Abstract
Egypt has the highest prevalence of antibodies to hepatitis C virus (HCV) in the world, estimated nationally at 14.7%. An estimated 9.8% are chronically infected. Numerous HCV prevalence studies in Egypt have published various estimates from different Egyptian communities, suggesting that Egypt, relative to the other nations of the world, might be experiencing intense ongoing HCV transmission. More importantly, a new national study provided an opportunity to apply established epidemiologic models to estimate incidence. Validated mathematical models for estimating incidence from age-specific prevalence were used. All previous prevalence studies of HCV in Egypt were reviewed and used to estimate incidence provided that there was sufficient age-specific data required by the models. All reports of anti-HCV antibody prevalence were much higher than any single other national estimate. Age was the strongest and most consistently associated factor to HCV prevalence and HCV RNA positivity. It was not possible to establish a prior reference point for HCV prevalence or incidence to compare with the 2009 incidence estimates. The modeled incidence from the national study and collectively from the modeled incidence from the previous community studies was 6.9/1,000 [95% confidence interval (CI), 5.5–7.4] per person per year and 6.6/1,000 (95% CI, 5.1–7.0) per person per year, respectively. Projected to the age structure of the Egyptian population, more than 500,000 new HCV infections per year were estimated. Iatrogenic transmission is the most likely, underlining exposure to the ongoing transmission. The study demonstrates the urgency to reduce HCV transmission in Egypt.
Keywords: incidence, iatrogentic transmission, parenteral transmission, Middle East and North Africa, mathematical modeling
Hepatitis C virus (HCV), first identified in 1989, is strictly a blood-borne RNA viral infection in the family Flaviviridae. Humans are the only reservoir for this viral infection. HCV infection most often leads to an asymptomatic chronic state, which can later progress to active liver disease, liver failure, or primary hepatocellular carcinoma. Treatment of HCV is costly, beyond the reach of most patients in less-developed countries, requires 48 or more weeks to complete, and has serious adverse effects and low efficiency. HCV in a family member can be socially and economically detrimental. There is no vaccine for HCV.
The Middle East and North Africa region (MENA) suffers from high prevalence of unnecessary medical injections and transfusions, reuse of needles and syringes, needle-stick injuries among health care workers, and skin scarifications (1–9). Public health systems are overstretched in several countries, leading to some careless attitudes toward safety measures (6). Standard precautions are not routinely implemented in public and even less so in private practices, such as among dentists (10–12). Injections are the preferred mode of therapy even when alternative modes are equally effective (13, 14). At 4.3 per year, MENA has the highest rate of injections per person per year of all regions (15). MENA has also the highest levels of all regions in the proportions of incident hepatitis B virus (58.3%), HCV (81.7%), and HIV (7.2%) infections attributable to contaminated injections (15). Blood transfusions are performed even when not medically indicated (16). The first documented HIV outbreak in renal dialysis centers in the history of the HIV epidemic occurred in Egypt (17), which has witnessed yet a second outbreak in recent years (18).
HCV currently infects ≈2% of the world's population (19). Collectively, among all nations, the percentage positive for HCV ranges from 0.01% in Scandinavia to 3% in North Africa, with a single unique exception, Egypt (19). In 1992, when HCV antibody testing became widely available, the prevalence of HCV in Egypt was reported to be 10.8% among first-time blood donors (20). Since this discovery, many prevalence estimates of HCV have been reported, mostly from rural communities located in the northern Nile Delta. Two more recent prospective studies estimating the incidence rate of new HCV cases have also been published, suggesting ongoing transmission in their respective communities (21, 22). For more than a decade, Egypt has been widely regarded as having an epidemic, with the highest recorded prevalence of HCV in the world (19). HCV is currently the most significant public health problem in Egypt.
Explanations for this unique epidemic in Egypt have been an ongoing subject of controversy. The iatrogenic role of parenteral antischistosomal therapy campaigns to control endemic schistosomiasis, which ceased some decades ago, is a widely held hypothesis (23). There may have been considerable other concurrent iatrogenic exposures at the time. More recent evidence suggests a continuation of iatrogenic exposures that is contributing to ongoing HCV transmission (24, 25).
The recently published Egyptian Demographic Health Survey (EDHS) in 2009 was a national probability sample of the resident Egyptian population. This report estimated an overall anti-HCV antibody prevalence of 14.7% (26). The number of Egyptians estimated to be chronically infected was 9.8%. This report provides a precise national prevalence estimate and includes additional data on patterns of HCV prevalence by gender, age, urban vs. rural, and between different regions of the country.
The primary objective of this study was to use the past and now current estimates of HCV prevalence in Egypt to characterize the magnitude of HCV infection transmission.
Methods
HCV Data.
A search of all published peer-reviewed literature (English language) from 1992 to 2010 on HCV and Egypt was made using the National Library of Medicine, PubMed, Google Scholar, Web of Science, Biological Abstracts, manual review of citations in search-identified publications, and in Egypt for reports available only locally. Studies that (i) reported HCV prevalence or incidence, (ii) described the serologic methods, (iii) were of cross-sectional or prospective epidemiologic design, and (iv) could be abstracted for the purposes of the study were included. In addition, age or 5-y age group–specific prevalence data were required for estimating age-specific incidence. Publications based on clinical populations were not included.
Modeling Incidence Estimates.
Prevalence in a given population was defined as the total number of persons identified as positive for anti-HCV antibody divided by all tested-negative plus all tested-positive persons in the population. Prevalence times 100 is often reported as a percentage. Prevalence is a proportion, at a given point in time, of a defined condition in a defined population. The magnitude of prevalence is a direct function of incidence and increases with the number of new incidence cases (27).
