Abstract
Models of learning-dependent sensory cortex plasticity require local activity and reinforcement. An alternative proposes that neural activity involved in anticipation of a sensory stimulus, or the preparatory set, can direct plasticity so that changes could occur in regions of sensory cortex lacking activity. To test the necessity of target-induced activity for initial sensory learning, we trained rats to detect a low-frequency sound. After learning, Arc expression and physiologically measured neuroplasticity were strong in a high-frequency auditory cortex region with very weak target-induced activity in control animals. After 14 sessions, Arc and neuroplasticity were aligned with target-induced activity. The temporal and topographic correspondence between Arc and neuroplasticity suggests Arc may be intrinsic to the neuroplasticity underlying perceptual learning. Furthermore, not all neuroplasticity could be explained by activity-dependent models but can be explained if the neural activity involved in the preparatory set directs plasticity.
Keywords: rat, instrumental learning, neurophysiology
The brain's ability to modify its own structure and function is currently used in diverse therapies involving remediation of language-learning impairment, reversing age-related cognitive decline, stroke rehabilitation, and others. These powers of neuroplasticity stem from the mammalian brain's ability to reorganize in response to experience (1–4). In sensory cortex, plasticity induced by sensory tasks optimizes the brain's capacity for future performance through amplifying activity that is most informative about reinforcement (4–6). Dominant models of this plasticity are activity-dependent (2, 7, 8), and associated learning is presumed to be a function of endogenous processes reliant on gene activation, because long-term plasticity and long-term memory require protein synthesis (9) following expression of immediate-early genes such as Arc (10, 11). Although models of sensory cortex plasticity are dependent on activity, these models fail to account for plasticity and behavioral phenomena observed shortly after learning.
A prediction of activity-dependent models is that the strongest and most selective population responses to a target stimulus should grow the most. However, in contrast to this prediction, cortical patterns of activity early after learning enter a weakly selective and highly responsive state that is subsequently refined into a highly selective and less responsive state (3). The same phenomenon is reflected behaviorally. If auditory stimuli are paired with nucleus basalis stimulation, acoustically induced changes in respiratory rate can initially be induced by a wide range of sound frequencies, and these induced respiratory changes become more frequency-specific with time (12). These findings suggest that cortical regions representing sensory stimuli other than the learned sensory stimulus may be recruited in brain plasticity shortly after learning. This conclusion contrasts with activity-dependent models. Although memory consolidation requires expression of immediate early genes like Arc (10, 13–15), it is unknown if Arc is required for plasticity in cortical regions that lack stimulus-induced activity. Although Arc expression has been observed with high levels of neuronal activity (16–21), such activity is not always sufficient (22–24). Direct tests linking Arc expression to neural activity require control over neural activity in a topographically specific manner, or a simultaneous assessment of action potential activity and Arc expression on a neuron-by-neuron basis.
In the present study, we test the hypothesis that sensory-evoked action potential responses are necessary for sensory remapping and Arc expression. We use the topography in rat auditory cortex to define regions in which a given sensory stimulus either produces robust activity or minimally affects action potential rates. Our results suggest a mechanism for plasticity in sensory cortex that is not simply activity-driven but may still be linked to Arc expression.
Results
Tone Detection Learning Quickly Reorganizes Activity in Primary Auditory Cortex.
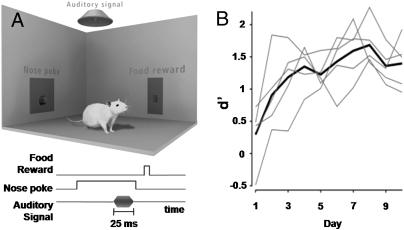
To induce cortical plasticity in a behaviorally relevant manner, rats were trained to hold a nose poke for at least 800 ms to earn a food pellet reward. Subsequently, animals were trained in an auditory detection task that used the same operant nose poke. The detection task required the rat to break its nose poke after the target sound and within reaction time limits to earn the food reward (Fig. 1A). The auditory target was a 50-dB, 5-kHz tone, and animal performance metrics were measured in terms of d′, a signal detection measure that increases with tone detectability as inferred from the rat's behavioral performance. Substantial improvements in target detection occurred through the fourth behavioral session (Fig. 1B).
