Abstract
The ability to control craving for substances that offer immediate rewards but whose long-term consumption may pose serious risks lies at the root of substance use disorders and is critical for mental and physical health. Despite its importance, the neural systems supporting this ability remain unclear. Here, we investigated this issue using functional imaging to examine neural activity in cigarette smokers, the most prevalent substance-dependent population in the United States, as they used cognitive strategies to regulate craving for cigarettes and food. We found that the cognitive down-regulation of craving was associated with (i) activity in regions previously associated with regulating emotion in particular and cognitive control in general, including dorsomedial, dorsolateral, and ventrolateral prefrontal cortices, and (ii) decreased activity in regions previously associated with craving, including the ventral striatum, subgenual cingulate, amygdala, and ventral tegmental area. Decreases in craving correlated with decreases in ventral striatum activity and increases in dorsolateral prefrontal cortex activity, with ventral striatal activity fully mediating the relationship between lateral prefrontal cortex and reported craving. These results provide insight into the mechanisms that enable cognitive strategies to effectively regulate craving, suggesting that it involves neural dynamics parallel to those involved in regulating other emotions. In so doing, this study provides a methodological tool and conceptual foundation for studying this ability across substance using populations and developing more effective treatments for substance use disorders.
Keywords: drug craving, cigarette smokers, emotion regulation, functional MRI, substance use
The ability to resist immediate gratification by controlling the impulse to consume a desirable—but, in the long run, unhealthy—substance is critical to both mental and physical health (1). This ability is perhaps no more important than for individuals with substance use disorders (SUDs), which are chronic relapsing conditions (2, 3) with staggering social costs (4). The strong desire to use drugs (known as drug craving) and failure to curb this desire are central to such disorders and are thought to be primary contributors to drug use in general (5–8). Three types of evidence directly link both craving and failure to regulate it to drug-taking behavior. First, across substance-using populations, prospective studies show that craving predicts relapse to drug taking following abstinence (9–15). Second, cognitive–behavioral therapies (CBT) include training in the cognitive regulation of craving, and are effective for treating SUDs (16). Third, across different forms of treatment, the deployment of cognitive strategies to reduce craving is associated with reduced relapse over time (13, 17–20).
In recent years, imaging studies have taken a step in elucidating the mechanisms underlying substance use by exposing drug users to conditioned drug-associated cues such as photos, videos, or stories depicting drug use (6). Such studies report that cue exposure reliably increases craving and activity in regions previously associated with emotion and the experience of drug effects, including the ventral tegmental area (VTA; e.g., ref. 21), ventral striatum (VS; e.g., refs. 21, 22), amygdala (21, 23), insula (23, 24), medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC; e.g., refs. 21–23), and anterior cingulate cortex (ACC; e.g., refs. 21, 22, 24) including its subgenual portion (sgACC; e.g., refs. 22, 25).
Although this research provides insight into the mechanisms by which drug cravings are generated, the mechanisms by which cravings are effectively regulated remain unclear, despite their relevance to SUDs and their treatment. The finding that craving depends on regions implicated in emotion raises the intriguing possibility that the cognitive regulation of craving could be similar to the cognitive regulation of emotion, a topic of increasing empirical interest. To date, the majority of studies on the cognitive regulation of emotion have examined negative emotions elicited by the presentation or anticipation of aversive stimuli (reviewed in ref. 26). These studies found that cognitive strategies can be used to decrease, maintain, or increase both emotional responses (such as negative emotion or fear) and activity in brain systems involved in generating emotion, such as the amygdala and/or insula (26, 27). In turn, the deployment of these strategies is associated with activity in a network of prefrontal regions implicated in cognitive control (28, 29), including the dorsolateral prefrontal cortex (dlPFC), dorsomedial prefrontal cortex (dmPFC), and ventral lateral prefrontal cortex (vlPFC) (26). A few studies have focused on the regulation of responses to positive images, food, or monetary reward and have reported similar findings (30–33).