Detecting new HCV infections is problematic. Soon after infection, HCV RNA viremia can be detected using nucleic acid testing (28), but rarely does this result in an acute symptomatic clinical illness. Symptoms, if and when they appear, may not be associated with disease onset, undermining the use of classic case–control studies to identify exposures. Direct estimation of incidence proportion or incidence rate requires prospective studies, which take considerable resources, large sample sizes, and time measured in years. National prospective studies would be impractical and doubtfully cost-effective.
The method used in this study for estimating incidence from prevalence was published by Leske et al. (29), Zou et al. (28), and others (30, 31). Zou et al. adapted the method for estimating HCV incidence from HCV prevalence in first-time blood donors. Using the notation of Leske et al. (29), incidence risk (∏) is a cumulative probability, which ranges from 0 to 1, of new HCV infections in a given time period, in this case either within a 5-y age group or single age group, and is estimated by:
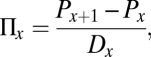 |
where ∏x is the cumulative probability of incidence cases for the age interval x, and x + 1 is the age interval in the next older age group, P is the prevalence proportion, and Dx is the age difference in the age group. For example, in 5-y age groups, Dx would equal 5.
There are several strict assumptions necessary to avoid distorted incidence estimates using this method:
Once positive for the anti-HCV antibody marker, an individual does not revert to anti-HCV antibody negative. Positive HCV antibody status is essentially irreversible and remains for the lifetime of the individual (30).
Incidence within an age interval is relatively stable. Age or ≤5-y age groups are sufficiently short periods for this assumption (30).
The biomarker of infection should appear shortly after infection. In HCV, there is a rather short delay from infection to the appearance of antibody, the so-called “window period,” usually considered to be approximately 50 days.
The sensitivity and specificity of anti-HCV antibody assays should not change significantly over time. Third-generation ELISA tests have replaced second-generation assays over the time period of this study. When incidence estimates were compared between studies using second-generation ELISA and studies using current third-generation assays, adjustments to second-generation were made using published sensitivity and specificity estimates for the two assays (32).
The demographic structure of the target population should be stable over time. The Egyptian population has grown over the study period, from 1992 to 2009, with only minor changes in the population age structure. This is a concern when there is significant migration, which is not the case in Egypt.
In general, when HCV prevalence is measured in Egyptian communities, it varies across 5-y age groups. Regression models were tested and used to smooth prevalence over age groups as described by Leske et al. (29) and in turn used the estimated prevalence for the beginning of each age interval. The SAS (SAS Institute) procedure FREQ with RISK DIFF included in the statement was used to calculate 95% confidence intervals (CI), as described by Zou et al. (28).
Incidence was estimated and tabulated from each report that met the criteria listed above. Data from the national sample (26) were used to generate an overall national estimate of ∏ (total population), ∏x,, and an overall estimate of the total population that would become infected with HCV in 1 y. Total population estimates for Egypt were obtained from the Center for Public Mobilization and Statistics (CAPMAS) (33) and related sources (34).
Given the importance of the EDHS nationally representative sample, needed for making a national representative estimate of incidence, the study design, sampling methods, and laboratory determination of HCV antibody and HCV RNA were scrutinized. The study design and sampling methods followed the strict guidelines set forward by the parent international Demographic Health Surveys (DHS) organization founded in 1984. DHS has since completed more than 240 studies in more than 85 countries, providing national representative data on health and population trends in developing countries (35). Laboratory methods for the detection of HCV antibody and HCV RNA were detailed in the EDHS report (26). Positive third-generation ELISA results were retested, and all dual-positives were confirmed by chemiluminescent microplate immunoassay. Quantitative real-time PCR was used at the Central Laboratory for the detection of HCV RNA. A stringent quality-control system was carried out in a separate government laboratory. The methodology used in the EDHS serology is robust, and it is unlikely that a known or unknown cross-reacting Flavivirus circulating in Egypt would have affected the prevalence measures.
An additional method to estimate incidence, given prevalence, was made using the data from each of the publications reviewed by study, whereby incidence was plotted against prevalence using each report as an observation. In this ecologic model, incidence was estimated for a given prevalence. Regression models were used to estimate the magnitude of association and curve fitting as described by Morgenstern (36).
Results
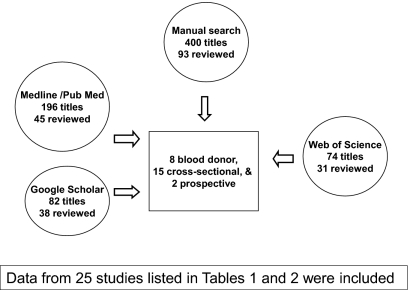
In total, 752 titles were found by using “hepatitis C” and “Egypt” as search words. There were 400 publications identified from searching individual references starting from the most recent studies that met all criteria and working back to 1992. Including the Internet databases, another 352 titles were screened. Only four reports were unique in addition to those identified manually. One report that met criteria for inclusion had to be obtained in Egypt. Fig. 1 shows the search results. Four study designs emerged from the reviewed literature that were included: blood donation studies, cross-sectional studies of rural communities, two national studies, and two prospective cohort studies. The reports that met the study criteria are listed by year, location, sample size, prevalence, incidence, and citation in Tables 1 and 2.
Fig. 1.
Publication search results for HCV epidemiologic studies in Egypt. Journal articles were screened for hepatitis C in Egypt, from which those that suggested clinical, treatment-related, or laboratory-based studies were excluded. Additional criteria for selection that were used included (i) reported HCV prevalence or incidence, (ii) described the serologic methods, (iii) were of cross-sectional or prospective epidemiologic design, and (iv) could be abstracted for the purposes of this study.
