Abstract
Increasing evidence supports the notion that spinal cord microglia activation plays a causal role in the development of neuropathic pain after peripheral nerve injury; yet the mechanisms for microglia activation remain elusive. Here, we provide evidence that NADPH oxidase 2 (Nox2)-derived ROS production plays a critical role in nerve injury-induced spinal cord microglia activation and subsequent pain hypersensitivity. Nox2 expression was induced in dorsal horn microglia immediately after L5 spinal nerve transection (SNT). Studies using Nox2-deficient mice show that Nox2 is required for SNT-induced ROS generation, microglia activation, and proinflammatory cytokine expression in the spinal cord. SNT-induced mechanical allodynia and thermal hyperalgesia were similarly attenuated in Nox2-deficient mice. In addition, reducing microglial ROS level via intrathecal sulforaphane administration attenuated mechanical allodynia and thermal hyperalgesia in SNT-injured mice. Sulforaphane also inhibited SNT-induced proinflammatory gene expression in microglia, and studies using primary microglia indicate that ROS generation is required for proinflammatory gene expression in microglia. These studies delineate a pathway involving nerve damage leading to microglial Nox2-generated ROS, resulting in the expression of proinflammatory cytokines that are involved in the initiation of neuropathic pain.
Keywords: spinal nerve transection, sulforaphane
Damage to the peripheral nerve often results in abnormal chronic pain and pain hypersensitivity that are generally referred to as “neuropathic pain.” It is known that hypersensitivity of pain-transmission neurons in the spinal cord is involved in the development of neuropathic pain, and this process has been referred to as central sensitization (1). Various pathological events are reported to precede central sensitization. These include, but are not limited to, p38 activation in spinal cord microglia and subsequent proinflammatory cytokine expression, NMDA receptor phosphorylation in spinal cord neurons, and altered expression of ion channels on neurons (2–5). In addition, a series of studies have implied that production of reactive oxygen species (ROS) in the spinal cord is involved in the induction of neuropathic pain. For example, systemic injection of ROS scavengers, such as phenyl-N-tert-butylnitrone (PBN) and 5,5-dimethylpyrroline-N-oxide (DMPO), relieved spinal nerve ligation-induced pain hypersensitivity in rats (6). In addition, spinal nerve ligation-induced phosphorylation of the NMDA receptor subunit 1 (NR1) in dorsal horn neurons was attenuated by ROS scavengers (7). These results suggested that ROS are critically involved in the development and maintenance of neuropathic pain; however, the exact primary source of ROS production has remained elusive.
Recently, a study using the mitochondrial ROS detector MT-Red showed that ROS are generated in the mitochondria of dorsal horn neurons (8). Production of mitochondrial ROS by intrathecal injection of the electron transport complex inhibitors antimycin A and rotenone in normal mice resulted in mechanical hyperalgesia (9). Based on these reports, it was argued that peripheral nerve injury generates superoxide in the mitochondria in dorsal horn neurons, which leads to phosphorylation of NR1 and thereby induces central sensitization. However, ROS production in other spinal cord cells, such as the microglia, after peripheral nerve injury has not been formally characterized, nor has the role of nonneuronal ROS in the development of neuropathic pain been addressed. In this regard, we characterized the cellular source of ROS in the spinal cord after spinal nerve transection (SNT), and investigated its mechanistic role in the development of neuropathic pain.
Results
Spinal Nerve Injury Induces ROS in Dorsal Horn Microglia.