Taken together, these two bodies of literature suggest that the effective regulation of craving might involve the use of systems implicated in emotion regulation, such as lateral PFC, to modulate activity in regions implicated in cue-induced drug craving, such as the VS. Recently, initial steps have been taken toward exploring this hypothesis by showing videos depicting substance use to cigarette smokers (34) or cocaine users (35) while asking them to “resist” or “inhibit” craving, respectively. Although both studies observed activity in at least some regions associated with control, the meaning of these activations is difficult to interpret for three reasons. First, neither study observed a significant reduction in craving. Second, in both studies participants were allowed to self-select one or more regulatory strategies that may be unlike those used to effectively treat SUDs in clinical contexts. Third, neither study included a control condition to determine whether regulation of craving for the abused substance was more or less effective, or involved neural systems different from regulation of craving for another appetitive stimulus for which there were no substance use issues. Such a condition is important if one wants to draw inferences about the specificity or generality of regulation-related changes in craving.
Thus, although prior work is suggestive, four key questions about the mechanisms by which clinically relevant cognitive strategies effectively reduce craving remain unclear. First, would the effective down-regulation of craving using treatment-relevant strategies be accompanied by increased activity in prefrontal control systems, and in turn, by decreased activity in the VS, VTA, and other craving-related regions? Second, is the regulation of craving for an abused substance different from an appetitive control stimulus? Third, are individual differences in the ability to regulate craving related to increased activity in control-related systems and decreased activity in craving-related systems? If the answers to the first and third questions are yes, then a fourth question naturally arises: Is the hypothesized inverse relationship between PFC control-related regions and self-reported craving mediated by changes in activity in craving-related regions (30, 36)? Or, put another way, does the PFC act on the striatum to modulate craving? Although such a relationship would be predicted by theories of both emotion regulation (36) and substance use (8, 37), this relationship has not been demonstrated empirically.
To address these questions, we collected functional MRI (fMRI) data while tobacco cigarette smoking participants completed a task that we previously developed to study the regulation of craving (the ROC task) using cognitive strategies that are used in CBT protocols (38). We selected cigarette smokers because cigarette smoking is the most lethal form of substance use: in the United States, more deaths are caused each year by tobacco use than by all forms of illegal drug use, alcohol use, motor vehicle injuries, suicides, and murders combined (39, 40). In addition, the relationship between craving and drug taking in this group is particularly robust (reviewed in ref. 38).
During the ROC task, participants viewed cigarette- and food-associated cues on two types of trials. On baseline trials, designed to isolate the neural correlates of cravings elicited by smoking and food cues, the instruction “NOW” directed participants to think about the immediate feelings associated with smoking or eating. We have shown previously that smokers report robust cue-induced cravings in this condition (38). On regulation trials modeled after prior work on delay of gratification, cognitive reappraisal, and CBT treatments for SUDs (1, 38, 41), the instruction “LATER” directed participants to think about the long-term consequences associated with smoking and eating high-fat foods. We have shown previously that smokers are able to decrease their craving for cigarettes and food using this strategy (38). After viewing each cigarette or food cue, and thinking about it in the instructed manner, participants rated how much they craved the type of stimulus seen on that trial (trial schematic in Fig. S1).
Results
Behavioral Ratings.
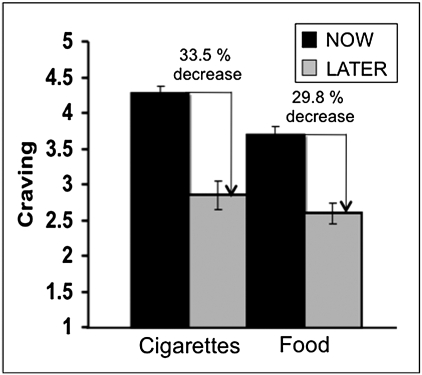
Upon arrival, participants reported their craving for food and cigarettes. These were not significantly correlated with any of the subsequent in-scanner ratings (detailed in SI Text). Analyses of in-scanner ratings revealed significant main effects of both instruction and cue type (Fig. 1): overall, significantly lower craving was reported on LATER compared with NOW instruction trials [F(1,20) = 44.16, P < 0.001] and on food compared with cigarette cue trials [F(1,20) = 11.10, P < 0.01]. These data demonstrate that, in general, regulation with cognitive strategies effectively reduced craving and that cigarette cues elicited stronger cravings than food cues, as would be expected for smokers.
