546. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 32.Storek J, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 33.O'keefe CL, et al. Molecular TCR diagnostics can be used to identify shared clonotypes after allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2004;32:1010–1022. doi: 10.1016/j.exphem.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Sykes M, et al. Effects of T cell depletion in radiation bone marrow chimeras. III. Characterization of allogeneic bone marrow cell populations that increase allogeneic chimerism independently of graft-vs-host disease in mixed marrow recipients. J Immunol. 1989;143:3503–3511. [PubMed] [Google Scholar]
- 35.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Exner BG, Chilton PM, Schanie C, Ildstad ST. CD45 congenic bone marrow transplantation: Evidence for T cell-mediated immunity. Stem Cells. 2004;22:1039–1048. doi: 10.1634/stemcells.22-6-1039. [DOI] [PubMed] [Google Scholar]
- 37.van Os R, et al. Immunogenicity of Ly5 (CD45)-antigens hampers long-term engraftment following minimal conditioning in a murine bone marrow transplantation model. Stem Cells. 2001;19:80–87. doi: 10.1634/stemcells.19-1-80. [DOI] [PubMed] [Google Scholar]
- 38.Negrin RS, et al. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant. 2000;6:262–271. doi: 10.1016/s1083-8791(00)70008-5. [DOI] [PubMed] [Google Scholar]
- 39.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.