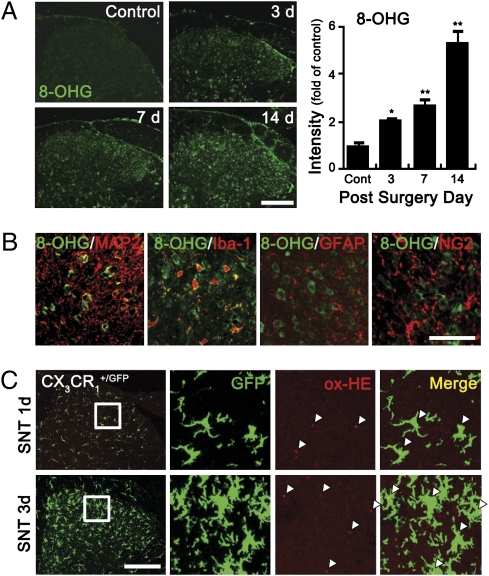
To characterize ROS-producing cells in the spinal cord after SNT, we assessed mitochondrial as well as nonmitochondrial ROS production, using 8-hydroxyguanine (8-OHG) antibody that detects oxidized nucleic acid, which results from cellular ROS damage. Three days after SNT, 8-OHG–immunoreactive cells were detected in the dorsal horn area, but not in naive control mice (Fig. 1A). The number and intensity of 8-OHG immunoreactive signals increased up to 14 d after SNT. To identify the ROS-damaged cell types, we immunostained the tissues with cell type-specific antibodies. The 8-OHG immunoreactive signals were detected in MAP2+ neurons and Iba1+ microglia in the spinal cord 7 d postinjury, but were not found in GFAP+ astrocytes or NG2+ oligodendrocyte precursor cells (Fig. 1B). These data show that ROS are generated not only in dorsal horn neurons but also in microglia after SNT. To confirm this, we tested ROS production in CX3CR1+/GFP mice, where GFP was expressed only in microglia in the spinal cord. In SNT-injured spinal cords 1 or 3 d postinjury, oxidized hydroethidine (ox-HE), a marker for intracellular superoxide, was detected mainly in GFP+ microglia (Fig. 1C), whereas the ox-HE+ signal was hardly detected in the spinal cord of sham-control mice. The number of ox-HE+/GFP+ microglia was 122 ± 11/mm2 and 197 ± 15/mm2 at 1 and 3 d postinjury, respectively, although the number of ox-HE+/GFP− cells was only 19 ± 6/mm2 and 22 ± 7/mm2. These data indicate that, immediately after SNT, more than 85% of ROS-producing cells in the spinal cord consist of microglia.
Fig. 1.
ROS are produced in the spinal cord microglia after L5 SNT. (A) Spinal cord sections of uninjured and SNT-injured mice at 3, 7, and 14 d (each group, n = 4) after L5 SNT were used for 8-OHG immunostaining. (Scale bar, 200 μm.) The 8-OHG+ cells were detected in spinal cord dorsal horn only after SNT. The intensity of 8-OHG immunoreactivity was measured, and the mean ± SEM values are presented (*P < 0.05; **P < 0.01). (B) 8-OHG+ (green) cells were immunostained with cellular markers (red) for neurons (MAP2), microglia (Iba-1), astrocyte (GFAP), and oligodendrocyte precursor cells (NG2). (Scale bar, 50 μm.) (C) Superoxide generation in SNT-injured CX3CR1+/GFP mice was detected with oxidized-hydroethidine (ox-HE) staining. Ox-HE signals (red) were mainly detected in the GFP+ microglia (green) and in the ipsilateral spinal cord of 1 and 3 d post-SNT. Enlarged images are shown in rectangles; ox-HE+ cells are marked with white triangles. (Scale bar, 200 μm.)
SNT Induces Nox2 Expression in Spinal Cord Microglia.
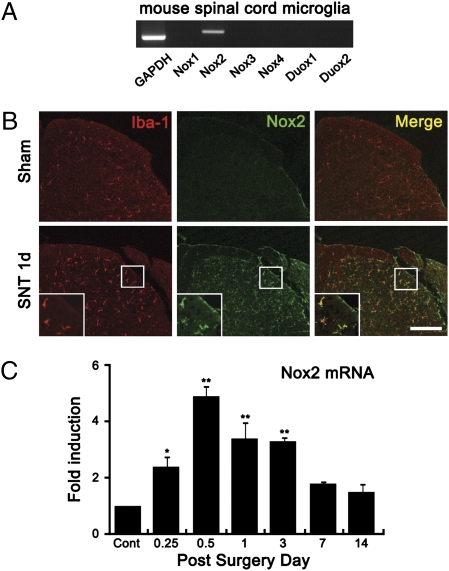
Because mitochondrial ROS production after SNT was detected mostly in neurons but not in microglia (8), we suspected that ROS were not generated by the mitochondria in microglia. In myeloid cells, as well as some other cell types, superoxide can be generated by cytoplasmic enzymes, specifically NADPH oxidases, including Nox1-5, Duox1, and Duox2 (10). In this study, transcripts of Nox2 (catalytic subunit gp91phox) were detected in primary mouse spinal cord microglia (Fig. 2A). Via immunohistochemistry, Nox2 was barely detectable in the spinal cord of sham-control mice but was induced 1 d post-SNT (Fig. 2B); the expression was maintained up to 3 d postinjury and declined thereafter (Fig. S1). Furthermore, Nox2 immunoreactivity mainly colocalized to Iba-1+ microglia (Fig. 2B) but not to MAP2+ neurons (Fig. S2). SNT-induced Nox2 expression was regulated at the mRNA level (Fig. 2C). Nox2 transcripts were detected as early as 6 h post-SNT, peaked at 12 h, and declined thereafter. These data suggested that SNT-induced Nox2 expression leads to ROS production in microglia after SNT.
