Abstract
Although stress-induced increases in inflammation have been implicated in several major disorders, including cardiovascular disease and depression, the neurocognitive pathways that underlie inflammatory responses to stress remain largely unknown. To examine these processes, we recruited 124 healthy young adult participants to complete a laboratory-based social stressor while markers of inflammatory activity were obtained from oral fluids. A subset of participants (n = 31) later completed an fMRI session in which their neural responses to social rejection were assessed. As predicted, exposure to the laboratory-based social stressor was associated with significant increases in two markers of inflammatory activity, namely a soluble receptor for tumor necrosis factor-α (sTNFαRII) and interleukin-6 (IL-6). In the neuroimaging subsample, greater increases in sTNFαRII (but not IL-6) were associated with greater activity in the dorsal anterior cingulate cortex and anterior insula, brain regions that have previously been associated with processing rejection-related distress and negative affect. These data thus elucidate a neurocognitive pathway that may be involved in potentiated inflammatory responses to acute social stress. As such, they have implications for understanding how social stressors may promote susceptibility to diseases with an inflammatory component.
Keywords: anterior cingulate, insula, inflammation, depression, disease
Psychological stress is intimately related to human health and well-being. It increases susceptibility to the common cold (1), elevates risk for several major diseases (2), and is a strong, independent predictor of morbidity and mortality. In a recent epidemiological study, for example, males experiencing high levels of stress were 32% more likely to die during a 22-y assessment period than were those experiencing low levels of stress (3).
Stress may affect health in part by up-regulating inflammatory processes that have been implicated in the onset or progression of several disorders, including asthma, rheumatoid arthritis, cardiovascular disease, and depression (4–8). Although many stressors can have this effect, animal and human research has shown that social stressors are particularly strong triggers of inflammation (9–11). Exposure to everyday social stress in humans, for example, is associated with elevated inflammatory activity (12–15) and with the up-regulated expression of genes that promote inflammation (16). In addition, controlled laboratory studies have shown that acute social stressors—specifically ones that involve social evaluation and the possibility of social rejection—elicit significant increases in proinflammatory cytokines, a key mediator of the inflammatory response (17, 18).
Despite evidence that social stressors may trigger inflammation, the neurocognitive pathways that underlie this effect remain unknown. In particular, no studies to date have investigated the neural regions associated with differences in inflammatory responding to acute social stress. The brain plays a critical role in appraising social stressors, as well as in modulating the immune system's response to stressors that involve social or physical threat (19–21). Differences in inflammatory responses to social stress may thus be explained, at least in part, by individual differences in activity in neural regions that process social threat-related information.
Insofar as stressors involving the possibility of social rejection up-regulate inflammatory activity (17, 18), neural regions involved in processing rejection-related distress may relate to individuals’ magnitude of inflammatory responses to social stress. On the basis of prior research, these brain regions include the dorsal anterior cingulate cortex (dACC) and the anterior insula. Exposure to an acute episode of social rejection or to rejection-related cues has been shown to activate both the dACC and the anterior insula (22, 23); greater activity in the dACC, in turn, has been associated with greater self-reported feelings of social distress (e.g., “I felt rejected”) (22, 24). Exposure to social-evaluative threat also has been shown to activate the dACC (25). Thus, neural regions associated with social rejection-related distress may play a role in inflammatory responses to stressors that involve elements of social-evaluative threat and rejection.
To examine whether neural regions involved in processing rejection-related information are associated with inflammatory responses to an episode of acute social stress, we administered two tasks to a sample of healthy young adult participants. First, we exposed participants (n = 124) to a laboratory-based social stressor, the Trier Social Stress Test (TSST) (26), which involves preparing and delivering an impromptu speech and performing difficult mental arithmetic in front of a nonresponsive, socially rejecting panel of raters. To quantify the magnitude of participants’ inflammatory response to this stressor, we collected oral fluids during the stressor and assessed two key markers of inflammatory activity—namely, a soluble receptor for tumor necrosis factor-α (sTNFαRII) and interleukin-6 (IL-6). In a subsequent session, a subset of these participants (n = 31) was scanned while they played a computerized ball-tossing game called “Cyberball” (27), in which participants were ultimately excluded by two other supposed players, leading to an experience of social rejection. We then examined how differences in neural activity during social rejection correlated with differences in inflammatory responses to the TSST. On the basis of prior research, we made two predictions. First, we hypothesized that the TSST would elicit significant increases in inflammatory activity. Second, we hypothesized that greater inflammatory responses to the TSST would be associated with greater neural activity in the dACC and anterior insula during social rejection.
