Abstract
Adult rats were trained to detect the occurrence of a two-element sound sequence in a background of nine other nontarget sound pairs. Training resulted in a modest, enduring, static expansion of the cortical areas of representation of both target stimulus sounds. More importantly, once the initial stimulus A in the target A-B sequence was presented, the cortical “map” changed dynamically, specifically to exaggerate further the representation of the “anticipated” stimulus B. If B occurred, it was represented over a larger cortical area by more strongly excited, more coordinated, and more selectively responding neurons. This biasing peaked at the expected time of B onset with respect to A onset. No dynamic biasing of responses was recorded for any sound presented in a nontarget pair. Responses to nontarget frequencies flanking the representation of B were reduced in area and in response strength only after the presentation of A at the expected time of B onset. This study shows that cortical areas are not representationally static but, to the contrary, can be biased moment by moment in time as a function of behavioral context.
Keywords: cortical representation, perceptual training, plasticity, primary auditory cortex
Successive-signal biasing (“prediction”), which is manifested in many behavioral studies in psychoacoustics and linguistics, very significantly contributes to the reception and production of rapidly successive inputs or actions (1–5). This study was designed to begin to reveal fundamental auditory system processes that could account for that biasing. Our primary initial goal was to apply a strategy in training by which an adult rat would be listening for the occurrence of specific stimuli in the context of (cued by the presentation of) other specific stimuli. In this initial study, we trained adult rats to respond to mark their recognition of the occurrence of a specific two-sound sequence, with the onsets of those sounds separated by 300 ms. We reasoned that in the context of this training, the rat might be biased for (“listen for” or “expect”) the first sound stimulus, A. If and only if it occurred, cortical networks would hypothetically be biased to favor the reception of the second component of the target-pair stimulus, B.
These studies show that training resulted in a modest static expansion of the representation of both sound elements of the target pair and, if and only if the first sound element of the target sound pair was delivered, in significant response biasing selective for the second sound stimulus in the target sequence.
Results
Behavioral Training.
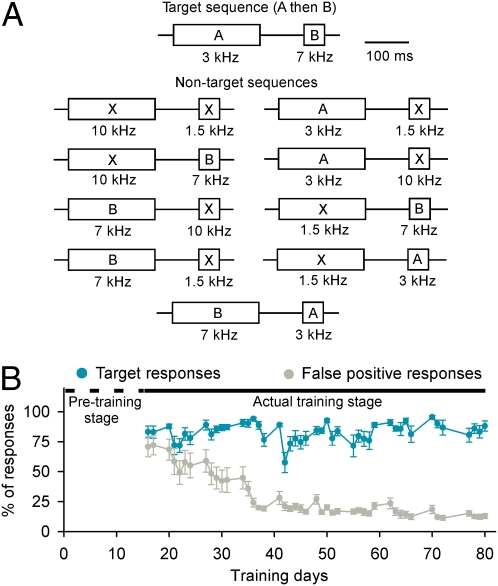
Rats in an experimental group (n = 6) were trained to identify a two-element auditory stimulus target presented with nine nontarget pairs (Fig. 1A) to receive food rewards. All initial stimuli in each pair were tones 200 ms in duration presented at 1.5, 3, 7, or 10 kHz. The initial stimulus in a target stimulus pair was 3 kHz (referred to as A). The second stimulus in each pair could again be 1.5, 3, 7, or 10 kHz. Second stimuli were 50 ms in duration and were presented 300 ms after the onset of the initial stimulus. The second stimulus in the target pair was 7 kHz (referred to as B). All 10 stimulus pairs (nine nontargets and one target) were presented randomly (i.e., the target pair was presented in 10% of trials; Materials and Methods).
Fig. 1.
Behavioral performance. (A) Schematic for tone sequences applied in training. The stimulus sequence with spectral separation of 3 and 7 kHz (i.e., A then B) was set as a target, whereas all others were set as nontargets. (B) Average target and nontarget responses on each training day. Rats (n = 6) were initially pretrained (dashed horizontal line) to make a nose-poke response after presentation of a target only (i.e., 100% target). During the actual training stage (solid horizontal line), rats were conditioned to make a discriminative response to a target from a set of distractors, with 10% of the target presentation rate. Error bars represent mean ± SEM.
In early days of training (Fig. 1B), rats responded on almost all trials, resulting in a high response rate to both target and nontarget stimuli. As training progressed, the nontarget rate for each training day progressively decreased, whereas the target response rate remained at a high level, indicating that rats were learning to identify the target pair. All rats mastered the behavior within 7–8 weeks of training. By that point, the daily nontarget (false-positive) response rate was consistently below 20% for all nontarget stimuli, whereas target (hit) response rates were consistently higher than 80%.