Fig. 2.
Nox2 expression is increased in spinal cord microglia after SNT. (A) Transcripts of Nox1, Nox2, Nox3, Nox4, Duox1, and Duox2 in primary mouse spinal cord microglia were detected by RT-PCR. (B) Spinal cord sections were immunostained with Iba-1 and Nox2 antibodies. Nox2 immunoreactivity signals were increased in Iba-1+ microglia in the spinal cord dorsal horn at 1 d after SNT. (Scale bar, 200 μm.) (C) Nox2 mRNA expression in mouse spinal cord after L5 SNT was measured by real-time RT-PCR. Total RNA was isolated from L5 spinal cord tissues of uninjured mice (n = 3) and SNT-injured mice at 6 h, 12 h, and 1, 3, 7, and 14 d after surgery (each group, n = 3). The Nox2 transcript level at each time point was normalized to the level of GAPDH and is presented as fold induction, compared with the Nox2 level of uninjured mice. Data are expressed as mean ± SEM (*P < 0.05; **P < 0.01).
SNT-Induced Pain Hypersensitivity and Microglia Activation Are Reduced in Nox2/gp91phox-Deficient Mice.
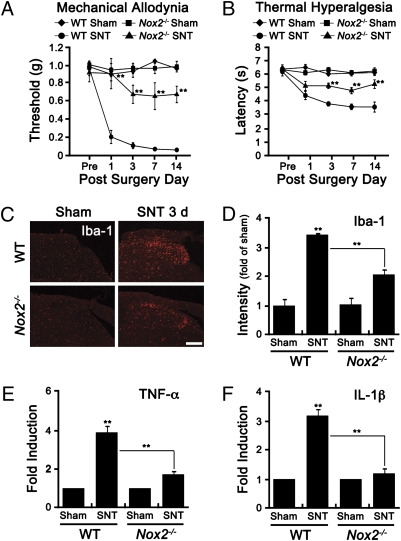
To assess the role of Nox2-derived ROS in pain hypersensitivity, we subjected WT and Nox2-deficient mice to SNT and measured mechanical allodynia and thermal hyperalgesia (Fig. 3 A and B). One day after SNT, the threshold for the mechanical stimuli decreased below 0.2 g in WT mice, which was maintained for up to 14 d (Fig. 3A). Likewise, latency for thermal stimuli decreased more than 40% in WT mice (Fig. 3B). However, in Nox2-deficient mice, SNT-induced mechanical allodynia and thermal hyperalgesia were significantly attenuated (Fig. 3 A and B). SNT-mediated ROS production in the spinal cord dorsal horn measured by 8-OHG immunostaining was also comparably attenuated in Nox2-deficient mice (Fig. S3 A and B). These data show that Nox2-mediated ROS production is involved in the induction of SNT-induced pain hypersensitivity.
Fig. 3.
SNT-induced neuropathic pain-like behaviors and microglia activation are reduced in Nox2-deficient mice. (A and B) Mechanical allodynia and thermal hyperalgesia were measured in SNT-injured WT (WT-SNT, n = 8), and Nox2−/− (Nox2−/−-SNT, n = 8) mice, or sham control mice (WT-Sham and Nox2−/−-Sham, n = 5). (C and D) Microglia activation in the ipsilateral L5 dorsal horn area of WT and Nox2−/− mice was measured by Iba-1 immunostaining. The intensity of Iba-1 immunoreactivity in the SNT-injured mice (SNT, n = 4) was measured and presented as fold-increase, compared with levels in sham-control mice (Sham, n = 4) (Scale bar, 200 μm.). (E and F) The mRNA expression of TNF-α and IL-1β in L5 spinal cord tissues of sham-operated control mice (Sham, n = 4) and SNT-injured mice (SNT, n = 4) at 3 d after surgery were measured by real-time RT-PCR. Data are expressed as mean ± SEM (*P < 0.05; **P < 0.01).