Results
Inflammatory Responses to Acute Social Stress.
As predicted, exposure to the TSST elicited significant increases (from baseline to post-TSST) in levels of both sTNFαRII, F(1, 123) = 9.78, P < 0.005, η2 = 0.074, and IL-6, F(1, 123) = 4.39, P < 0.05, η2 = 0.034 (see Fig. 1). These effects did not differ as a function of gender, ethnicity, or body mass index (BMI) (all Ps > 0.17). Changes in sTNFαRII and IL-6 were positively correlated, r = 0.53, P < 0.001. Thus, exposure to the TSST, which involves elements of social-evaluative threat and rejection, triggered significant increases in inflammatory activity.
Fig. 1.
Levels of the inflammatory markers. Shown are (A) a soluble receptor for tumor necrosis factor-α (sTNFαRII) and (B) interleukin-6 (IL-6) at baseline (Baseline) and following exposure to the Trier Social Stress Test (Post-TSST), expressed as mean ± SEM. The TSST elicited significant increases in both sTNFαRII and IL-6. (C) The relation between sTNFαRII and IL-6 responses to the TSST, where response is calculated as post-TSST minus baseline (n = 124).
Neural Correlates of Inflammatory Responses to Acute Social Stress.
Next, we examined participants who had been scanned while they played Cyberball (n = 31) to test associations between their neural responses to social rejection and their inflammatory responses to acute social stress. As with the full sample, exposure to the TSST in this neuroimaging subsample elicited significant increases in both sTNFαRII, F(1, 30) = 12.58, P < 0.001, η2 = 0.30, and IL-6, F(1, 30) = 12.36, P < 0.001, η2 = 0.29. These effects did not differ by gender, ethnicity, or BMI (all Ps > 0.1). In addition, changes in levels of sTNFαRII and IL-6 were again significantly and positively correlated, r = 0.53, P < 0.005.
To examine the hypothesis that greater inflammatory responses to the TSST would be associated with greater neural responses to social rejection, we used a region of interest (ROI)-based approach that involved selecting the dACC and anterior insula as a priori anatomical ROIs. Activity in these brain regions during social exclusion (vs. inclusion) was unrelated to baseline levels of sTNFαRII and IL-6 (all Ps > 0.1). Consistent with hypotheses, however, greater increases in sTNFαRII in response to the TSST were significantly associated with greater activity in the dACC ROI (r = 0.48, P < 0.005), left anterior insula ROI (r = 0.34, P < 0.05), and right anterior insula ROI (r = 0.30, P < 0.05; see Fig. 2). Changes in IL-6 were unrelated to activity in the dACC and left anterior insula ROIs (Ps > 0.2), but were marginally related to activity in the right anterior insula ROI (r = 0.26, P = 0.08).
Fig. 2.
Relations between inflammatory responses to the Trier Social Stress Test (TSST), as indexed by changes in levels of a soluble receptor for tumor necrosis factor-α (sTNFαRII), and activity in the (A) dorsal anterior cingulate cortex (dACC) ROI, (B) left anterior insula ROI, and (C) right anterior insula ROI. Greater activity in these brain regions during social exclusion (vs. inclusion) was significantly associated with greater sTNFαRII responses to the TSST (n = 31).
To more fully explore these effects, we supplemented the anatomical ROI analyses with whole-brain regression analyses to examine which neural regions were associated with sTNFαRII and IL-6 responses to the TSST (P < 0.001, 20-voxel extent threshold). Consistent with the ROI analyses, and as shown in Table 1, greater TSST-induced increases in sTNFαRII were associated with greater activity in the dACC (r = 0.67, P < 0.001; see Fig. 3) and left anterior insula (r = 0.63, P < 0.001) during social exclusion (vs. inclusion), as well as with several other areas in the cortex, midbrain, and cerebellum. IL-6 was unrelated to activations in higher cortical, paralimbic, or limbic areas (Table S1). Finally, no brain regions were negatively correlated with changes in sTNFαRII or IL-6.