It might be noted that false-positive rates significantly differed for different nontarget pairs. In general, rates were lower (well below 10%) when initial stimuli were 1.5 or 10 kHz. Not surprisingly, more false-positive responses were recorded when the initial stimulus was the correct 3 kHz (ANOVA, P < 0.05–0.00001). For this condition, false-positive rates were closer to (but still below) 20%.
Cortical Representation of Sound Frequency.
Immediately after training cessation, neural responses in A1 of trained rats were documented in detail using conventional extracellular unit recording and characteristic frequency (CF) mapping procedures (Materials and Methods). At all the same recording sites, additional datasets and maps were derived in each animal using a two-stimulus paradigm in which a probe tone located in the normal position of the second stimulus of the target pair was preceded by an initial target (i.e., 3 kHz) or nontarget (1.5, 7, or 10 kHz) stimulus element. Cortical CF (tonotopic) maps and neural responses recorded using these stimulus paradigms were then compared with those derived in the identical manners in naive age-matched controls (n = 8).
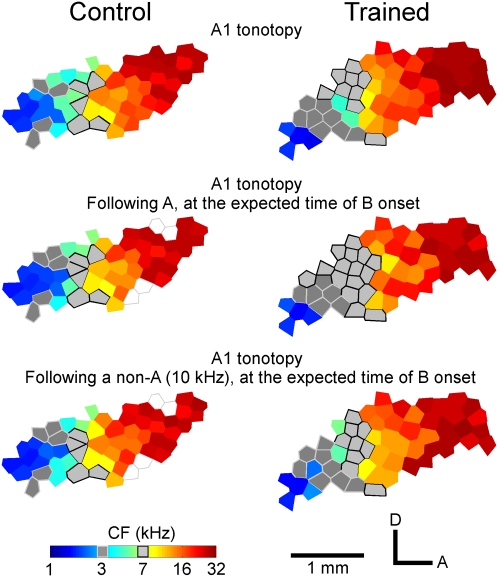
Consistent with earlier studies (6, 7), the frequency representation of A1 determined using a conventional mapping procedure was complete and orderly in control rats, with isofrequency representational bands oriented approximately orthogonal to an orderly sound frequency representational (tonotopic) gradient (Fig. 2 Top Left). A1 maps determined with two-stimulus paradigms were similar to those generated using a conventional mapping procedure (Fig. 2 Middle or Bottom Left vs. Top Left and Fig. S1).
Fig. 2.
Representative auditory cortical CF maps. The color of each polygon indicates the CF for neurons recorded at that site (Bottom Left, scale). Dark or light-gray polygons indicate recording sites with CF values of 3 or 7 kHz ± 0.25 octaves. Unfilled polygons indicate that CFs were not determined at these sites. A, anterior; D, dorsal.
In experimental rats, however, the zones of representation of both 3 and 7 kHz were differentially enlarged vs. control rats (Fig. 2 Top Right vs. Top Left). There were no significant differences in the representations of other sound frequency domains. When a map of sound frequency was defined in experimental rats for the moment after a non-A stimulus (e.g., after a 10-kHz tone in the examples illustrated in Fig. 2 Bottom Right at the time of the expected occurrence of the second sound of a stimulus pair), that CF map was similar to the map derived from recording at the same sample sites in A1 in the conventional manner (Fig. 2 Bottom Right vs. Top Right). Put another way, the presentation of this (or other in other derived maps) nontarget initial sound element(s) resulted in no detectable biasing in the cortex at the time of the expected onset of any second pair element. By contrast, for experimental but not control rats (Fig. 2 Middle Right vs. Middle Left and Fig. 3A, arrow), the area of representation of the second element (B, 7 kHz) of the target stimulus A (3 kHz) and then B (7 kHz) was substantially further enlarged in A1 at the moment that B would be expected to occur if it was preceded by A (3 kHz).