Table 1.
Anti-HCV antibody screening in blood donors at various locations and times in Egypt
| Year* | Sample size | Study type | Prevalence (%) | Incidence per 1,000 | Location | HCV assay | First author (reference) |
| 1992 | 13,483 | Blood donors | 10.8 | 3.7 | Cairo | Second generation | Kamel (20) |
| 1992 | 90 | Blood donors | 14.4 | —† | Cairo | Second generation | Darwish (50) |
| 1992 | 1,837 | Blood donors | 14.5 | — | Suez Canal | Second generation | el Gohary (51) |
| 1993 | 163 | Blood donors | 13.6 | — | Cairo | Second generation | Darwish (52) |
| 1993 | 2,644 | Blood donors | 24.8 | — | National | Second generation | Arthur (53) |
| 1995 | 188 | Blood donors | 26.6 | — | Cairo | Second generation | Bassily (54) |
| 1999 | 3,608 | Blood donors | 8.8 | — | National | Second generation | Tanaka (55) |
| 2009 | 55,922 | Blood donors | 7.4–17.7 | — | Nile Delta | Third generation | Ismail (37) |
*Year of investigation, not year of publication.
†Age-specific data could not be abstracted.
Table 2.
Studies of HCV in different communities, including the results of the EDHS study on a representative national sample
| Year* | Sample size | Study type | Prevalence (%) | Incidence per 1,000 | Location | HCV assay | First author (reference) |
| 1993 | 726 | Military recruits | 33.4 | —† | Alexandria | Second generation | Farghaly (39) |
| 1994 | 1,258 | Rural, CS | 18.7 | 6.4 | Nile Delta | Second generation | Kamel (44) |
| 1995 | 796 | Rural, CS | 40.0 | 15.0 | Nile Delta | Second generation | Darwish (45) |
| 1995‡ | 796 | Rural, CS | 40.0 | 15.0 | Nile Delta | Second generation | Rao (56) |
| 1996 | 155 | Rural, CS | 24.3 | — | Nile Delta | Second generation | Darwish (57) |
| 1996 | 7,357 | National, CS | 13.5 | — | National | Second generation | Mohamed (38) |
| 1997 | 506 | Rural, CS | 10.3 | — | Nile Delta | Second generation | el-Sayed (58) |
| 1997 | 3,888 | Rural, CS | 24.3 | 13.3 | Nile Delta | Second generation | Abdel-Aziz (46) |
| 1997‡ | 3,888 | Rural, CS | 24.3 | 13.3 | Nile Delta | Second generation | Habib (59) |
| 1999 | 6,031 | Rural, CS | 8.7 | 5.2 | Upper Egypt | Second generation | Nefeh (47) |
| 1999‡ | 6,031 | Rural, CS | 8.7 | 5.2 | Upper Egypt | Second generation | Medhat (42) |
| 2003 | 1,422 | Rural, CS | 25.8 | 10.0 | Nile Delta | Not given | el-Sadawy (48) |
| 2003 | 4,020 | Rural, CS | 11.8 | 7.4§ | Nile Delta | Third generation | Arafa (49) |
| 2007 | 1,520 | Rural, CS | 10.9 | — | Nile Delta | Third generation | Eassa (60) |
| 2008 | 11,126 | National, CS | 14.7 | 6.9 | National | Third generation | El-Zanaty (26) |
| 2005 | 6,734 | Rural, P | See note.¶ | 3.1/0.8 | ND/UP | Third generation | Mohamed (21) |
| 2008 | 2,171 | Rural, P | See note.¶ | 5.2 | Nile Delta | Third generation | Saleh (22) |
The two available prospective studies were placed at the bottom of the table. Incidence was estimated from prevalence reports that included age-specific data using the methods described by Leske et al. (29) and Zou et al. (28). CS = cross-sectional study design; ND = Nile Delta; UP = Upper Egypt (area south of the Nile Delta).
*Year of investigation, not year of publication.
†Age-specific data could not be abstracted.
‡Identical study population used in two publications.
§Interpolated from Lehman and Wilson (30).
¶Prospective study design: prevalence not estimated by design.
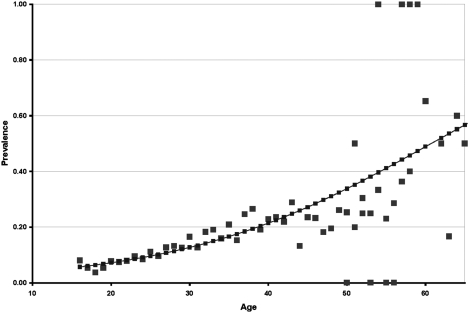
As shown in Table 1, the earliest report of anti-HCV antibody prevalence (10.8%) in Egypt was in 1992 (20). This study examined a large number of healthy first-time volunteer blood donors in Cairo. HCV prevalence in these blood donors increased monotonically with age (Fig. 2). For example, at age 20 y the prevalence was 7.5% and increased steadily to 20% at age 40 y.
Fig. 2.
Prevalence for anti-HCV antibody by age in 17,286 first-time healthy blood donors in 1992 by age (20). Logistic regression (solid line) demonstrates a monotonic increase of prevalence with age. Prevalence in ages 50 y and older had fewer data, causing statistical instability. Logistic regression: χ2 = 571.96, SE = 0.0025, df = 1, P = 0.000, r2 = 0.81, and 1/e(– α – β x), where α = −3.8063 and β = 0.0627.