Table 1.
Neural activity during social exclusion (vs. inclusion) that was significantly associated with greater inflammatory responses to the Trier Social Stress Test (TSST), as indexed by changes in levels of a soluble receptor for tumor necrosis factor-α (sTNFαRII) (n = 31)
| Brain region | Brodmann area | MNI coordinates | Cluster size (k) | t | r (TNF) |
| Paralimbic | |||||
| dACC | BA 24 | −6 18 34 | 351 | 4.58 | 0.67 |
| dACC | BA 24 | 12 38 0 | 29 | 4.45 | 0.63 |
| Anterior insula | −34 –22 28 | 45 | 4.44 | 0.63 | |
| Parahippocampal gyrus | BA 36 | −22 –20 −30 | 31 | 4.13 | 0.60 |
| Cortex | |||||
| Middle frontal gyrus | BA 10/46 | −30 52 12 | 99 | 4.54 | 0.64 |
| Inferior frontal gyrus | BA 45 | 50 26 20 | 23 | 3.80 | 0.59 |
| Precuneus | BA 30 | 4 –46 −4 | 454 | 6.08 | 0.71 |
| Fusiform gyrus | BA 37 | −40 –60 −12 | 28 | 4.41 | 0.63 |
| Lingual gyrus | BA 18 | 26 –74 0 | 296 | 6.33 | 0.71 |
| Lingual gyrus | BA 18 | −4 –70 −8 | 107 | 4.36 | 0.63 |
| Fusiform gyrus | BA 18 | 22 –98 −10 | 21 | 3.75 | 0.58 |
| Subcortical and cerebellum | |||||
| Caudate | 18 18 14 | 79 | 4.34 | 0.61 | |
| Thalamus | −20 –28 14 | 51 | 4.30 | 0.62 | |
| Midbrain (substantia nigra) | −12 –12 −12 | 20 | 4.16 | 0.62 | |
| Medulla | 6 –32 −48 | 30 | 4.09 | 0.61 | |
| Cerebellum | 32 –48 −36 | 49 | 4.41 | 0.64 | |
| Cerebellum | −4 –70 −34 | 35 | 4.23 | 0.62 | |
| Cerebellum | −28 –44 −24 | 21 | 4.05 | 0.60 | |
| Cerebellum | −22 –72 −34 | 45 | 4.00 | 0.59 | |
The brain regions listed represent those that were significantly related to TSST-induced changes in sTNFαRII, thresholded at P < 0.001, 20 voxels. dACC is the dorsal anterior cingulate cortex; BA refers to putative Brodmann's area; MNI coordinates identify the local maxima of particular brain activations, reported in Montreal Neurological Institute (MNI) format; Cluster size (k) is the number of voxels in each activation cluster that was significantly associated with greater sTNFαRII responses to the TSST; t is the t-value at those coordinates (local maxima); r (TNF) is the correlation coefficient describing the strength of association between the activation cluster and sTNFαRII response to the TSST.
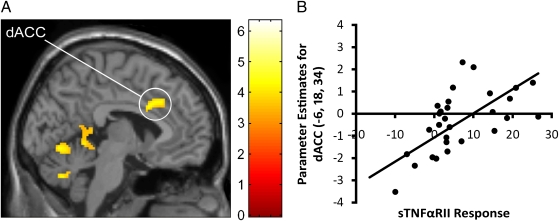
Fig. 3.
(A) Neural activity in the dorsal anterior cingulate cortex (dACC) during social exclusion (vs. inclusion) that correlated positively with inflammatory responses to the Trier Social Stress Test (TSST), as indexed by changes in levels of a soluble receptor for tumor necrosis factor-α (sTNFαRII). (B) Scatterplot showing the relation between mean activity in the dACC cluster (thresholded at P < 0.001, 20 voxels) and sTNFαRII responses to the TSST. Greater activity in the dACC was significantly associated with greater sTNFαRII responses to the TSST (n = 31).