Fig. 1.
Mean craving reported by smokers as a function of cue type (cigarette/food) and instruction type (NOW/LATER). Overall, reported craving was significantly decreased on LATER vs. NOW instruction trials, across both stimulus types, and was significantly greater for cigarette compared with food cues, across both instructions. Error bars represent ±SEs. When expressed as percent drop relative to craving reported on NOW trials, regulation-related drops in craving on LATER trials were only marginally different between cigarettes and food (P < 0.07).
Next, we determined how regulation affected craving for each cue type individually. We observed a significant stimulus × strategy interaction [F(1,20) = 7.02, P < 0.05] reflecting a greater modulation of craving on NOW vs. LATER trials for cigarettes compared with food cues. Inspection of Fig. 1 shows, however, that cigarette and food cues differed in baseline craving reported on NOW trials [t(20) = 4.52, P < 0.001]. To correct for this baseline difference and to ensure that craving decreases did not appear larger for cigarette cues simply because reported craving was overall greater, we calculated regulation-related decreases in craving as the percentage (rather than the absolute) decrease in craving on LATER relative to NOW trials. Comparison of these values showed that the percentage drop in craving due to regulation was only marginally greater for cigarettes than food [33.52% for cigarettes, 29.76% for food; t(20) = 1.96, P < 0.07]. This result is important because it suggests that whereas smokers experience greater cravings for cigarettes than other appetitive cues, when instructed to regulate their cravings using a cognitive strategy, these individuals are at least as—if not more—effective at doing so for cigarettes as they are for other appetitive stimuli. Finally, none of these effects differed between functional runs (see SI Text for additional details).
Imaging Results.
Main effects of regulation.
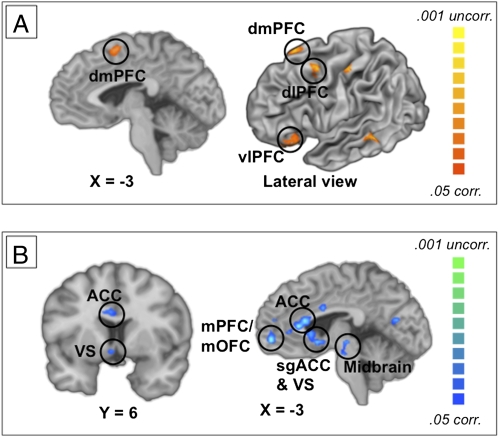
To test our first prediction—that effective down-regulation of craving increases activity in prefrontal control systems and decreases activity in craving-related regions—we used contrasts to identify regions that were more or less active when participants regulated craving on LATER trials compared to NOW trials. As shown in Fig. 2 and Table S1, our first prediction was confirmed: we observed increased activation in regions previously associated with cognitive control in general (28, 29) and with regulating negative emotion in particular (26), including dmPFC, dlPFC, and vlPFC (Fig. 2A). This was accompanied by decreased activation in regions previously associated with emotion in general (42) and with craving in particular (6), including VS, amygdala, sgACC, and VTA (Fig. 2B). It should be noted that we do not discuss the main effect of cue type (food vs. cigarettes) in this paper because it is not relevant to the theoretical issues discussed. The results of this analysis do not change the interpretation of the data presented here in any way, and will be reported elsewhere.
Fig. 2.
Regions active during or modulated by the cognitive regulation of craving. (A) Medial (Left) and lateral (Right) views of brain regions that showed greater activation in the LATER vs. NOW trials, when participants used a cognitive strategy to reduce their craving. Highlighted activations are shown in regions previously implicated in regulation of aversive emotion. (B) Medial (Left) and coronal (Right) views of brain regions that showed reduced activation in LATER vs. NOW trials. Highlighted reductions are shown in regions previously reported in studies of cue-induced craving or emotion. corr, Corrected for multiple comparisons; uncorr, uncorrected for multiple comparisons.
Whole-brain interaction.