HCV prevalence varied considerably, from 8.8% to 26.6%, in the reports of blood donors, owing primarily to differences in the age structure of the blood donors tested, as shown in Table 1. Blood donor studies were reported from 1992 to 2009. In a 2009 study on a large number of first-time volunteer blood donors in a central urban area in the Nile Delta, a trend in HCV prevalence over time was reported (37). In this study, the prevalence of HCV using third-generation assay in 2000 was 17.7%. Each year after 2000, the prevalence of HCV declined until the study completion in 2007, when 7.4% of donors were HCV positive.
As shown in Table 2, prevalence ranged from 8.7% to 40% between cross-sectional studies reported from 1993 to 2008. With two exceptions, all studies were conducted in different rural villages, mostly in the Nile Delta. All reports showed a similar age-specific increase, with HCV prevalence as shown in Fig. 2. Some differences between studies therefore could be explained by the different range of ages included in the respective studies, given the strong association of age with HCV prevalence. Collectively, the Nile Delta communities had a higher prevalence than the single estimate from Upper Egypt (the region of Egypt south of the Nile Delta), which suggested that Upper Egypt had a lower HCV prevalence than the Nile Delta.
The 1996 report of 13.5% HCV prevalence as a national estimate was from a secondary publication of data and incompletely documented (38). For example, estimates were given without SEs or CIs. Only 10 of the 26 governorates of Egypt were included. Age-specific prevalence data could not be abstracted from this report.
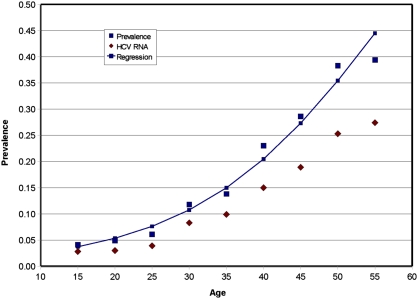
The EDHS data were based on a representative national sample, completed in 2008 and published in 2009 (26). As shown in Table 2, the overall country HCV prevalence was 0.147 ± 0.005. Fig. 3 shows the 5-y age group–specific HCV prevalence from the national study. Prevalence increased with each 5-y age group. The 2008 study also included the prevalence of HCV RNA, also shown in Fig. 2, which also increased sharply with age. Note, however, that HCV RNA prevalence, as expected, was consistently lower than HCV prevalence in every age group but that this difference increased slightly by age.
Fig. 3.
Prevalence of anti-HCV antibody positive and the prevalence of HCV RNA positive by 5-y age groups from the EDHS national sample (26). This graph was prepared from data abstracted from the EDHS national sample (26). The line was fitted by logistic regression for anti-HCV antibody prevalence. Logistic regression: χ2 = 1177.97, df = 1, SE = 0.0024, P = 0.000, r2 = 0.96, and 1/e(– α – β x), where α = −4.4 β = 0.0758.
The data in Fig. 3 represent both urban and rural populations; the prevalence in the 20–24-y age group was 0.05, which is less than the blood donor data for the same age group more than a decade previously, 0.078 (Fig. 2). Prevalence also increased by age. Comparing the regression lines in Figs. 2 and 3, HCV prevalence increased more sharply with age in the national survey. SEs are provided in the EDHS report (26).
Two prospective studies were completed in 2005 and 2008 (21, 22). The incidence reported from both studies demonstrated ongoing transmission of HCV. However, there were large differences in incidence reported, as shown in Table 2. This can be best explained by differences in study design and the respective target populations. The 2005 study started as a cross-sectional design in two rural communities, one in the Nile Delta and the other in Upper Egypt. The incidence from this report was 3.1/1,000 and 0.8/1,000 for the Nile Delta site and Upper Egypt site, respectively. Intrafamilial transmission was suggested by the authors. The 2008 study was a prospective study of pregnant females at a large urban medical center in the Nile Delta. The incidence was 5.2/1,000, and iatrogenic transmission was reported.
Incidence Estimates from HCV Prevalence.
Incidence among the first-time blood donors in 1992 was estimated to be 3.7/1,000 (95% CI, 3.66–4.4). The lack of age-specific prevalence data provided in the other reports of blood donors prevented incidence estimation.
Among the studies shown in Table 2, modeled incidence estimates are given for all prevalence studies that published age-specific data. In all cases, a logistic regression was found to fit the age-specific data best. The derived incidence estimates also varied among these studies, ranging from 5.2/1,000 to 15/1,000 persons per year.
A logistic regression was found to be the best fit for age-specific HCV prevalence, as shown in Fig. 3. The logistic regression coefficients (where α = −4.4 and β = 0.0758) were used to estimate the incidence from the 2008 national sample. The overall HCV incidence estimated by this model was 6.9/1,000 persons per year. As shown in Table 2, this estimate was lower compared with most of the other cross-sectional prevalence studies but slightly higher than incidence estimates from the two prospective studies.
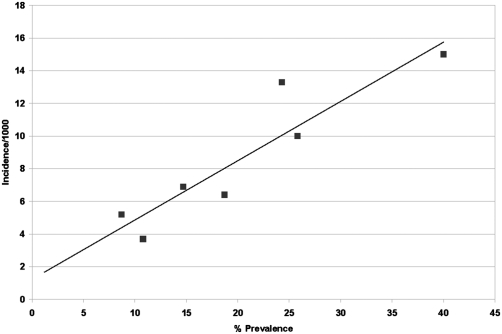
The variation of incidence derived from the individual cross-sectional studies can be best explained by their respective prevalence (given control for age). This is demonstrated in Fig. 4, which plots incidence as a function of prevalence for each cross-sectional study for which incidence could be estimated (n = 7). A linear regression with coefficients of α = 1.2 and β = 0.36 was found to have the best fit. Note that the r2 = 0.85 or 85% of the variation in incidence was due to variation in prevalence. The estimated incidence using this relationship for a prevalence of 14.7%, the national estimate, was 6.5/1,000 persons per year.