Discussion
The present study demonstrates that a social stressor involving social-evaluative threat and rejection elicits significant increases in inflammatory activity, as indexed by both sTNFαRII and IL-6 (see also refs. 17 and 18). To examine the neurocognitive pathways that might underlie this effect, we focused on brain regions previously implicated in processing rejection-related distress and negative affect. Anatomical ROI analyses revealed that greater activity in the dACC and bilateral anterior insula during social exclusion (vs. inclusion) was associated with greater sTNFαRII responses to the laboratory-based social stressor; greater activity in the right anterior insula was marginally related to increases in IL-6. These associations were consistent with whole-brain analyses, which confirmed that greater activity in the dACC and left anterior insula was associated with greater increases in sTNFαRII. Considered together, these data demonstrate that neural responses to social rejection are associated with potentiated inflammatory responses to an episode of acute social stress.
Interestingly, the relations between neural activity and inflammatory responding were found despite the fact that the neuroimaging session and social stressor session took place several weeks apart. This result suggests that these neural patterns of responding represent at least a moderately stable trait that, in turn, is involved in potentiated inflammatory responses to social stress. Consistent with this formulation, greater social rejection-induced dACC activity during an fMRI session has been associated with greater self-reported distress during daily social interactions (28). Moreover, there is considerable evidence that individual differences in inflammatory responses to stress are relatively stable over time (29, 30).
It is also important to note that although dACC and bilateral anterior insula activity was significantly related to sTNFαRII, IL-6 was related only to right anterior insula activity (P = 0.08). At least three explanations are possible. First, variability in TSST-induced inflammatory responses was substantially greater for sTNFαRII (SD = 11.03) than for IL-6 (SD = 3.02), making it easier to detect associations for sTNFαRII. Second, because TNF responses occur earlier in the inflammatory cascade, our assessment time points may have been more appropriate for capturing TSST-induced changes in sTNFαRII. Finally, the dACC and anterior insula may simply not be directly involved in IL-6 responses to acute social stress.
One critical question raised by the present findings concerns why neural sensitivity to social rejection would relate to inflammatory responding. There are several possible reasons. First, to the extent that physical threats are more likely to occur in situations that involve social threat or rejection, social rejection may trigger inflammatory activity to manage the possibility of injury. Inflammatory cytokines are released in response to impending (or actual) physical assault because they can accelerate wound healing and reduce risk for infection (10, 17). Cytokines also induce a constellation of behaviors called sickness behaviors, which promote recuperation, limit transmission of pathogens to others, and reduce risk for additional conflict (10, 31). Neural regions that process rejection-related information may thus be involved in inflammatory responding because they help organisms mount preparatory responses to potential physical injury. A second reason for why neural responses to social rejection might relate to inflammatory responding is based on overlapping neural circuitry underlying physical and social pain (22). To the extent that physical pain (or the possibility of physical pain) triggers inflammatory responding to manage physical injury, social rejection may be capable of triggering these processes as well because it utilizes some of the same pain-related neural systems.
A second question raised by these findings concerns how the dACC and anterior insula are involved in generating inflammatory responses to stress. Relevant to this issue is a growing body of research suggesting that the ACC and anterior insula function as an integrated circuit (32, 33), forming a key node in the visceromotor network (34). Both regions appear to be involved in high-level representation of visceral states (35), which may include neural encoding of peripheral inflammation (36–38), and they are connected to the periphery in several ways that give these paralimbic structures the ability to modulate inflammatory activity. For example, both the ACC and the anterior insula have extensive efferent connections to the hypothalamus (39), enabling them to influence inflammatory activity via endocrine pathways. In addition, these regions project to the brainstem autonomic control nuclei (40), where peripheral inflammatory processes can be regulated by sympathetic and parasympathetic activity (20).
Although we did not assess health outcomes in the present study, it is possible that individual differences in magnitude of inflammatory responses to social stress may have implications for health (41). Specifically, they may help to explain the considerable variability that has been observed in susceptibility to disorders with an inflammatory component, including asthma (4), arthritis (5), cardiovascular disease (6), certain types of cancer (7), and depression (8). Risk for depression, for example, increases substantially following rejection-related life events (42, 43), but not all people who experience rejection become depressed. Greater neural responses to rejection may be associated with greater inflammatory activity, which is subsequently reflected in the pathogenesis of inflammatory-related disorders such as depression (44).