To test the second prediction, and to determine whether the effects of craving regulation varied by cue types, we performed a whole-brain interaction analysis (CN vs. CL) vs. (FN vs. FL). Two types of interactions were of a priori theoretical interest, and we limited our analysis to identifying regions showing one of them. First, we were interested in regions showing larger increases during regulation (i.e., a larger LATER > NOW effect) for one type of stimulus as compared with the other, which could be indicative of greater demands for control. Only one region showed such an effect: Activation in dmPFC was stronger for LATER than for NOW trials for food compared with cigarettes. Second, we were interested in regions showing larger regulation-related drops in activity (i.e., a larger NOW > LATER effect) for one type of stimulus as compared with the other. Again, only one region showed such an effect: For food compared with cigarette stimuli, activation in the postcentral gyrus was greater on NOW compared with LATER trials. As we had no a priori expectations about dmPFC being more active when regulating responses to food or the precentral gyrus showing greater regulation-related modulation for food, we simply report these findings and offer no post hoc interpretation of them. What these results importantly show, however, is that the main effects of strategy for control and craving-related regions (i.e., activations in control-related regions and modulations in craving-related regions) are not qualified by an interaction with stimulus type (Table S2).
Correlations with individual differences in regulatory ability.
To test our third prediction—that individual differences in the ability to decrease craving would relate to increased activity in control-related systems and decreased activity in craving-related systems—we computed a robust whole-brain correlation of activity in the NOW vs. LATER contrast, with the mean decrease in craving reported by each participant on LATER trials as compared with NOW trials. Because individual difference correlations can be difficult to detect (43), and because the proportional decreases in craving were similar for cigarette and food cues, we maximized power for this analysis by first computing this correlation collapsing across cue types. We found that decreases in self-reported craving correlated with decreases in VS activity (R2 = 0.47) and with increases in left dlPFC activity (R2 = 0.56; Table S3). To then confirm that these results did not differ across cue types, we used the Fisher z test to determine whether the observed correlations significantly differed as a function of cue type. In both the VS and the dlPFC, no significant differences were observed (P > 0.1).
Test of prefrontal–striatal mediation of craving.
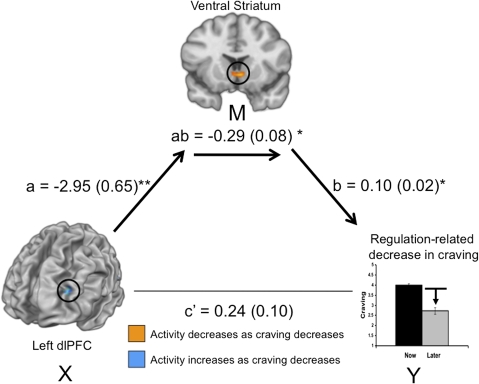
Finally, we addressed the fourth prediction, which was related to the third. The correlations reported above are consistent with either of two possible kinds of functional relationships between regulation-related drops in craving and prefrontal or VS activity. The first possibility is that cognitive strategies exert parallel effects on each brain region, and that prefrontal cortex and the VS are independently related to changes in craving. The second possibility is that the inverse relationship between dlPFC activity and craving is mediated by changes in VS activity, as predict based on prior findings of frontal–subcortical pathways mediating regulation of aversive emotion (36, 44). Given that we wished to test a strong a priori hypothesis that prefrontal cortex would act on the striatum to influence craving, a mediation analysis, which specifically tests for such a relationship, was deemed to be most appropriate (relative to connectivity analyses that only examine covariances between two regions, for example). Therefore, to test this prediction, we performed a formal mediation analysis, collapsing across cues types, given that the analysis reported above found that correlations of brain activity and craving did not vary as a function of cue type. This analysis explicitly tested whether the relationship between activation in dlPFC and regulation success scores (denoted X and Y in Fig. 3) can be explained by values of activation in VS (denoted M in Fig. 3). We found that the VS fully mediated the relationship between dlPFC and reported craving (Fig. 3).
Fig. 3.