Fig. 1.
Instrumental training for tone detection task. (A) Operant chamber used for training rats in tone detection task. Devices include a nose poke, food pellet dispenser, and speaker. The chronological sequence of the instrumental detection task for a 5-kHz 50-dB tone is shown. (B) For rats in the 14-d group, d′ results vs. number of training days. Thin lines plot results from individual animals, whereas the thick line shows the group average. ANOVA shows a significant effect of training day on d′ (P < 0.005), and post hoc testing finds that days 1 and 2 are significantly lower than all days, with d′ greater than or equal to that on day 4.
Animals were studied either 23 h after their first session of tone detection (1-day) or 23 h following 2 wk of continual training (14-d). All animals in the 1-day group reached learning criteria during that first session. These criteria required the rat to detect the tone in 15 trials in which the tone was presented 400 ms after the nose poke initiation and, concurrently, to have fewer than five false-positive results. Trials with the delays of 400 and 800 ms between the nose poke and tone were interleaved. The d′ was calculated a posteriori in all animals.
Before microelectrode mapping of primary auditory cortex (A1), optical intrinsic signal imaging was conducted to divide A1 into a low-frequency (LF) region and a high-frequency (HF) region (Fig. S1A). Microelectrode penetrations were centered in the A1 portions of the LF and HF regions (Fig. S1B). At each location, action potential responses to a large battery of tones within the rat audiogram were recorded (Fig. S1C). Data collected from these recording were used to construct frequency-intensity plots for each individual penetration (Fig. S1D).
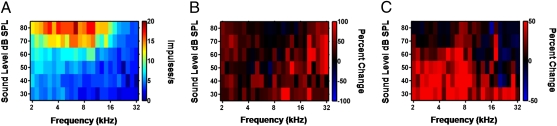
Frequency-intensity plots were pooled within groups. The control group plot (Fig. 2A) shows unrefined A1 tuning with activity broadly distributed across frequencies of high intensity. One day of training resulted in an increase in action potential rate of 32.39% averaged across all frequencies and intensities (Fig. 2B). Changes in the frequencies and intensities close to the target [i.e., those less than 8 kHz and less than 70-dB sound pressure level (SPL)], were significantly weaker (t test, P < 0.01) and less than half the average of those observed at other frequencies and intensities in the plot.
Fig. 2.
Early plasticity in A1 is not organized around the learned tone. (A) Population frequency-intensity plot for the control group is shown. Each pixel color codes the average A1 response to a tone of the corresponding frequency and intensity. (B) Percent change in A1 activity after 1 d of tone detection as compared with controls. Changes are significantly stronger at frequency intensities away from the target. (C) Percent change in A1 activity in the 14-day group compared with the 1-d group. Changes are significantly stronger close to the target. Pixels at the ends of the scale may exceed scale limits.
Responses to tonal sounds were significantly enhanced in the target section of the frequency-intensity plot in 14-d animals relative to the nontarget section (t test, P < 0.001), although increases were still observed in the nontarget section (Fig. 2C). These findings indicate that cortical modifications made in response to operant training of auditory detection tasks have at least two distinctive components. Immediately following learning, response changes are strongest at frequencies and intensities other than those close to the target stimulus. In contrast, more extensive rehearsal shifts the responses toward stimuli close in frequency and intensity to those of the target.
Ascending Auditory Input Does Not Predict Plasticity in A1 Regions.