A limitation of the present study is that the associations observed between neural activity and inflammatory responding were correlational and, as such, causality cannot be determined. Furthermore, additional research is needed to examine whether neural responses to social rejection are uniquely related to differences in inflammatory responding or, alternatively, whether they are part of a more general “stress” system that can be activated by several types of negative events that also relate to inflammatory responding. Nevertheless, across several studies and different sets of emotional stimuli, the dACC and anterior insula are the primary sites of neural activation that correlate with stress-related physiological responding (see refs. 24, 25, and 45–47). The emerging neurocognitive account, therefore, is that brain regions involved in processing social rejection-related information are associated with a variety of biological responses to social and physical threat. These brain regions may thus have important implications for health in general and susceptibility to inflammatory diseases in particular.
Materials and Methods
Participants.
Advertisements for a study of psychological responses to stress were posted on the University of California, Los Angeles (UCLA) campus. Respondents were screened to recruit those in good physical and mental health. Prospective participants were excluded if they had a diagnosed physical or mental health problem; were experiencing a cold, viral infection, or other inflammatory condition (e.g., asthma, bronchitis, thyroid problems, gingivitis, etc.); were exhibiting symptoms consistent with a cold or infection (e.g., sore throat, runny nose, sweating, coughing, bleeding gums, etc.); were taking psychiatric medications or medications affecting cardiovascular or endocrine function; were seeing a mental health professional; or were pregnant or breastfeeding. One hundred twenty-four individuals (54 males, 70 females) met these criteria and received $60 for participating in the laboratory social stressor component of the study. These participants ranged in age from 18 to 36 (M = 21.25, SD = 2.62; males, M = 21.40, SD = 3.36; females, M = 21.13, SD = 1.88) and were ethnically diverse (i.e., 34.7% Asian American, 34.7% European American, 11.3% Hispanic/Latino, 7.3% Middle Eastern, 4.0% African American, and 8.0% “mixed” or other).
Participants from this sample were subsequently invited to participate in a neuroimaging study. Respondents were screened to determine if they met the fMRI-related inclusion criteria of being right-handed, not claustrophobic, and free of bodily metals (except dental fillings, which were allowed) or other conditions that could have prevented them from being scanned (e.g., pacemaker). Thirty-three individuals met these criteria and received an additional $20 for their time. Data for one individual was unusable as an extreme outlier (i.e., >3 SDs below the mean on neural activity) and another individual had been previously administered the Cyberball task; as a result, they were omitted from the MRI analyses. The final neuroimaging subsample thus consisted of 31 participants (12 males, 19 females) who ranged in age from 18 to 36 (M = 21.30, SD = 3.10; males, M = 22.17, SD = 4.57; females, M = 20.72, SD = 1.41). The ethnic diversity of this subsample was representative of the larger sample (i.e., 35.5% Asian American, 25.8% European American, 16.1% Hispanic/Latino, 6.5% Middle Eastern, 6.5% African American, and 9.6% mixed or other). All participants provided written informed consent and all procedures were preapproved by the UCLA Institutional Review Board. The present report associates the neuroimaging and TSST-related inflammatory data for these participants.
Laboratory Social Stressor Paradigm.
Laboratory social stressor sessions were scheduled between 2:30 and 4:30 PM to minimize variability due to diurnal variation in inflammatory activity and cortisol production (48, 49). Participants were told to not eat, exercise, or consume caffeine for at least 1 h before their session.
Information about participants’ social and interpersonal functioning (not part of the present study) was collected upon arrival. Participants were then escorted into the laboratory for the TSST, a widely used laboratory stress task that has been shown to up-regulate inflammatory activity (10, 11). Following a baseline rest period of 10 min, participants were asked to prepare (5 min) and deliver (5 min) a speech on why they would be a good administrative assistant. The speech was delivered to an unresponsive, socially rejecting panel of two raters who behaved nonverbally as if they found the speech to be lacking in quality. Participants were then asked to complete difficult mental arithmetic out loud (5 min). Specifically, they were asked to start at 2,935 and to count backward by 7’s and by 13’s while being urged to go faster by an apparently exasperated experimenter. These tasks were followed by a 30-min recovery period, during which time participants completed a packet of questionnaires (not part of the present study).