Mediation model for the association between dorsolateral prefrontal cortex (dlPFC), ventral striatum (VS), and regulation-related decreases in craving, whereby VS is a complete mediator of the dlPFC–craving relationship. Path coefficients are shown next to arrows indicating each link in the analysis, with SEs in parentheses. Path a refers to the path from dlPFC to VS; path b refers to the direct link between VS and craving; and path c’ refers to the total association between dlPFC and craving, without the mediator VS. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Taken together, these findings provide direct support for the hypothesis that clinically relevant cognitive strategies can effectively reduce craving via the effects of prefrontal control systems on the VS and other systems implicated in reward learning and emotion. As such, these findings have implications for our basic understanding of affect-regulatory mechanisms and of substance use disorders, as well as for studies that translate this work to clinical contexts.
With respect to basic mechanisms, the present study builds on prior work in two ways. First, conceptually, our approach builds on prior work on delay of gratification in children and temporal (or delay) discounting, showing that healthy as well as drug-dependent adults prefer immediate smaller rewards compared with larger delayed rewards (45, 46), but that cognitive strategies can influence and even reverse this preference (47). Whereas such studies have focused on what our choices reveal about our relative desires for immediate as opposed to delayed rewards, the present work focuses on how we can actively take control over the affective impulses that motivate these choices.
This leads to the second point. To our knowledge, no prior studies have shown that the same prefrontal systems support the effective regulation of cravings elicited by two different types of appetitive stimuli, in this case cigarettes and food. This is important, because it suggests that the vlPFC and dmPFC regions (that were engaged during regulation of craving for both cue types), as well as the dlPFC–striatal pathway (which mediated successful regulation), play general roles in the regulation of different kinds of appetitive desires. This fits with prior studies on the regulation of negative emotion (26) and positive emotion (30, 32) showing activation of these prefrontal systems, and suggests that common prefrontal–subcortical dynamics support the use of cognition to regulate responses to various kinds of affective cues, including drug cues.
Importantly, this mechanism is consistent with a prevailing hypothesis in the substance use literature— that a prefrontal–striatal pathway is involved in the control of craving in general, and compulsive drug use in particular (8, 37, 48). However, the behavioral results indicate that although smokers showed greater craving for cigarettes, they were at least as effective at downregulating these cravings as they were at downregulating their cravings for another appetitive stimulus, food. This is consistent with the hypothesis that smoking does not result from a general inability to control appetitive impulses but, rather, from the failure to effectively deploy regulatory strategies that could be effective if one were motivated to use them. The imaging results dovetailed with this conclusion by showing that the same prefrontal–striatal pathway mediated the effective regulation of cravings elicited by cigarette and food cues.
With respect to clinical contexts, the present study has at least three kinds of translational implications. First, it builds on initial attempts to study the regulation of responses to drug-related stimuli (34, 35) that did not observe significant reductions in craving. Here, we showed that craving could be curbed effectively via a single, clinically relevant strategy that involved cognitively reinterpreting the meaning of an initially craved substance in terms that highlighted its potential longer-term deleterious effects on one's health. In general, such cognitive strategies are known to be effective for regulating emotion in the laboratory and field (26, 49), in children and adults (1, 26), and for treating psychiatric (50) as well as substance use disorders (18, 51).
Second, the results of this study identify neural mechanisms by which cognitive strategies reduce craving, and in turn, a potential mechanism by which cognitive therapies can successfully decrease drug craving and use over time. In this regard, the present findings have great practical importance for understanding nicotine dependence in particular, which has been identified as the leading preventable cause of disease and death in the United States, contributing to ~430,000 deaths every year (52). However, future work is needed to replicate and generalize these results across larger populations of smokers and individuals with other SUDs.
Third and last, future translational research could use the present findings as a model, and the method used here as a tool, for testing the hypothesis that impaired prefrontal control over subcortical systems generally characterizes substance-using populations, as has been suggested previously (8, 37, 53). For example, for a given population, this method could be adapted for comparing and testing the efficacy of specific cognitive strategies for regulating cravings induced either by cues or by negative emotions that also may trigger them. Activity in the brain systems identified here could also be used to measure baseline differences in regulatory ability and to track the effects of treatment on their function. In either case, the ultimate goal is to develop more effective treatments for SUDs.
Methods
Participants.