It is well known that microglia activation and subsequent proinflammatory cytokine expression are responsible for enhanced pain hypersensitivity caused by peripheral nerve injury (11). To delineate the mechanisms underlying the pain-inducing effects of Nox2-derived ROS in microglia, we tested spinal cord microglia activation in SNT-injured mice. Microglia activation marker CD11b mRNA expression was up-regulated 9-fold in WT and 2-fold in Nox2-deficient mice at 3 d postinjury (Fig. S3C). SNT-induced expression of Iba-1, another microglia activation marker, was also attenuated in Nox2-deficient mice (Fig. 3C). Iba-1 fluorescence intensity in the spinal cord dorsal horn of SNT-injured mice was reduced by more than 50% in Nox2-deficient mice (Fig. 3D). In addition, SNT-mediated induction of TNF-α and IL-1β mRNA, two proinflammatory cytokines that are involved in central sensitization (12), was significantly reduced in Nox2-deficient mice (Fig. 3 E and F). These data show that Nox2-mediated ROS generation is involved in SNT-induced spinal cord microglia activation and proinflammatory cytokine expression.
Intrathecal Sulforaphane Administration Induces HO-1 Expression and Nrf-2 Activation Specifically in Spinal Cord Microglia.
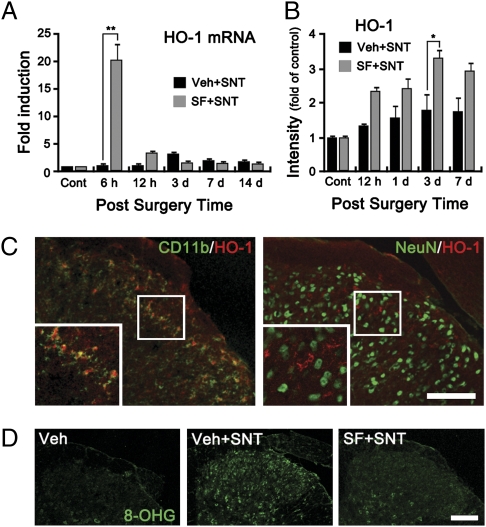
After SNT, Nox2 expression was detected mainly in microglia but not in dorsal horn neurons (Fig. 2B and Fig. S2). This observation suggests that microglial Nox2 expression contributes to SNT-induced pain hypersensitivity but does not exclude the contribution of Nox2 from other cell types, e.g., peripheral immune cells. To address this issue, we directly introduced the antioxidant sulforaphane to WT mice intrathecally. Sulforaphane is known to exert its antioxidant effect by inducing nuclear translocation of Nrf-2 with subsequent heme oxygenase-1 (HO-1) expression in myeloid cells, including the microglia (13). In the spinal cord of SNT-injured mice, sulforaphane injection enhanced the translocation of Nrf2 to the nuclear fraction (Fig. S4A). The SNT-induced Nrf2 immunoreactivity mostly colocalized to the CD11b+ microglia (Fig. S4B). Sulforaphane injection also up-regulated the HO-1 transcript in spinal cord at 6 h postinjury (Fig. 4A), and the expression of HO-1 in the spinal cord was detected as early as 12 h after sulforaphane injection with peak expression at day 3 (Fig. 4B). Once again, HO-1 expression was detected mostly in microglia and not in neurons (Fig. 4C). In addition, sulforaphane injection almost completely blocked SNT-induced ROS generation in the SNT-injured mouse spinal cord (Fig. 4D). These data indicate that intrathecal introduction of sulforaphane inhibits SNT-induced microglial ROS production.
Fig. 4.
Administration of sulforaphane inhibits SNT-induced ROS production in spinal cord microglia. (A) Transcripts of HO-1 in the L5 spinal cord tissues were measured by real-time RT-PCR at 6 h, 12 h, and 3, 7, and 14 d after surgery (each group, n = 4). Sulforaphane or vehicle solutions were injected intrathecally 5 min before surgery. (B) HO-1 expression in the spinal cord dorsal horn of vehicle- or sulforaphane-injected mice at 12 h and 1, 3, and 7 d (each group, n = 4) post-SNT were characterized by immunohistochemistry. The intensity of HO-1 signal in a designated area of the dorsal horn in each mouse was quantified, and presented as fold-increase, compared with the level of the uninjured control mice (*P < 0.05; **P < 0.01). (C) Spinal cord sections of sulforaphane-injected mice at 3 d post-SNT were immunostained for HO-1 and CD11b or NeuN antibodies. HO-1 signals in the ipsilateral dorsal horn were mostly detected in the CD11b+ microglia but not in NeuN+ neurons. (Scale bar, 200 μm.) (D) ROS damage was detected by 8-OHG staining at 7 d after surgery in the spinal cord dorsal horn area of vehicle-injected sham-operated mice (Veh, n = 4), vehicle-injected SNT-injured mice (Veh+SNT, n = 4), and sulforaphane-injected SNT-injured mice (SF+SNT, n = 4). (Scale bar, 200 μm.)