Inflammatory Activity.
Inflammatory responses to the TSST were assessed by measuring inflammatory markers when participants arrived for the study (baseline) and 30 min after beginning the TSST (post-TSST). We focused on markers of the proinflammatory cytokines tumor necrosis factor-α (TNF-α) and IL-6 because they have been examined extensively in studies of stress and health. These cytokines are acute phase proteins that play key roles in initiating and mediating inflammatory activity. Moreover, they are believed to underlie several major disorders (4–8). Because laboratory-based social stressors trigger the localized expression of inflammatory markers in the mouth (i.e., gingival crevicular fluid) (50, 51), we assessed TNF-α and IL-6 activity in oral mucosal transudate (OMT). OMT is a filtrate of blood plasma that has been validated for measuring inflammatory activity. For example, levels of sTNFαRII (a receptor for TNF-α) are highly correlated with those obtained from plasma (52); also, they have been used in prior research assessing the effects of acute stress on inflammatory biology (53, 54). In the present study, OMT samples were obtained using an OraSure Collective Device (Epitope), which consists of an absorbent pad and a storage vial. For each collection, the pad was placed between the participant's lower cheek and gum for 2 min and then inserted into the vial for storage.
Vials containing the OMT samples were immediately refrigerated and then transferred to a −80 °C freezer for storage. Assays were conducted at the Center for Interdisciplinary Research in Immunology and Disease at UCLA and were run in duplicate. sTNFαRII was measured using the Quantikine Human sTNFαRII enzyme immunoassay (ELISA) kit manufactured by R&D Systems. IL-6 was measured using the IMx automated microparticle enzyme immunoassay system (Abbot). The inter- and intraassay coefficients of variation were ≤4.1 and 7.5%, respectively, for sTNFαRII, and <9.0 and 3.3%, respectively, for IL-6. For data analyses, sTNFαRII values were normally distributed at each time point and thus were not transformed. IL-6 values for each collection time point were log-transformed (after a constant of 2 was added to each measurement) to correct for nonnormality. Finally, TSST inflammatory response scores were computed for each participant by subtracting baseline levels of sTNFαRII and IL-6 from post-TSST levels of these markers (i.e., post-TSST minus baseline). One participant was an outlier (>2.5 SDs) on sTNFαRII response and was thus recalculated to be at 2.5 SDs from the sample mean to normalize the influence this observation would have on tests of the primary hypotheses. Preliminary analyses were run to test for differences in inflammatory reactivity as a function of gender, ethnicity, and BMI. To test for TSST-induced changes in levels of sTNFαRII and IL-6, we conducted two parallel repeated-measures ANOVAs with one within-subjects factor (time) of two levels (baseline, post-TSST).
fMRI Paradigm.
fMRI scans were obtained while participants completed the Cyberball task (27). Participants were told they would be playing a virtual ball-tossing game with two other individuals. In reality, however, they interacted with two virtual players whose actions were controlled by a preset computer program. At the beginning of each game, participants saw a computer screen displayed through fMRI-compatible goggles. Cartoon images representing the other players were displayed in the upper left- and right-hand corners of the screen. The participant was represented by a cartoon hand, located in the bottom-center position of the screen. To increase the personal nature and believability of the task, the participant's name was displayed below the hand, and names for the other two players were displayed below their respective images. After 9 s, one virtual player started the game by throwing the ball to either the other virtual player or the participant. After receiving a pass, the participant could elect to throw the ball to either of the two other players by pressing one of two keys on a button box. Brief random delays of 0.5–3.0 s were inserted before each virtual player made a throw to enhance participants’ belief that they were playing with other people.
Participants were scanned while they played two games of Cyberball, consisting of 60 throws each. In the first game (inclusion), participants played with the other players for the entire game (≈140 s). During this game, the virtual players threw the ball to the participant ≈50% of the time. In the second game (exclusion), participants received seven passes (lasting ≈50 s) and were then excluded from receiving passes for the remainder of the game (≈60–90 s). Participants were debriefed following this scanning sequence to reveal the true nature of the study and the reason for using deception.
fMRI Data Acquisition and Processing.