Twenty-one cigarette-smoking subjects participated in this study (9 women and 12 men, aged 18–45 y, mean 26.8. SD 8.94). Participants were recruited via posters and electronic bulletin board ads from the general New York City population. Participants were considered cigarette smokers if they reported smoking more than 10 cigarettes/d, 7 d/wk, for at least 1 y (mean cigarettes/wk 110, SD 35.93, range 70–175). Participants reported starting to smoke regularly between 14 and 28 y of age (mean age of onset 16.95, SD 3.27), and smoked between 1 and 30 y (mean years 9.30, SD 8.63). All participants reported completing high school; years of secondary education ranged from 1 to 5 y (mean years secondary education = 3.19, SD 1.40). Participants were excluded if they were left-handed, did not speak English, or reported body mass index (kg/m2) of more than 28, or any of the following conditions: dependence on substances (other than nicotine), neurological or psychiatric disorders, use of prescription (psychiatric and/or nonpsychiatric) medication that could affect brain function in the past 6 months, medical conditions that might alter cerebral function, cardiovascular disease or diabetes, head trauma with loss of consciousness for more than 30 min, pregnancy, claustrophobia, or any implants contraindicated in MRI. Participants also were excluded if they reported any food allergies or aversion to any of the pictured food stimuli presented in this experiment. Before participation, all participants gave informed consent in accordance with the Columbia University Institutional Review Board. After completion of the study, they were paid $70 for their participation.
ROC Task.
In this single-session study, participants were exposed to photographic images of cigarettes and food that were previously shown to induce craving for these substances (38). On each trial, participants were directed to think about these stimuli in one of two ways. NOW cues instructed participants to consider the immediate consequence of consuming the pictured substance. LATER cues instructed participants to consider the long-term consequences of repeatedly consuming the substance; this strategy is often used in cognitive–behavioral treatment for SUDs. Our prior work indicates that the latter strategy decreases reported craving for both food and cigarettes (38). There were 100 trials (50 food, 50 cigarette) with intertrial intervals jittered around 4 s and a pseudorandomized presentation order.
Strategy training.
Before scanning, participants were instructed in and trained to use each strategy. Participants then experienced eight sample trials, during which they were asked to practice using the strategies while looking at photographs of cigarettes and food that were not used during the scanning session. During training, participants were also trained to use a Likert-type five-point scale to indicate the extent of their craving after each stimulus presentation, as has been used previously (24, 38, 54, 55). The scanning session began once participants indicated to the experimenter that they could use these strategies while looking at the stimuli, and that the directions were understood.
Scanning session.
To minimize effects of circadian rhythms on cue reactivity, scans were all performed in the midafternoon (3:00–5:00 PM). In addition, participants were instructed to abstain from smoking or eating for 2 h before the scanning session, and confirmed their abstinence status upon arrival via a carbon monoxide reading (Vitalograph Breath CO monitor 29700; Vitalograph, Inc). During the scanning session, participants completed five functional runs consisting of 20 trials each. As Fig. S1 illustrates, each trial began with a fixation cross that was displayed for an intertrial interval jittered around 4 s. This interval was followed by a 2-s instructional cue (NOW or LATER) followed by a 6-s presentation of the stimulus (a picture of food or cigarettes). Following another delay period jittered around 3 s, participants next indicated how much they wanted to consume the substance at that moment using a rating scale of 1 (not at all) to 5 (very much) that appeared onscreen for up to 3 s or until the participants indicated a response. Exposure to study stimuli and the order of the instructional cues and photos was counterbalanced across participants. A total of 100 trials lasting ≈18 s each were completed. Finally, participants completed individual difference measures, rerated all of the stimuli, were paid for their time, and were debriefed.
Data Acquisition and Analysis.
Stimulus presentation was controlled using E-Prime software (PST Inc.). An LCD projector displayed stimuli on a back-projection screen mounted in the scanner suite. Responses were made with the right hand on a five-finger button-response unit (Avotec Inc. and Resonance Technologies). Response data were subjected to a 2 (Cue: Food vs. Cigarette cues) × 2 (Strategy: NOW vs. LATER) repeated-measures ANOVA, with an alpha level of P < 0.05. The relative drop in self-reported craving between the NOW and LATER conditions was calculated for each subject and used to correlate with brain activation in subsequent analyses.
fMRI Data Acquisition and Analysis.