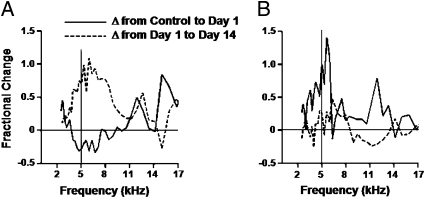
Plasticity in representation of the target tone in the two learning groups was further explored in the topographically different regions defined by intrinsic signal. Activity in the strongest 30 ms of the response vs. frequency was plotted for controls in the LF region (Fig. S2A) and HF region (Fig. S2B) for stimuli within one-half octave of the target. These stimuli were all at the intensity of the target sound in the task. Representation of the target tone differed substantially between these two regions. In the 1-day group, responses to the target decreased in the LF region (rank sum test, P < 0.002; Fig. 3A). In contrast, responses to stimuli close in frequency to the target increased in the HF region (P = 0.002), along with responses to other tones (Fig. 3B). Continued performance of the task between the 1st and 14th days resulted in further significant increases in responses to sounds similar to the target in both regions (LF region, P < 0.001; HF region, P = 0.046). The difference between the two regions appears to be mainly based on the response suppression observed in the LF region in the 1-d group, a region that was defined by its a priori strong response to the target.
Fig. 3.
Ascending auditory input does not predict plasticity in A1 regions. (A) Fractional increase in response strength from control group to 1-day group (solid line) and from 1-day group to 14-day group (dashed line) in recordings from the LF region. The solid vertical line indicates target frequency. There is a fractional change at 5 kHz: control vs. 1-day, −0.27 ± 0.11; 1-day vs. 14-day, 0.71 ± 0.37. (B) Change measures in the HF region. There is a fractional change at 13 kHz: control vs. 1-day, 1.01 ± 0.62; 1-day vs. 14-day, 0.25 ± 0.14.
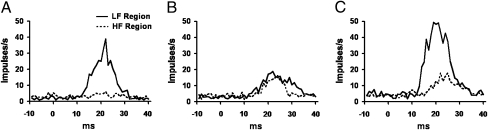
Regional responses to the target were examined by plotting neuronal action potential rate against time in response to stimuli within one-sixth octave of the target stimulus and at the target intensity. In the LF region of control animals (Fig. 4A), a robust and significant response was observed (t test, P < 0.001). The HF region, however, lacked any change in activity (t test, P = 0.933). Following training, the LF region of 1-d animals trended toward a reduction in the total level of activity (Fig. 4B) but did not reach statistical significance (P = 0.08). There was, however, a significant increase in the duration of elevated tone-evoked activity by 9 ms in 1-d animals (Kolmogorov–Smirnov test, P < 0.05). The HF region of 1-d animals greatly increased its response to the target (t test, P < 0.001).
Fig. 4.
Temporal responses to the target stimulus at different learning time points. (A) Impulse rate vs. time relation for recordings in the LF region (solid line) and HF region (dashed line) of control animals in response to the target stimulus. (B) Temporal responses to target stimulus in 1-day animals. (C) Temporal responses to target stimulus in 14-day animals. Peak peri-stimulus time histogram values were as follows: control LF, 39.09 ± 3.04; HF, 6.07 ± 2.17; 1-day LF, 18.98 ± 7.61; 1-day HF, 16.37 ± 5.93; 14-day LF, 49.43 ± 27.76; HF 14-day, 17.96 ± 5.50.
In the LF region of 14-d animals (Fig. 4C), activity in response to the target was increased relative to that in 1-d animals (P < 0.001) and the duration of tone-evoked activity remained elevated. The HF region of 14-day animals also showed an additional increase in activity (P < 0.001), although this effect was apparently attributable to a further increase in the duration of activity, because the peak levels increased only slightly. In addition to activity induced by auditory stimuli, average background rates were measured before the target stimulus and were found to increase relative to control (t test, P < 0.001) in the 1-day and 14-day groups for the LF region as well as in the 14-day group HF region (LF region: control = 1.86 action potentials per second, 1-day = 2.88, 14-day = 4.23; HF region: control = 3.48, 1-day = 3.99, 14-day = 4.76).