Fig. 4.
Relationship between estimated incidence and overall HCV prevalence from six community cross-sectional studies (44–49). The age-specific prevalence was abstracted from each of six cross-sectional reports. Prevalence from the EDHS (26) was also included as a seventh observation. In turn, these data were used as described in Methods to generate an estimate of report-specific incidence, which can be seen in Table 2. Report-specific prevalence and incidence points were plotted on a linear x-y graph with percentage prevalence on the x axis and incidence per 1,000 per year on the y axis. Linear regression: α + βx, where α = 1.23 and β = 0.363, r2 = 0.85 was the best fit and demonstrates an internal validation of the model used to generate a national incidence estimate.
At the time of this study, CAPMAS (33) reported the current Egyptian population to be 77,156,000 million. The number of new HCV infections, using the EDHS age structure estimated from the national study sample, would be 537,066 (95% CI, 512,372–577,782) persons per year. This estimation is shown in Table 3. Of these, ≈70,000 would be pediatric infections or in those aged <20 y.
Table 3.
Estimation of HCV incidence
| Age group (y) | Estimated* population | Regression estimated prevalence | Incidence estimate | No. of incident cases | Prevalence HCV RNA | No. of viremic cases |
| <5 | 9,081,000 | 0.0122 | 0.0011 | 10,064 | —† | —† |
| 5–9 | 8,489,000 | 0.0178 | 0.0016 | 13,556 | — | — |
| 10–14 | 8,155,000 | 0.0258 | 0.0023 | 18,647 | — | — |
| 15–19 | 9,123,000 | 0.0372 | 0.0032 | 29,608 | 0.028 | 227,437 |
| 20–24 | 7,828,000 | 0.0534 | 0.0045 | 35,611 | 0.030 | 241,362 |
| 25–29 | 6,405,000 | 0.0762 | 0.0063 | 40,133 | 0.039 | 256,447 |
| 30–34 | 5,073,000 | 0.1075 | 0.0084 | 42,742 | 0.083 | 404,513 |
| 35–39 | 4,587,000 | 0.1496 | 0.0110 | 50,323 | 0.099 | 436,540 |
| 40–44 | 4,154,000 | 0.2045 | 0.0137 | 56,915 | 0.150 | 638,216 |
| 45–49 | 3,588,000 | 0.2730 | 0.0162 | 58,297 | 0.189 | 731,047 |
| 50–54 | 2,948,000 | 0.3542 | 0.0181 | 53,430 | 0.253 | 802,450 |
| 55–59 | 2,568,000 | 0.4449 | 0.0189 | 48,506 | 0.274 | 720,681 |
| 60–64 | 1,702,000 | 0.5393 | 0.0183 | 31,216 | — | — |
| 65–69 | 1,385,000 | 0.6310 | 0.0166 | 23,026 | — | — |
| 70–74 | 1,016,000 | 0.7141 | 0.0142 | 14,383 | — | — |
| 75–79 | 645,000 | 0.7849 | 0.0114 | 7,370 | — | — |
| 80–84 | 298,000 | 0.8420 | 0.0088 | 2,632 | — | — |
| 85–89 | 92,000 | 0.8862 | 0.0066 | 607 | ||
| 90–94 | 17,000 | 0.9192 | 0.0000 | 0 | ||
| 95–99 | 2,000 | 0.0000 | 0 | |||
| 100+ | 0 | |||||
| 77,156,000 | 537,066‡ | 4,458,693 |
Using logistic regression, HCV prevalence was estimated for those aged <15 y and >56 y using the data from EDHS (26). Incidence estimates for each 5-y age group was calculated as described in Methods. The number of new or incident cases is a product of the incidence estimate and population in each 5-y age group, respectively. The number of viremic cases was a product of HCV RNA prevalence and the respective 5-y age group population.
*Estimated from the age structure of the 2005 Egyptian population (34) and the current population estimated of Egypt from CAPMAS (33).
†No population estimates of the number of viremic persons were made for these age groups.
‡See text for 95% CI for the total number of incident cases.
The 2008 prevalence data for HCV RNA can also be used to estimate the number of persons who are HCV RNA positive in each age group. Combined, this is 4,458,691 persons. This is an underestimated human reservoir of HCV in Egypt, because those aged <15 y and >55 y were not included.
Discussion
The major conclusion of this study is that the incidence of HCV in Egypt seems to be continuing at a rate of ≈6.9/1,000 persons per year, indicative of possibly ongoing hyperepidemic transmission. This estimate was modeled from the 2008 national HCV survey, which has a well-documented representative sample design with small SEs (26). A similar estimate of incidence was made using an ecologic model from the available, mostly rural, community studies. This result is, in part, due to the classic relationship between prevalence and incidence (27) and provides a measure of internal validly to the study. Results from the two published prospective studies confirm ongoing transmission at these levels, at least in the Nile Delta. However, estimates from these reports cannot be inferred to the general population.
Increasing HCV prevalence was consistently found to be associated with age in all reports. Because of this strong association, the differences in prevalence in most studies could be explained by differences in the age ranges included and location of study. All cross-sectional results including reports on blood donors had higher HCV prevalences than any single other country in the world.
It bears notice that the EDHS study showed the age-specific relationship between HCV prevalence and HCV RNA prevalence. This in turn was used to estimate the number of Egyptians positive for HCV RNA, or essentially infectious. Because the younger and older age groups were not included, this is an underestimate of the human HCV reservoir in Egypt. This also delineates the potential magnitude of the future disease burden due to chronic liver disease and its squelae.