Sulforaphane Reduces Neuropathic Pain Behavior via HO-1 Induction.
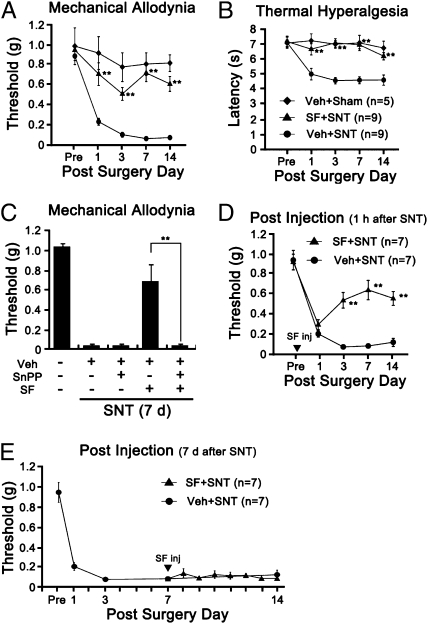
To test the effects of microglial ROS on pain hypersensitivity, mechanical allodynia and thermal hyperalgesia were measured with or without sulforaphane injection. Sulforaphane injection 5 min before SNT injury reduced mechanical allodynia by 70%, and almost completely inhibited the induction of thermal hyperalgesia (Fig. 5 A and B). To test whether the analgesic effect of sulforaphane was due to HO-1 up-regulation in microglia, we treated mice with protoprophyrin (SnPP), a potent HO-1 inhibitor together with sulforaphane. Administration of SnPP alone had no effect on the induction of mechanical allodynia after SNT (Fig. 5C), whereas it almost completely blocked the analgesic effects of sulforaphane, demonstrating that sulforaphane exerts its analgesic function via HO-1 induction. In addition, sulforaphane injection 1 h postinjury was able to inhibit mechanical allodynia (Fig. 5D), whereas injection at a later time point (7 d postinjury) failed to reverse the ongoing SNT-mediated pain hypersensitivity (Fig. 5E). These data show that blocking microglial ROS generation at an early time point inhibits the development of pain hypersensitivity, thus implying that microglial ROS contribute to the induction of neuropathic pain after SNT.
Fig. 5.
Sulforaphane reduces SNT-induced neuropathic pain behavior via HO-1 expression. (A and B) Mechanical allodynia and thermal hyperalgesia were measured using the SNT-injured mice with vehicle injection (Veh+SNT, n = 9), SNT-injured mice with sulforaphane injection (SF+SNT, n = 9, 10 mg/kg, in 3% DMSO, i.t.), and sham-operated mice with vehicle injection as the negative control (Veh+Sham, n = 5). (C) SnPP blocks the analgesic effects of sulforaphane. Sulforaphane (50 mg/kg) was i.p. injected into SNT-injured mice with or without SnPP (50 μmol/kg in 5% DMSO with PBS) at 1 h postsurgery. After 2 d, SnPP was injected (i.p.) again; and mechanical allodynia was measured on day 7 after surgery. (D and E) Sulforaphane inhibit the induction, but not the maintenance, of neuropathic pain. Mechanical allodynia was measured up to 14 d using SNT-injured mice with (SF+SNT) or without (Veh+SNT) sulforaphane injection (50 mg/kg, i.p.). Sulforaphane was given either 1 h (D) or 7 d (E) after SNT. Data are expressed as mean ± SEM (*P < 0.05; **P < 0.01).
Sulforaphane Reduces Microglia Activation and Proinflammatory Cytokine Expression in the Spinal Cord After SNT.