To examine the spatial reorganization of A1 responses to target stimuli, Voronoi tessellation maps of the target responses for each A1 reconstruction were computed. A representative map for a control animal is shown with the HF region and LF region divided (Fig. S3A). As expected, virtually all activity in response to target was restricted to the LF region. A normalized population frequency histogram for target rates from all penetrations made in control animals was also generated to show the distribution of firing rates for each region (Fig. S3D). As demonstrated in the representative map, the organization of target responses was changed in 1-day animals (Fig. S3B) because responses to the target were no longer restricted to the LF region. Spatial organization of responses at this time was widely distributed over the area of each region and appeared to lack clustering. The population frequency histogram also showed a clear shift in firing rates within the HF region (Fig. S3E), and 23% of the penetrations had responses stronger than the strongest HF region target responses in controls. In the 14-day animals, clustering of the target response was observed, with organization in the HF region being spatially localized along the HF/LF region border in these animals (Fig. S3C). Shifts for both regions were also observed in the 14-day population frequency histogram (Fig. S3F).
Arc Expression Cannot Be Predicted by Ascending Auditory Input to A1 Regions.
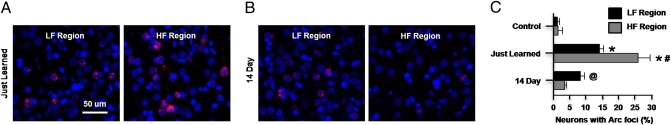
Baseline levels of Arc expression, specifically the percentage of neuronal nuclei with Arc foci, were low within both the LF and HF regions (LF = 1.14 ± 0.66%, HF = 1.35 ± 1.35%; Fig. S4A). Arc expression was significantly elevated in both regions in animals that had just learned the tone detection task (just learned group, Materials and Methods) compared with caged controls (P < 0.002 for both) (Fig. 5 A and C). Additionally, Arc expression was higher in the HF region compared with the LF region (P = 0.001). This pattern was reversed in the 14-day group, in which Arc expression was elevated above baseline only for the LF region (P < 0.05 for control vs. LF region comparison) (Fig. 5 B and C).
Fig. 5.
Arc expression cannot be predicted by ascending auditory input to A1 regions. (A) Arc mRNA expression in the just learned animals. (B) Arc mRNA expression in the 14-day animals. Arc mRNA expression is shown in red, and DAPI staining of cell nuclei is shown in blue. (C) Percentage of neurons with Arc foci. Statistical analysis results for two-way ANOVA are as follows: *Significant difference from control from the same region (P ≤ 0.001); #significant difference from LF region of just learned group (P = 0.001); @significant difference from HF region of just learned group (P < 0.001).
Although the increases in Arc expression in A1 of animals that had just learned the task were robust, such changes did not generalize to the entire cortex, because the percentage of Arc-expressing neurons remained low in the perirhinal cortex, which is located just ventral of the auditory-responsive fields (0.92 ± 0.13%) (25, 26).
Discussion
The present study directly tests whether strong action potential activity in A1 is necessary for physiological plasticity and Arc expression. A region with weak responses to the target stimulus showed strong response enhancement and Arc expression immediately after learning, whereas a region with strong target responses showed response suppression and weaker Arc expression. Further task practice led to response enhancement in both regions, with preferential Arc expression in the region with initial strong target responses. This dissociation between the topography of early and later learning-related physiological changes and Arc expression extends the understanding of the phenomenology and mechanisms supporting adult behaviorally induced sensory cortex plasticity.
A central argument in this study is that the HF region lacked strong action potential responses to the target stimulus in control animals. The lack of action potential rate changes does not preclude the occurrence of subthreshold activity (27–29) or the occurrence of such a response in unanesthetized animals. However, comparisons of awake and anesthetized responses in other species support the idea that in awake control animals, responses to the target sound in the HF region would be substantially weaker than in the LF region (30, 31). For these reasons, the action potential activity in response to the target stimulus should be close to an order of magnitude stronger in the LF region than in the HF region during the learning trials. Accordingly, the high-activity (i.e., LF) region initially has weaker Arc expression and physiological plasticity than the low-activity (i.e., HF) region.