The temptation to infer temporal changes between the first report in 1992 or other previous studies and the 2008 national prevalence should be discouraged. No report was discovered in the search of literature that was designed to demonstrate temporal trends, with the possible exception of the 2009 blood donor study (37). This study included a large number of first-time volunteer blood donors in the Mansoura area (Dakahlia) recruited over a period of 8 y. During this time HCV prevalence among these donors declined from 17.7% to 7.4%. There was no analysis in this study for age structure changes that may have occurred over time. Increasing numbers of younger donors would have reduced prevalence. Nor was urban/rural residence assessed. Increasing numbers of urban residences could also have reduced prevalence. It is not possible to know whether there were changes in attitudes among the self-selected volunteers over time or whether recruitment methods of blood donors became more discerning given that better recruitment methods would reduce collection costs. As in all cross-sectional designs, the time of infection by HCV cannot be known from serology. Finally, self-selected volunteer blood donors in Mansoura are not representative of any other group or community in the Nile Delta or the whole of the country. The EDHS HCV prevalence estimate for the Mansoura was 17.8%, which is larger than the 7.4% HCV prevalence of these blood donors in 2008.
The only report to date based on a national representative sample was EDHS (26). This study was limited by design to report only cross-sectional prevalence estimates at a point in time from which no temporal inferences can be made. In conclusion, no other suitable historical estimate for HCV prevalence for Egypt could be found for which valid historical comparisons could be made. Given the natural history of HCV, an estimate of 6.9/1,000 per person per year, and the limits of epidemiologic study designs, demonstrating changes in HCV incidence over time would be challenging and costly. The issue of how to demonstrate the efficacy of prevention interventions to reduce HCV incidence in light of this should be urgently studied.
Our modeling of the EDHS age-specific data to obtain a broad national estimate of incidence is based on stated assumptions. These were the same assumptions used by Lehman and Wilson (30), who published age group–specific HCV incidence using data from a single rural community in the Nile Delta. Others have validated this model to estimate HIV incidence (31). Because our estimate of HCV incidence is based on a representative national sample, inference can be made to the entire Egyptian population. Moreover, the number of new cases, using the current population estimate from CAPMAS and the population age structure of the EDHS sample, is based on low sampling errors. The number of new HCV infections per person per year is large and is likely the highest in the world for a national population. Moreover, the inability to include newly infected persons who were in the “window” period before the development of HCV-specific antibodies at the time that the blood specimens were taken, results in ascertainment bias and, accordingly, underestimation of the true incidence (28).
Regardless of the stated strict assumptions, the results in this study should serve as a strong rationale for recognizing that HCV transmission is ongoing and supports the justification to confirm and validate the estimated 6.9/1,000 new cases of HCV per year at the national level.
Currently it is not possible, owing to the study design limits of the available EDHS data, to make firm causal inferences regarding the current principal mode of HCV transmission in Egypt or historical trends of HCV prevalence. For now, it is prudent for policy makers and public health professionals to target prevention measures at all HCV transmission routes. This is especially true for current and ongoing iatrogenic transmission of HCV in Egypt. The latter is emphasized on the basis of a significant body of literature available on current iatrogenic routes of HCV transmission in Egypt (39–42). For example, this includes excessive numbers of nontherapeutic injections (24), poor or nonexistent infection control in health and dental care facilities (25), and unacceptable HCV seroconversion rates in hemodialysis units (17, 43). Considering the size of estimated new cases of HCV and the current overwhelming demand by Egyptian patients seeking treatment for HCV infection, reduction of HCV transmission, specifically iatrogenic transmission, should be given a higher public health priority.
Acknowledgments
We thank Dr. Shimian Zou (Transmissible Disease Department, Jerome H. Holland Laboratory for the Biomedical Sciences, American Red Cross, Rockville, MD) for invaluable comments, discussions, and encouragement; and anonymous reviewers for comments and suggestions. This study was supported by the William Fulbright Scholarship program and Qatar National Research Fund.
Footnotes
The authors declare no conflict of interest.
References
- 1.Khawaja ZA, Gibney L, Ahmed AJ, Vermund SH. HIV/AIDS and its risk factors in Pakistan. AIDS. 1997;11:843–848. doi: 10.1097/00002030-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 2.World Bank Group . World Bank Update 2005: HIV/AIDS in Pakistan. Washington, DC: World Bank; 2005. [Google Scholar]
- 3.Yerly S, et al. Nosocomial outbreak of multiple bloodborne viral infections. J Infect Dis. 2001;184:369–372. doi: 10.1086/322036. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Raddad L, et al. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa. Time for Strategic Action. 2010. Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. World Bank/UNAIDS/WHO Publication. [Google Scholar]