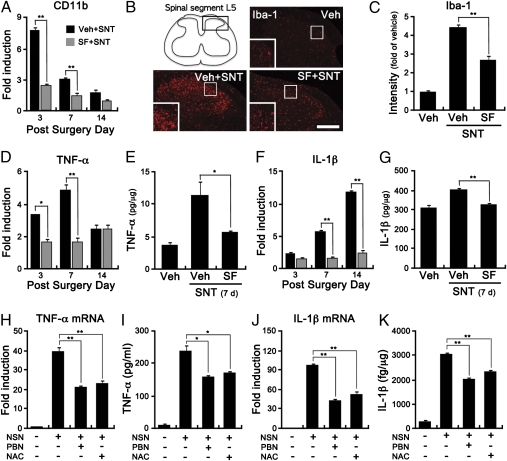
It was reported that superoxide generated in dorsal horn neurons leads to central sensitization by inducing phosphorylation and activation of NR-1 (7). Although intrathecal sulforaphane injection had strong analgesic effects in our study, it did not have much of an effect on NR-1 phosphorylation in the spinal cord (Fig. S5). These data suggest that microglial ROS contribute to pain hypersensitivity independently of neuronal superoxide. It has been reported that ROS works as an upstream signaling molecule for the expression of proinflammatory cytokines in macrophages (14). We have also found reduced proinflammatory cytokine expression in Nox2-deficient mice after SNT (Fig. 3 E and F). Previous reports, combined with our Nox2-deficient mice data, suggest the possibility that ROS play a role in microglia activation and proinflammatory cytokine expression in the spinal cord, which may, in turn, sensitize pain transmission in the spinal cord. Indeed, in our study, sulforaphane injection inhibited microglia activation after SNT (Fig. 6 A–C). Accordingly, SNT-induced expression of TNF-α and IL-1β in the spinal cord was attenuated by sulforaphane injection (Fig. 6 D–G). In addition, we tested the effects of ROS on expression of these cytokines using primary spinal cord microglia. Previously, we reported that necrotic sensory neurons (NSN) induce proinflammatory cytokine expression in microglia and suggested it as a putative mechanism for nerve injury-induced microglia activation (15). NSN induced the expression of TNF-α and IL-1β transcripts by 40- and 100-fold, respectively (Fig. 6 H and J). Upon pretreatment with PBN or N-acetylcysteine (NAC) ROS scavengers, NSN-induced TNF-α and IL-1β expression was significantly reduced, both at the mRNA (Fig. 6 H and J) and protein levels (Fig. 6 I and K). These data indicate that ROS are involved in inflammatory cytokine expression in the spinal cord microglia after SNT, and thus suggest the possibility that microglial ROS induce SNT-mediated pain hypersensitivity partly via enhancing proinflammatory cytokine expression in the spinal cord.
Fig. 6.
Sulforaphane reduces microglia activation and proinflammatory cytokine expression in the spinal cord after SNT. (A) Total RNA was prepared using L5 spinal cord tissues of SNT-injured mice with (SF+SNT) or without (Veh+SNT) sulforaphane injection (10 mg/kg, i.t), and used for real-time RT-PCR to measure CD11b transcripts. The mRNA levels of CD11b in each group were compared with the level of sham-operated mice and were presented as fold-increase. (B and C) Microglia were immunostained with anti–Iba-1 using L5 spinal cord sections of SNT-injured mice with or without sulforaphane injection. Sham-operated mice with vehicle injection (Veh) served as the control. (Scale bar, 200 μm.) Fluorescent densities of Iba-1+ cells were presented (*P < 0.05; **P < 0.01). (D and F) The mRNA expression of TNF-α and IL-1β gene was measured by real-time RT-PCR using L5 spinal cord tissue from sham-operated mice, and SNT-injured mice with or without sulforaphane injection at 3, 7, 14 d after surgery. (E and G) Protein levels of TNF-α and IL-1β were measured by ELISA. The ipsilateral L5 hemispinal cord tissues were prepared from vehicle-injected mice (Veh, n = 4), and SNT-injured mice with or without sulforaphane injection (each group, n = 4). (H and J) Primary cultured rat spinal cord microglia were incubated with NSN for 3 h with or without ROS scavenger PBN (1 mM) and NAC (10 mM). The mRNA expression of TNF-α and IL-1β were measured by real-time RT-PCR. (I and K) Primary microglia were stimulated by NSN with PBN (1 mM) and NAC (10 mM). After 12 h, medium (for TNF-α) and cell lysate (for IL-1β) were collected and used in an ELISA (*P < 0.05; **P < 0.01).
Discussion
There is a growing body of evidence supporting a causal role of microglia activation in the development of nerve injury-induced neuropathic pain; yet the mechanisms are far from being understood. In this study, we report the finding that ROS production in spinal cord microglia is required for the nerve injury-induced microglia activation and subsequent development of pain hypersensitivity. A previous study has implicated ROS in neuropathic pain, in which SNT injury induced superoxide production in spinal cord neurons at 7 d postinjury (8). However, our work emphasizes that ROS production in spinal cord microglia immediately after nerve injury is critical for the induction of pain hypersensitivity. In our study, we found that ROS are generated mainly in microglia at early time points (1 and 3 d postinjury). These data show that, after SNT, ROS production in dorsal horn microglia precedes ROS generation in neurons. Pain hypersensitivity develops within 3 d after SNT (15, 16), which indicates that microglial ROS are involved in the induction of neuropathic pain.