Participants were scanned in a GE Signa Twin Speed Excite HD scanner (GE Medical systems). Functional images were acquired with a T2*-weighed EPI BOLD sequence with a TR of 2,000 ms, TE of 34 ms, flip angle of 90°, 64 × 64 in-plane matrix, field of view of 22.4 cm, and 28 4-mm slices in an ascending-interleaved order. High resolution SPGR structural images were also acquired with a TR of 9,700 ms, TE of 2,300 ms, flip angle of 20°, 256 × 256 in-plane matrix, field of view 24 cm, and ~182 1-mm slices covering the entire brain of the subject (range: 176–192).
Following prior protocols established in our laboratory (36), functional images were subjected to preprocessing using SPM5 (Wellcome Department of Cognitive Neurology), warped to the Montreal Neurological Institute template and smoothed using a 6-mm kernel FWHM. After preprocessing, we extracted the global time course from white matter in each volume, and removed “spikes” (e.g., volumes in which the global time course was above or below 3 SD from the mean, after accounting for low-frequency drifts). The data were then subjected to first-level statistical analysis with a standard GLM model. We used a boxcar regressor for the instruction cue and picture presentation period. The instruction cues were not modeled separately, as they were highly correlated to the picture period because of their shortness and regularity (2 s, always right before the stimulus). To address a possible question about the soundness of this methodology, we modeled the instructions separately, and found that the data were not substantially different. The rating period was modeled with an event-related regressor, but is not of interest here. We also used motion parameters and high-pass filter parameters as additional regressors of no interest. We then performed second-level random effects analysis using robust regression to localize regions of activation across subjects. The robust regression approach iteratively reweights the regression matrix using a bisquare weighting function (robustfit.m in the statistics toolbox of MATLAB software, Mathworks). This approach allows a limited number of strong outliers to be down-weighted automatically, thus showing a more accurate outcome in cases in which the sampled measure (DV) cannot be supposed to have a normally distributed error. Such robust procedures are therefore particularly resistant to outliers (56) and have been used previously with fMRI data (36, 57). Similarly, whole-brain robust correlations were computed to assess the relationship between self-reported craving and brain activation.
To correct for multiple comparisons we used AlphaSim, a Monte Carlo simulation bootstrapping program implemented in the AFNI library. AlphaSim takes into account the voxel-wise and cluster-volume thresholds to establish a false discovery rate of 5%. Only regions with corrected P < 0.05 at a combined height and cluster level were considered to be significantly activated or deactivated in the whole-brain analysis. Several regions that were previously reported in studies of cue-induced drug craving, the VS and amygdala, were selected as a priori regions of interest and were considered significant at an uncorrected whole-brain threshold of P < 0.005. In addition, as the IFG and dlPFC are often reported in studies of emotion regulation, they were also selected a priori and considered significant at this uncorrected threshold.
Regions in which activity in the NOW vs. LATER contrast correlated with drop in self-reported craving (e.g., “regulation success”) were subjected to mediation analyses using the robust regression weights to exclude outliers. This mediation analysis used the standard three-variable path model (58). Mediation analyses test whether the relationship between any two variables (activation in dlPFC and regulation success) can be explained by the values from a third variable (activation in VS). If VS is a true mediator of the dlPFC-regulation success relationship, then this relationship will become insignificant when VS is controlled for in the model. According to standard convention, “a” refers to the dlPFC-VS effect, “b” refers to the VS-regulation success effect, and “c” refers to the direct dlPFC-regulation success effect, controlling for the mediator VS. The product “a*b” tests the significance of the direct mediator. As is customary, we used a bootstrapping test for the statistical significance of the product “a*b” (36, 42, 59, 60).
Supplementary Material
Acknowledgments
We thank the participants in this study, Michaela Bamdad for help in recruiting, Stephen Dashnaw for MRI operations, Tor Wager for use of mediation software, and members of the SCAN Unit for helpful commentary. This work was supported by a National Science Foundation Graduate Research Fellowship (to H.K.), by National Institute on Drug Abuse Grant R01 DA022541 (to K.N.O.), and by National Institute of Mental Health Grant R01 MH076137 (to K.N.O.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007779107/-/DCSupplemental.