- 5.Laraqui O, et al. Assessing knowledge, attitude, and practice on occupational blood exposure in caregiving facilities, in Morocco (in French) Med Mal Infect. 2008;38:658–666. doi: 10.1016/j.medmal.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Zafar A, Aslam N, Nasir N, Meraj R, Mehraj V. Knowledge, attitudes and practices of health care workers regarding needle stick injuries at a tertiary care hospital in Pakistan. J Pak Med Assoc. 2008;58:57–60. [PubMed] [Google Scholar]
- 7.Hossini CH, et al. [Knowledge and attitudes of health care professionals with respect to AIDS and the risk of occupational transmission of HIV in 2 Moroccan hospitals] Sante. 2000;10:315–321. [PubMed] [Google Scholar]
- 8.Yemen Ministry of Health . National Strategic Framework for the Control and Prevention of HIV/AIDS in the Republic of Yemen. Sana, Yemen: Yemen Ministry of Health; [Google Scholar]
- 9.Kennedy M, O'Reilly D, Mah MW. The use of a quality-improvement approach to reduce needlestick injuries in a Saudi Arabian hospital. Clin Perform Qual Health Care. 1998;6:79–83. [PubMed] [Google Scholar]
- 10.Askarian M, Mirzaei K, McLaws ML. Attitudes, beliefs, and infection control practices of Iranian dentists associated with HIV-positive patients. Am J Infect Control. 2006;34:530–533. doi: 10.1016/j.ajic.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Askarian M, Mirzaei K, Cookson B. Knowledge, attitudes, and practice of Iranian dentists with regard to HIV-related disease. Infect Control Hosp Epidemiol. 2007;28:83–87. doi: 10.1086/509851. [DOI] [PubMed] [Google Scholar]
- 12.Kabbash IA, et al. Risk perception and precautions taken by health care workers for HIV infection in haemodialysis units in Egypt. East Mediterr Health J. 2007;13:392–407. [PubMed] [Google Scholar]
- 13.Janjua NZ, Hutin YJ, Akhtar S, Ahmad K. Population beliefs about the efficacy of injections in Pakistan's Sindh province. Public Health. 2006;120:824–833. doi: 10.1016/j.puhe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Altaf A, et al. Determinants of therapeutic injection overuse among communities in Sindh, Pakistan. J Ayub Med Coll Abbottabad. 2004;16:35–38. [PubMed] [Google Scholar]
- 15.Hauri AM, Armstrong GL, Hutin YJ. The global burden of disease attributable to contaminated injections given in health care settings. Int J STD AIDS. 2004;15:7–16. doi: 10.1258/095646204322637182. [DOI] [PubMed] [Google Scholar]
- 16.Mujeeb SA. Blood transfusion—a potential source of HIV/AIDS spread. J Pak Med Assoc. 1993;43:1. [PubMed] [Google Scholar]
- 17.Hassan NF, et al. HIV infection in renal dialysis patients in Egypt. AIDS. 1994;8:853. doi: 10.1097/00002030-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 18.El Sayed NM, et al. Epidemic transmission of human immunodeficiency virus in renal dialysis centers in Egypt. J Infect Dis. 2000;181:91–97. doi: 10.1086/315167. [DOI] [PubMed] [Google Scholar]
- 19.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamel MA, et al. High HCV prevalence in Egyptian blood donors. Lancet. 1992;340:427. doi: 10.1016/0140-6736(92)91508-6. [DOI] [PubMed] [Google Scholar]
- 21.Mohamed MK, et al. Intrafamilial transmission of hepatitis C in Egypt. Hepatology. 2005;42:683–687. doi: 10.1002/hep.20811. [DOI] [PubMed] [Google Scholar]
- 22.Saleh DA, et al. Incidence and risk factors for hepatitis C infection in a cohort of women in rural Egypt. Trans R Soc Trop Med Hyg. 2008;102:921–928. doi: 10.1016/j.trstmh.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank C, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 24.Talaat M, et al. Overview of injection practices in two governorates in Egypt. Trop Med Int Health. 2003;8:234–241. doi: 10.1046/j.1365-3156.2003.01015.x. [DOI] [PubMed] [Google Scholar]
- 25.Talaat M, et al. Evolution of infection control in Egypt: Achievements and challenges. Am J Infect Control. 2006;34:193–200. doi: 10.1016/j.ajic.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 26.El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Egyptian: Ministry of Health. Cairo: El-Zanaty and Associates and Macro International; 2009. p. 431. [Google Scholar]
- 27.Kleinbaum D, Kupper L, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York: John Wiley & Sons; 1982. p. 519. [Google Scholar]
- 28.Zou S, Fang CT, Dodd RY. A method for estimating incidence rate of infectious diseases among first-time blood donors. Transfusion. 2008;48:1827–1832. doi: 10.1111/j.1537-2995.2008.01750.x. [DOI] [PubMed] [Google Scholar]
- 29.Leske MC, Ederer F, Podgor M. Estimating incidence from age-specific prevalence in glaucoma. Am J Epidemiol. 1981;113:606–613. doi: 10.1093/oxfordjournals.aje.a113138. [DOI] [PubMed] [Google Scholar]
- 30.Lehman EM, Wilson ML. Epidemic hepatitis C virus infection in Egypt: estimates of past incidence and future morbidity and mortality. J Viral Hepat. 2009;16:650–658. doi: 10.1111/j.1365-2893.2009.01115.x. [DOI] [PubMed] [Google Scholar]
- 31.Saidel T, Sokal D, Rice J, Buzingo T, Hassig S. Validation of a method to estimate age-specific human immunodeficiency virus (HIV) incidence rates in developing countries using population-based seroprevalence data. Am J Epidemiol. 1996;144:214–223. doi: 10.1093/oxfordjournals.aje.a008916. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Hamid M, et al. Comparison of second- and third-generation enzyme immunoassays for detecting antibodies to hepatitis C virus. J Clin Microbiol. 2002;40:1656–1659. doi: 10.1128/JCM.40.5.1656-1659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CAPMAS Center for Public Mobilization and Statistics. 2009 Available at: http://www.capmas.gov.eg/. Accessed September 2, 2009. [Google Scholar]