In our effort to elucidate the mechanism underlying SNT-induced ROS generation, we found that Nox2 is induced in spinal cord microglia after SNT. Nox2 generates superoxide that can be converted to other ROS molecules, including hydrogen peroxide (17). Currently, it is not clear how peripheral nerve injury induces Nox2 transcript expression in spinal cord microglia. Thus far, various molecules, e.g., fractalkine, glutamate, and ATP, have been implicated in the signal transmission from injured peripheral nerve to spinal cord microglia (18, 19). We have also reported that Toll-like receptor 2 (TLR2) is involved in nerve injury-induced spinal cord microglia activation, suggesting the possibility that damaged neuron-derived endogenous TLR2 agonist may activate microglia via TLR2 (15). Although it is pure speculation, we hypothesize that an endogenous TLR2 agonist released from the damaged sensory neurons might induce Nox2 transcripts in spinal cord microglia, this notion needs to be investigated in future studies.
The fact that SNT-induced mechanical allodynia and thermal hyperalgesia were attenuated in Nox2-deficient mice suggests that microglial ROS contribute to neuropathic pain induction. Although these data do not formally exclude the contribution of Nox2 in other cell types, Nox2 immunoreactivity in the spinal cord was detected mainly in microglia and not in neurons. Based on these data, we propose that the behavioral phenotype observed in the Nox2-deficient mice is attributed to the Nox2 deletion in microglia. This conclusion is further corroborated by our data using sulforaphane, in which intrathecal injection of sulforaphane reduced microglial ROS generation and development of neuropathic pain after SNT. Once again, the pain-relieving effects of sulforaphane were dependent on HO-1 expression, and sulforaphane induced HO-1 expression was detected only in spinal cord microglia and not in neurons. These data support the idea that sulforaphane exerts its effects on spinal cord microglia and not on neurons. Taken together, the data suggest that Nox2-derived microglial ROS is required for maximal induction of neuropathic pain behavior after SNT.
Sulforaphane is an isothiocyanate molecule, a natural compound found in cruciferous vegetables, and is well known for its antioxidant effects (20). Sulforaphane exerts its antioxidant effects by increasing HO-1 expression via Nrf2 activation (13, 21). Carbon monoxide and biliverdin, which are generated in the process of HO-1 heme catabolism, act as strong suppressors of superoxide production and ROS scavengers, respectively (22). Previous studies have shown that sulforaphane inhibits microglia activation by regulating Nrf2-dependent gene expression (13). Due to the importance of microglia activation in neurological diseases, sulforaphane has been tested in other neurological disease models. For example, systemic administration of sulforaphane reduced LPS-induced inflammation in the brain (13). In an intracerebral hemorrhage model, sulforaphane reduced oxidative stress-induced brain damage by activating Nrf2 signaling (23). In this study, we found that sulforaphane has strong analgesic effects on SNT-induced pain hypersensitivity, suggesting additional therapeutic potential for this molecule in the treatment of neuropathic pain. Its analgesic efficacy observed postinjury and its safety verified in a clinical trial (24) further underscore its therapeutic potential.
Currently, it is not clear how microglial ROS contribute to the development of neuropathic pain. In this study, we found that TNF-α and IL-1β expression in the spinal cord after SNT was reduced in Nox2-deficient mice and also in sulforaphane-injected WT mice. The data from primary spinal cord glial cells suggest that ROS generation is required for expression of these proinflammatory cytokines. TNF-α and IL-1β have been implicated in the development of neuropathic pain, as these cytokines are expressed in spinal cord microglia after peripheral nerve injury (15, 16). Blocking these cytokines using neutralizing antibodies or inhibitors decreased neuropathic pain induced by nerve injury (25, 26). In isolated spinal cord slices, TNF-α and IL-1β enhanced the frequency of spontaneous excitatory synaptic transmission in lamina II neurons, and IL-1β reduced inhibitory synaptic transmission, as well (12). Excitatory synaptic transmission in lamina II neurons is regulated by MCP-1 (27). Based on these reports, it was proposed that TNF-α and IL-1β released from activated microglia after SNT induces MCP-1 expression in astrocytes, which, in turn, regulates the excitability of pain transmission neurons in the dorsal horn (28). Regardless of the exact mechanism for TNF-α/IL-1β–induced central sensitization, microglial ROS generated after SNT may increase pain hypersensitivity, at least in part, by up-regulating proinflammatory cytokine expression in the spinal cord.