To evaluate the possible mechanisms that could result in increased responsiveness of the HF region neurons caused by the first learning session, we ask “What do the HF region neurons experience in their inputs during learning?” The target stimulus causes subthreshold activity mediated by cortical horizontal connections in A1 (27–29). Neuromodulators should be released 100–300 ms after the target stimulus, because the neuromodulator nuclei are active after stimuli associated with reinforcement (32–34). The preparatory set, or the neural activity caused by anticipation and expectation of the stimulus, is carried by frontal and other associational cortices and has been studied in working memory and attentional contexts (35). The preparatory set activity, presumably projected from nearby associational cortices in the temporal lobe, has been shown to bias primary sensory cortex responses modestly (36, 37). We consider the possible contributions from combinations of these three inputs.
Pairing neuromodulators with sensory stimuli can cause plasticity (38–40). However, neuromodulatory activity does not have specificity for auditory cortex, and Arc expression was specific to auditory fields. Another cue, either the subthreshold activity or preparatory set, must direct the plasticity and result in the observed topographic specificity, which is field-selective but not frequency-selective. The subthreshold neuronal activation may provide this field-selective but not frequency-selective cue. All pathways and associated responses from the target stimulus, however, should be strongly target-specific (i.e., only responses to LFs should be enhanced in the HF region). However, the potentiation in the HF region lacks target-frequency specificity, suggesting that target-induced subthreshold activity plus neuromodulator release cannot account for all observed plasticity. Additionally, subthreshold activity should be even stronger in the LF region, which had less plasticity. An alternative possibility is that signals relating to the anticipation of the sensory stimulus, which may optimally work to sample broadly to enhance a detection task, are generated in auditory association cortices and project to the HF region neurons to work in conjunction with the neuromodulators to direct the cortical plasticity. We regard this scenario as the most likely set of neural factors responsible for the neuroplasticity.
An associated question at the cellular level is what molecular changes contribute to the long-lasting neuroplasticity across A1 and to perceptual learning. We hypothesized that changes in cortical responsiveness would involve the expression of immediate-early genes, notably Arc, because Arc expression is necessary for stabilizing changes in neural responsiveness into long-term plasticity and consolidating learning into long-term memory (10, 13, 15). We observed that Arc expression is topographically aligned with the learning-induced physiological changes observed both 1 and 14 days after learning, even as the topography of these effects reverses. Because perceptual decisions are believed to be based on discriminability of neurophysiological responses in sensory cortex (41, 42), our findings show that Arc expression fulfills some necessary requirements for contributing to the neuroplasticity underlying perceptual learning.
Our work, en toto, creates an experimentally testable hypothesis of how neuroplasticity in primary sensory cortex develops. During initial task learning, neural activity associated with the preparatory set biases circuits in primary sensory cortex. When combined with neuromodulator release, and possibly subthreshold target-induced activity, the neurons express immediate early genes, notably Arc. The change in gene expression leads to a nonselective increase in responsiveness within 24 h. Continued task practice drives activity- and Arc-dependent selective changes in responsiveness that progressively define the target responses from background activity. In contrast to activity-dependent models, this work supports a stronger role for conscious cognitive processes involved in the preparatory set in reshaping primary sensory cortex.
Materials and Methods
Operant Training.
Male Sprague–Dawley rats, 55–58 postnatal d of age on arrival, were pretrained to hold a nose poke for 800 ms and rewarded with food pellets. Tone detection required animals to end the nose poke after a 5-kHz, 50-dB, 25-ms target tone played either 400 or 800 ms after nose poke onset. The learning criterion was a 75% hit rate in any 20 consecutive releases in the early time window. The d′ was determined by the hit and false-alarm rates.
Optical Intrinsic Signal Imaging.
A1 was identified using optical intrinsic signal imaging. Rats were anesthetized for intrinsic imaging and microelectrode procedures using i.p. pentobarbital. Cranial bone was thinned over A1. Images were collected as described previously (26). A 4-s sound stimulus was used, and the speaker was positioned 12 in in front of the nose on the interaural plane and 0° vertical. The first and third seconds of the stimulus contained 1 s of repeating 5- or 13-kHz, 25-ms stimuli. These two seconds were alternated with 1 s of randomized bracketing-frequency tones. The border between the LF and HF regions occurred at roughly 58% of the distance across their rostral/caudal borders.