Data were acquired on a Siemens Allegra 3T head-only scanner. High-resolution structural T2-weighted echo-planar images (spin echo, TR = 5,000 ms, TE = 33 ms, matrix size 128 × 128, 36 axial slices, FOV = 20 cm, 3-mm thick, skip 1 mm) were acquired coplanar with the functional scans. During the Cyberball task, two functional scans were acquired (echo planar T2*-weighted gradient echo, TR = 3,000 ms, TE = 25 ms, flip angle = 90°, matrix size 64 × 64, 36 axial slices, FOV = 20 cm, 3-mm thick, skip 1 mm), each lasting 2 min 30 s. Head movements were restrained with foam padding and surgical tape that was placed across each participant's forehead.
The imaging data were analyzed using SPM’99 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK). Images for each participant were realigned to correct for head motion using a 6-parameter affine “rigid-body” transformation, normalized (12-paramater affine transformation) into a standard stereotactic space, and smoothed with an 8-mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio.
The design was modeled using a boxcar function convolved with a canonical hemodynamic response function. For each participant, periods of inclusion and exclusion were modeled as epochs on the basis of the length of that participant's inclusion and exclusion episodes. These episodes were individually timed and slightly different between participants, due to the random time delay assigned to the tossing behavior of the virtual players and differences in how long it took participants to throw a caught ball. Neural activity during the inclusion and exclusion episodes was an average of the sustained neural activity that occurred during each episode. After the task was modeled for each participant, planned comparisons were computed as linear contrasts to investigate neural activity during the exclusion compared with the inclusion episode. Random effects analyses of the group were computed using the contrast images generated for each participant.
fMRI Analyses.
Relations between neural responses to social rejection and inflammatory responses to the social stressor were examined in two ways. First, we conducted ROI analyses on the basis of a priori hypotheses concerning the specific anatomical brain regions that we predicted would be associated with inflammatory responses to acute social stress. Second, we conducted whole-brain analyses to explore in more detail the neural regions associated with inflammatory responses to social stress.
For the ROI analyses, we created anatomical ROIs for brain regions known to be involved in processing rejection-related distress and negative affect—namely, the dACC and anterior insula. The ROIs were constructed in PickAtlas (55) using templates from the atlas of Tzourio-Mazoyer et al. (56). The dACC ROI used a rostral boundary of y = +32 on the basis of criteria established by Vogt et al. (57) and a caudal boundary of y = 0 on the basis of summary data indicating that the majority of physical pain study activations occur anterior to that coordinate. To create an ROI for the anterior insula, the insula was divided at its midpoint (y = 0), which corresponds to the approximate boundary between the dysgranular and granular sectors (58, 59).
The MarsBaR toolbox (http://marsbar.sourceforge.net) was then used to extract mean parameter estimates (that model the amplitude of the BOLD response during exclusion vs. inclusion), averaged across all voxels in the ROI. Standard statistical software (SPSS 17.0) was used to conduct correlation analyses to test associations between neural activity during social rejection and inflammatory responses to the TSST. To determine if activity in the dACC and anterior insula was associated with the magnitude of inflammatory response to the TSST, mean parameter estimates from these anatomical ROIs were correlated with reactivity scores for the inflammatory markers sTNFαRII and IL-6. One-tailed tests were used given a priori hypotheses concerning the relation of these brain regions to increased inflammatory activity.
We then performed whole-brain regression analyses to more fully explore associations between neural activity and changes in levels of sTNFαRII and IL-6. All whole-brain regression analyses were thresholded at P < 0.001, 20 voxels, which is comparable to the false-discovery rate correction of P < 0.05 that is commonly used in behavioral research (60).
Supplementary Material
Acknowledgments
We thank Michael Irwin, Keely Muscatell, Mary-Frances O'Connor, Aoife O'(tm)Donovan, and Elliot Berkman for their assistance, as well as the Brain Mapping Center, the Cousins Center for Psychoneuroimmunology, and the Center for Interdisciplinary Research in Immunology and Disease at the University of California, Los Angeles. This research was supported by a Society in Science: Branco Weiss Fellowship (to G.M.S.) and by National Institutes of Health Grants T32MH019925 (to G.M.S. and N.I.E.) and AG030309 and MH56880 (to S.E.T.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009164107/-/DCSupplemental.