References
- 1.Mischel W, et al. “Willpower” over the life span: Decomposing self-regulation. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsq081. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLellan AT, Lewis DC, O'Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R. Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institutes of Health . Understanding Drug Abuse and Addiction. Bethesda, MD: National Institutes of Health, National Institute on Drug Abuse; 2008. pp. 1–4. Info Facts. [Google Scholar]
- 5.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: Can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R, Li CSR. Imaging stress- and cue-induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, et al. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: Insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crits-Christoph P, et al. Predictors of sustained abstinence during psychosocial treatments for cocaine dependence. Psychother Res. 2007;17:240–252. [Google Scholar]
- 10.Doherty K, Kinnunen T, Militello FS, Garvey AJ. Urges to smoke during the first month of abstinence: Relationship to relapse and predictors. Psychopharmacology (Berl) 1995;119:171–178. doi: 10.1007/BF02246158. [DOI] [PubMed] [Google Scholar]
- 11.Galloway GP, et al. How long does craving predict use of methamphetamine? Assessment of use one to seven weeks after the assessment of craving. Substace Abuse. Res Treat. 2008;1:63–79. [PMC free article] [PubMed] [Google Scholar]
- 12.Litt MD, Cooney NL, Morse P. Reactivity to alcohol-related stimuli in the laboratory and in the field: Predictors of craving in treated alcoholics. Addiction. 2000;95:889–900. doi: 10.1046/j.1360-0443.2000.9568896.x. [DOI] [PubMed] [Google Scholar]
- 13.O'Connell K, Schwartz J, Gerkovich M, Bott M, Shiffman S. Playful and rebellious states vs. negative affect in explaining the occurrence of temptations and lapses during smoking cessation. Nicotine Tob Res. 2004;6:661–674. doi: 10.1080/14622200410001734049. [DOI] [PubMed] [Google Scholar]
- 14.Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: Further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 16.Dutra L, et al. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- 17.Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. J Consult Clin Psychol. 1989;57:443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychol. 2007;26:77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- 19.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 20.Carroll KM, Nich C, Frankforter TL, Bisighini RM. Do patients change in the way we intend? Treatment-specific skill acquisition in cocaine-dependent patients using the Cocaine Risk Response Test. Psychol Assess. 1999;11:77–85. [Google Scholar]
- 21.Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry. 2002;159:954–960. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- 22.David SP, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin TR, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brody AL, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 26.Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 33.Wang GJ, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brody AL, et al. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkow ND, et al. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 38.Kober H, Kross EF, Mischel W, Hart CL, Ochsner KN. Regulation of craving by cognitive strategies in cigarette smokers. Drug Alcohol Depend. 2010;106:52–55. doi: 10.1016/j.drugalcdep.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Annual smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 1997–2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–628. [PubMed] [Google Scholar]
- 40.McGinnis JM, Foege WH. Actual causes of death in the United States. JAMA. 1993;270:2207–2212. [PubMed] [Google Scholar]
- 41.Carroll K. Therapy Manuals for Drug Addiction. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. Rockville, MD: National Institute of Drug Abuse; 1998. [Google Scholar]
- 42.Kober H, et al. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman M, Berkman E, Wager T. Correlations in social neuroscience aren't voodoo. Perspect Psychol Sci. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 46.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 47.Mischel W, Baker N. Cognitive appraisals and transformations in delay behavior. J Pers Soc Psychol. 1975;31:254–261. [Google Scholar]
- 48.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 49.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 50.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Carroll KM. In: Cognitive-Behavioral Therapies. The American Psychiatric Publishing Textbook of Substance Abuse Treatment. 4th Ed. Galanter M, Kleber HD, editors. Arlington, VA: American Psychiatric Publishing; 2008. pp. 349–360. [Google Scholar]
- 52.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults—United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1157–1161. [PubMed] [Google Scholar]
- 53.Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 54.Lee JH, Lim Y, Wiederhold BK, Graham SJ. A functional magnetic resonance imaging (FMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- 55.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 58.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 59.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 60.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.