- 34.United Nations, Department of Economic and Social Affairs, Population Division . World Population Prospects: The 2008 Revision. New York: United Nations; 2008. [Google Scholar]
- 35.Measure DHS . Demographic and Health Surveys. 2010. Available at: http://www.measuredhs.com/start.cfm. Accessed October 14, 2009. [Google Scholar]
- 36.Morgenstern H. Uses of ecologic analysis in epidemiologic research. Am J Public Health. 1982;72:1336–1344. doi: 10.2105/ajph.72.12.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ismail AM, Ziada HN, Sheashaa HA, Shehab El-Din AB. Decline of viral hepatitis prevalence among asymptomatic Egyptian blood donors: A glimmer of hope. Eur J Intern Med. 2009;20:490–493. doi: 10.1016/j.ejim.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Mohamad M. Epidemiology of HCV in Egypt. Afro-Arab Liver J. 2004;3:41–52. [Google Scholar]
- 39.Farghaly AG, Barakat RM. Prevalence, impact and risk factors of hepatitis C infection. J Egypt Public Health Assoc. 1993;68:63–79. [PubMed] [Google Scholar]
- 40.Farghaly AG, Mansour GA, Mahdy NH, Yousri A. Hepatitis B and C virus infections among patients with gingivitis and adult periodontitis: Seroprevalence and public health importance. J Egypt Public Health Assoc. 1998;73:707–735. [PubMed] [Google Scholar]
- 41.Lurie Y, et al. Acute hepatitis C in Israel: A predominantly iatrogenic disease? J Gastroenterol Hepatol. 2007;22:158–164. doi: 10.1111/j.1440-1746.2006.04491.x. [DOI] [PubMed] [Google Scholar]
- 42.Medhat A, et al. Hepatitis c in a community in Upper Egypt: Risk factors for infection. Am J Trop Med Hyg. 2002;66:633–638. doi: 10.4269/ajtmh.2002.66.633. [DOI] [PubMed] [Google Scholar]
- 43.Hassan AA, Khalil R. Hepatitis C in dialysis patients in egypt: Relationship to dialysis duration, blood transfusion, and liver disease. Saudi J Kidney Dis Transpl. 2000;11:72–73. [PubMed] [Google Scholar]
- 44.Kamel MA, et al. The epidemiology of Schistosoma mansoni, hepatitis B and hepatitis C infection in Egypt. Ann Trop Med Parasitol. 1994;88:501–509. doi: 10.1080/00034983.1994.11812897. [DOI] [PubMed] [Google Scholar]
- 45.Darwish NM, Abbas MO, Hady SI, Mohammed TA. Study of the high prevalence of HCV in Egypt. J Egypt Public Health Assoc. 1995;70:397–414. [PubMed] [Google Scholar]
- 46.Abdel-Aziz F, et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: Population description and HCV prevalence. Hepatology. 2000;32:111–115. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- 47.Nafeh MA, et al. Hepatitis C in a community in Upper Egypt: I. Cross-sectional survey. Am J Trop Med Hyg. 2000;63:236–241. [PubMed] [Google Scholar]
- 48.el-Sadawy M, et al. Hepatitis C virus infection at Sharkia Governorate, Egypt: Seroprevalence and associated risk factors. J Egypt Soc Parasitol. 2004;34(1,)(Suppl)):367–384. [PubMed] [Google Scholar]
- 49.Arafa N, et al. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J Hepatol. 2005;43:418–424. doi: 10.1016/j.jhep.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 50.Darwish NM, Abbas MO, Abdelfattah FM, Darwish MA. Hepatitis C virus infection in blood donors in Egypt. J Egypt Public Health Assoc. 1992;67:223–236. [PubMed] [Google Scholar]
- 51.el Gohary A, et al. High prevalence of hepatitis C virus among urban and rural population groups in Egypt. Acta Trop. 1995;59:155–161. doi: 10.1016/0001-706x(95)00075-p. [DOI] [PubMed] [Google Scholar]
- 52.Darwish MA, et al. Risk factors associated with a high seroprevalence of hepatitis C virus infection in Egyptian blood donors. Am J Trop Med Hyg. 1993;49:440–447. doi: 10.4269/ajtmh.1993.49.440. [DOI] [PubMed] [Google Scholar]
- 53.Arthur RR, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271–274. doi: 10.1016/s0035-9203(97)90070-5. [DOI] [PubMed] [Google Scholar]
- 54.Bassily S, Hyams KC, Fouad RA, Samaan MD, Hibbs RG. A high risk of hepatitis C infection among Egyptian blood donors: the role of parenteral drug abuse. Am J Trop Med Hyg. 1995;52:503–505. doi: 10.4269/ajtmh.1995.52.503. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka Y, et al. Exponential spread of hepatitis C virus genotype 4a in Egypt. J Mol Evol. 2004;58:191–195. doi: 10.1007/s00239-003-2541-3. [DOI] [PubMed] [Google Scholar]
- 56.Rao MR, et al. Further evidence for association of hepatitis C infection with parenteral schistosomiasis treatment in Egypt. BMC Infect Dis. 2002;2:29. doi: 10.1186/1471-2334-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darwish MA, Faris R, Clemens JD, Rao MR, Edelman R. High seroprevalence of hepatitis A, B, C, and E viruses in residents in an Egyptian village in The Nile Delta: A pilot study. Am J Trop Med Hyg. 1996;54:554–558. doi: 10.4269/ajtmh.1996.54.554. [DOI] [PubMed] [Google Scholar]
- 58.el-Sayed HF, Abaza SM, Mehanna S, Winch PJ. The prevalence of hepatitis B and C infections among immigrants to a newly reclaimed area endemic for Schistosoma mansoni in Sinai, Egypt. Acta Trop. 1997;68:229–237. doi: 10.1016/s0001-706x(97)00097-1. [DOI] [PubMed] [Google Scholar]
- 59.Habib M, et al. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248–253. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 60.Eassa S, Eissa M, Sharaf SM, Ibrahim MH, Hassanein OM. Prevalence of hepatitis C virus infection and evaluation of a health education program in el-ghar village in zagazig, egypt. J Egypt Public Health Assoc. 2007;82:379–404. [PubMed] [Google Scholar]