References
- 1.Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen NR, Kristensen TS, Schnohr P, Grønbaek M. Perceived stress and cause-specific mortality among men and women: Results from a prospective cohort study. Am J Epidemiol. 2008;168:481–491. doi: 10.1093/aje/kwn157. [DOI] [PubMed] [Google Scholar]
- 4.Kay AB. Asthma and inflammation. J Allergy Clin Immunol. 1991;87:893–910. doi: 10.1016/0091-6749(91)90408-g. [DOI] [PubMed] [Google Scholar]
- 5.Choy EHS, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: Meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 8.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avitsur R, Powell N, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Immunol Allergy Clin North Am. 2009;29:285–293. doi: 10.1016/j.iac.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Kiecolt-Glaser JK, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiecolt-Glaser JK, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 14.Davis MC, et al. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: Implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuligni AJ, et al. A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosom Med. 2009;71:329–333. doi: 10.1097/PSY.0b013e3181921b1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerson SS, Gable SL, Irwin MR, Aziz N, Kemeny ME. Social-evaluative threat and proinflammatory cytokine regulation: An experimental laboratory investigation. Psychol Sci. 2009;20:1237–1244. doi: 10.1111/j.1467-9280.2009.02437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denson TF, Spanovic M, Miller N. Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychol Bull. 2009;135:823–853. doi: 10.1037/a0016909. [DOI] [PubMed] [Google Scholar]
- 19.Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sternberg EM. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–328. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 23.Kross E, Egner T, Ochsner K, Hirsch J, Downey G. Neural dynamics of rejection sensitivity. J Cogn Neurosci. 2007;19:945–956. doi: 10.1162/jocn.2007.19.6.945. [DOI] [PubMed] [Google Scholar]
- 24.Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wager TD, et al. Brain mediators of cardiovascular responses to social threat, part II: Prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 27.Williams KD, Jarvis B. Cyberball: A program for use in research on interpersonal ostracism and acceptance. Behav Res Methods. 2006;38:174–180. doi: 10.3758/bf03192765. [DOI] [PubMed] [Google Scholar]
- 28.Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: Implications for stress-elicited changes in immune related health. Brain Behav Immun. 2003;17:407–414. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 30.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 31.Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 32.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 36.Harrison NA, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capuron L, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ongür D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol. 1998;401:480–505. [PubMed] [Google Scholar]
- 40.An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 41.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 42.Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60:789–796. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- 43.Slavich GM, Thornton T, Torres LD, Monroe SM, Gotlib IH. Targeted rejection predicts hastened onset of major depression. J Soc Clin Psychol. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slavich GM, O'Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: A psychobiological model of social rejection and depression. Neurosci Biobehav Rev 35. 2010 doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, et al. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gianaros PJ, et al. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane RD, et al. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 48.Haack M, Pollmächer T, Mullington JM. Diurnal and sleep-wake dependent variations of soluble TNF- and IL-2 receptors in healthy volunteers. Brain Behav Immun. 2004;18:361–367. doi: 10.1016/j.bbi.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 50.Deinzer R, et al. Acute stress effects on local Il-1beta responses to pathogens in a human in vivo model. Brain Behav Immun. 2004;18:458–467. doi: 10.1016/j.bbi.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 51.Weik U, Herforth A, Kolb-Bachofen V, Deinzer R. Acute stress induces proinflammatory signaling at chronic inflammation sites. Psychosom Med. 2008;70:906–912. doi: 10.1097/PSY.0b013e3181835bf3. [DOI] [PubMed] [Google Scholar]
- 52.Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin Diagn Lab Immunol. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- 54.O'Connor MF, Irwin MR, Wellisch DK. When grief heats up: Pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47:891–896. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 56.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 57.Vogt BA, Berger GR, Derbyshire SWG. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ongür D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 59.Bonthius DJ, Solodkin A, Van Hoesen GW. Pathology of the insular cortex in Alzheimer disease depends on cortical architecture. J Neuropathol Exp Neurol. 2005;64:910–922. doi: 10.1097/01.jnen.0000182983.87106.d1. [DOI] [PubMed] [Google Scholar]
- 60.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.