Microelectrode Mapping.
Regions of A1 were reconstructed with 2-MΩ microelectrodes. Target sampling density was 20–25 penetrations per square millimeter, and the location of each penetration was marked on an image of the cortical surface. A hydraulic microdrive (FHC) was used to sample 500 μm from the cortical surface. Cortical activity was recorded using a TDT audio/physiology workstation. Tones were adjusted in frequency and intensity until auditory responses were identified by audio/visual means on an oscilloscope. Depth was adjusted to optimize auditory responses. A battery of randomized 25-ms tonal sounds with raised cosine ramps was then presented. The presentation rate was 10 Hz, and the battery was divided into two blocks. Block 1 consisted of pure-frequency sounds ranging from 2 to 32 kHz using full-tone spacing at intensities 30- to 80-dB SPL in 10-dB steps, with four repetitions. Block two ranged from 2.5 to 25 kHz with semitone spacing and 12 repetitions each, and tone intensities were adjusted based on the rat audiogram (43) with five 25-kHz sets at 50-dB SPL.
Construction of Frequency-Intensity Plots and Peri-Stimulus Time Histogram (PSTH) Curves.
Action potentials were defined as positive-going threshold crossings at 5.5 multiples of noise rms, with a 1-ms dead time. Penetrations were considered part of A1 if they met three criteria: structure in frequency or time in the frequency-intensity plot, latency characteristic of A1, and positioned within the cochleotopic gradient of A1. Population frequency-intensity plots were constructed by averaging action potential rates for each tone across all A1 penetrations per animal and then across animals in each experimental group. PSTHs were constructed using data from the second stimulus block. Comparisons of fractional change plots in Fig. 3 were done in the one-octave region of sound stimuli centered at the 5-kHz target frequency, 3.53–7.07 kHz, so that 13 frequencies were used for the rank sum comparison. Estimates of duration of response were obtained by subtracting the prestimulus rate and integrating the response. The time of the peak of the integral was the duration, and by normalizing to a peak of 1, the integrals could be compared with Kolmogorov–Smirnov distribution tests.
Dye-Marking A1 and Training After Surgical Recovery.
The dorsal, ventral, anterior, and posterior boundaries of A1 regions were identified on pretrained rats using optimal intrinsic signal imaging and marked by making 0.5-mm burr holes through the thinned skull and lowering a 30-gauge needle loaded with NeuroTrace DiI tissue-labeling paste (Molecular Probes). The animals were recovered for 3 days and returned to training regiments for an additional 3 days before being used for gene expression studies. Performance of 1-day animals was continuously monitored, and 7 min after reaching learning criteria, they were removed from the operant chamber, immediately anesthetized, and decapitated, and the brain was frozen in dry ice-chilled isopentane within 2 min. Seven minutes was an optimal time for detecting learning-induced Arc foci of transcription according to the cellular compartment analysis of temporal activity using fluorescence in situ hybridization (catFISH) method (44, 45), because Arc foci are present from 3 to 15 min after initiation of transcription (45). Thus, any Arc foci induced at the beginning of the session attributable to the novelty of the tone would have dissipated by the time the brains were harvested. Frozen brains were then stored at −80 °C until being prepared for cryosectioning. Harvesting brains of the 14-day animals was time-matched to the average of the 1-day group [i.e., 25 min from the beginning of the behavioral session (range: 20–35 min)]. Cage controls were allowed to recover from surgery, and brains were harvested in the same manner as in animals in other groups, but they did not receive any training.
Cryosectioning and In Situ Hybridization.
Serial 20-μm-thick coronal sections containing A1 were stained for Arc mRNA according to the catFISH method described elsewhere (45). Briefly, the tissue was fixed in paraformaldehyde and hybridized overnight at 56 °C with a full-length Arc anti-sense riboprobe tagged with digoxigenin. After several washes, including treatment with RNase A, endogenous peroxidase activity was quenched with 2% H2O2. The slides were incubated for 2 h with antidigoxigenin peroxidase-conjugated antibody (1:500; Roche Products), and the stain was visualized using the CY3 TSA fluorescence system (PerkinElmer). The nuclei were counterstained with DAPI (Invitrogen).