In conclusion, our data show that SNT induces the generation of ROS in spinal cord microglia via Nox2, and Nox2-derived ROS in the spinal cord microglia contribute to the development of neuropathic pain. In addition, we report that sulforaphane has a strong analgesic effect on neuropathic pain by inhibiting microglial ROS, which may have important therapeutic implications.
Materials and Methods
Detailed materials and methods are presented in SI Materials and Methods.
Animals and Surgery.
Nox2−/− (gp91phox−/−) and CX3CR1+/GFP mice with a C57BL/6 background were purchased from Jackson Laboratories. WT (C57BL/6), CX3CR1+/GFP and Nox2−/− male mice aged 8–10 wk and weighing 23–25 g were used for experimentation. For SNT surgery, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and the L5 spinal nerve was transected as previously described (16). For intrathecal (i.t.) sulforaphane administration, mice were injected under sodium pentobarbital anesthesia by direct lumbar puncture between L5 and L6 vertebrae of the spine using a 10-μL Hamilton syringe (Hamilton Bonaduz AG) as described in SI Materials and Methods.
Behavioral Analysis.
All animal experiment procedures were reviewed and approved by the Institutional Animal Care and Use Committee, Seoul National University. A 50% withdrawal threshold was measured using a set of von Frey filaments (0.02–6 g, Stoelting), following an up–down method (29). Heat hyperalgesia was determined using plantar test instruments (7370; Ugo Basile), following a method of Hargreaves et al. (30). A detailed description of the behavioral methods is given in SI Materials and Methods.
Histology.
Immunohistochemistry and detection of superoxide using hydroethidine were performed as described in SI Materials and Methods.
Primary Spinal Cord Microglia Culture.
Primary rat spinal cord microglia were prepared as previously described (15).
Real-Time RT-PCR.
Real-time PCR was performed using a 7500 Real-Time PCR system (Applied Biosystems). Relative mRNA levels were calculated according to the 2-ΔΔCt method (31). All ΔCt values were normalized to GAPDH. All real-time RT-PCR experiments were performed at least three times, and the mean ± SEM values are presented unless otherwise noted. The PCR primer sequences used in this study are listed in Tables S1 and S2.
Statistical Analysis.
Data are represented as mean ± SEM. The statistical significance of differences was analyzed using the PASW statistical program (SPSS Inc.). Statistical analyses were performed using a Student t test and one-way or two-way ANOVAs, followed by Tukey's post hoc tests. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2009-0081467, 2008-0062413, 313-2008-2-C00749), Republic of Korea, and by an award from the Ellison Medical Foundation (AG-SS-2190-08, to M.I.S).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009926107/-/DCSupplemental.
References
- 1.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svensson CI, et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 3.Fitzsimmons BL, et al. Role of spinal p38alpha and beta MAPK in inflammatory hyperalgesia and spinal COX-2 expression. Neuroreport. 2010;21:313–317. doi: 10.1097/WNR.0b013e32833774bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Yang L, et al. Peripheral nerve injury induces trans-synaptic modification of channels, receptors and signal pathways in rat dorsal spinal cord. Eur J Neurosci. 2004;19:871–883. doi: 10.1111/j.0953-816x.2004.03121.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim HK, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 9.Kim HY, Chung JM, Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: The effect of mitochondrial electron transport complex inhibitors. Neurosci Lett. 2008;447:87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 11.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 12.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Innamorato NG, et al. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 14.Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 15.Kim D, et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- 16.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence? Nat Med. 2004;10(Suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 18.Verge GM, et al. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- 19.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol Cell Biochem. 2006;291:21–28. doi: 10.1007/s11010-006-9190-y. [DOI] [PubMed] [Google Scholar]
- 23.Zhao X, et al. Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke. 2007;38:3280–3286. doi: 10.1161/STROKEAHA.107.486506. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro TA, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 25.Sommer C, et al. Anti-TNF-neutralizing antibodies reduce pain-related behavior in two different mouse models of painful mononeuropathy. Brain Res. 2001;913:86–89. doi: 10.1016/s0006-8993(01)02743-3. [DOI] [PubMed] [Google Scholar]
- 26.Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 27.Gao YJ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 30.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.