Image Collection and Stereological Analysis.
Mosaics of A1 image stacks (z stacks) were collected on a Zeiss AxioImager/Apotome system. Neurons were segmented from within the channel labeling cell nuclei. Putative glial cells were excluded from the analysis. Segmented neurons were classified with Axiovision imaging software (Zeiss) using an optical dissector method, which minimizes sampling errors attributable to partial cells and stereological concerns (46). Cells were classified as Arc+ when Arc mRNA at the foci of transcription was present on at least three planes.
Supplementary Material
Acknowledgments
We thank D. Polley, C. Constantinidis, J. Hegdé, H. Cui, R. Morris, and R. Metherate for providing critical comments on this work. We thank Rebecca Nalloor and Chris Bunting for technical support. This work was supported by National Institutes of Health Grants 5R01NS055173 (to D.T.B.) and R21MH083188 (to A.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008604107/-/DCSupplemental.
References
- 1.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guíc-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 2.Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- 3.Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci. 2006;26:6589–6602. doi: 10.1523/JNEUROSCI.0457-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buonomano D, Merzenich M. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:97–102. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 8.Cruikshank SJ, Weinberger NM. Evidence for the Hebbian hypothesis in experience-dependent physiological plasticity of neocortex: A critical review. Brain Res Brain Res Rev. 1996;22:191–228. doi: 10.1016/s0165-0173(96)00015-x. [DOI] [PubMed] [Google Scholar]
- 9.Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 10.Guzowski JF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messaoudi E, et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberger NM, Miasnikov AA, Chen JC. Sensory memory consolidation observed: Increased specificity of detail over days. Neurobiol Learn Mem. 2009;91:273–286. doi: 10.1016/j.nlm.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Wang KH, et al. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Ploski JE, et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Link W, et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyford GL, et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 18.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 19.Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: Evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke SN, et al. Differential encoding of behavior and spatial context in deep and superficial layers of the neocortex. Neuron. 2005;45:667–674. doi: 10.1016/j.neuron.2005.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher BR, Calhoun ME, Rapp PR, Shapiro ML. Fornix lesions decouple the induction of hippocampal arc transcription from behavior but not plasticity. J Neurosci. 2006;26:1507–1515. doi: 10.1523/JNEUROSCI.4441-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23:6443–6451. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzowski JF, et al. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci USA. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazdarjanova A, et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- 25.Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- 26.Kalatsky VA, Polley DB, Merzenich MM, Schreiner CE, Stryker MP. Fine functional organization of auditory cortex revealed by Fourier optical imaging. Proc Natl Acad Sci USA. 2005;102:13325–13330. doi: 10.1073/pnas.0505592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- 29.Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- 30.Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol. 1999;415:460–481. doi: 10.1002/(sici)1096-9861(19991227)415:4<460::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 31.Blake DT, Merzenich MM. Changes of AI receptive fields with sound density. J Neurophysiol. 2002;88:3409–3420. doi: 10.1152/jn.00233.2002. [DOI] [PubMed] [Google Scholar]
- 32.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 33.Richardson RT, DeLong MR. Context-dependent responses of primate nucleus basalis neurons in a go/no-go task. J Neurosci. 1990;10:2528–2540. doi: 10.1523/JNEUROSCI.10-08-02528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao SS, O'Shaughnessy DM, Johnson KO. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J Neurophysiol. 1993;70:444–447. doi: 10.1152/jn.1993.70.1.444. [DOI] [PubMed] [Google Scholar]
- 37.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 40.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 41.Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- 42.Hernández A, Zainos A, Romo R. Neuronal correlates of sensory discrimination in the somatosensory cortex. Proc Natl Acad Sci USA. 2000;97:6191–6196. doi: 10.1073/pnas.120018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hear Res. 1994;73:244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 44.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 45.Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West MJ. Stereological methods for estimating the total number of neurons and synapses: Issues of precision and bias. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.