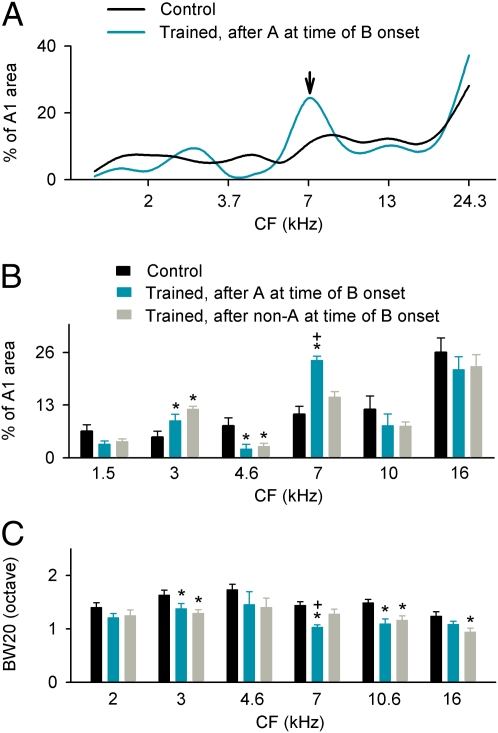
Fig. 3.
Cortical frequency representation and response specificity. (A) Percent cortical areas representing different sound frequencies determined under various stimulus conditions. Here, percent A1 area representing each particular frequency (a total of 15 frequencies from 1–30 kHz at a 0.3-octave interval) was defined as the total percentage of sites with CFs in a 0.5-octave range centered at that frequency. The arrow shows a distribution peak for representation of 7 kHz for trained rats. (B) Percent cortical areas tuned to different frequencies. Error bars represent mean ± SEM. *P < 0.05–0.001 compared with controls; +P < 0.01 compared with values of trained rats determined after non-A at time of B onset. (C) Comparisons of BW20 at different CF ranges. *P < 0.05–0.001 compared with controls; +P < 0.01 compared with values of trained rats determined after non-A at time of B onset.
These basic effects are illustrated in an analysis by frequency band in Fig. 3B. The representations for experimental rats at frequency bands centered at 3 and 7 kHz were significantly enlarged relative to controls (Fig. 3B, cyan or gray vs. black bars; ANOVA, both P < 0.002). That overrepresentation was greater (repeated-measures ANOVA, P < 0.01) at the moment when the second target element (7 kHz) was (i) expected and (ii) preceded by the first target-pair element (3 kHz) [Fig. 3 A (arrow) and B (cyan bar at 7 kHz)].
These results show that training resulted in a modest, enduring, static, areal overrepresentation of the two frequencies presented in the target sequence in training. Most importantly, the overrepresentation of the second target element sound (7 kHz) was still further selectively amplified at its expected time of occurrence if and only if it was preceded by the first pair element (3 kHz). In striking contrast, all control maps and all maps derived following the presentation of nontarget tones in the A position were similar to normal maps.
We calculated tuning curve bandwidths 20 dB above threshold (BW20s) to define the tuning specificity of receptive fields in these different control and experimental groups. Again, bandwidths were defined in the usual way and for that moment after presentation of an initial target- or nontarget-pair element that corresponded with the time of expected occurrence of a second sound-pair element. No significant differences in BW20 were recorded for control rats measured using a conventional mapping procedure as compared with a time epoch following the delivery of 1.5, 3, 7, or 10 kHz (ANOVA, P > 0.3). By contrast, BW20s determined under all conditions were significantly narrower in trained animals and significantly smaller still in the expected time of occurrence of the second element of a target pair (B) if and only if it was preceded by the first target stimulus element (A) (repeated-measures ANOVA, P < 0.001).
BW20s were further analyzed as a function of cortical CF categories to determine better where these effects were most strongly expressed (Fig. 3C). We found that BW20s defined for static maps in experimental rats were consistently smaller than in control rats for CF bands centered at 3, 7, 10.6, or 16 kHz (ANOVA, P < 0.03–0.0001). Effects at other CF bands (centered at 2 or 4.6 kHz) were not consistently significant (ANOVA, both P > 0.3). Again, the narrowing of BW20s for A1 sites with CFs centered at 7 kHz was greater if the stimulus was preceded by the first target-pair element (3 kHz) (Fig. 3C, cyan bar at 7 kHz; repeated-measures ANOVA, P < 0.01).
A1 Neuronal Responses.
A1 response magnitudes were also substantially biased as a function of stimulus context in trained rats. In general, responses at many recording sites were significantly greater for neurons with CFs near 7 kHz if and only if its occurrence was preceded, in an appropriate time relationship, by a (target) 3-kHz stimulus element. By contrast, responses did not significantly differ from those in controls when they were preceded by any non-A stimulus (i.e., by 1.5-, 7-, or 10-kHz tones).
By contrast, responses at other cortical sites were likely to be significantly reduced in amplitude if their best frequencies flanked 7 kHz. Again, responses recorded under other stimulus conditions were not significantly differ from those recorded from controls. For cortical sites with CFs that were still further removed from the target stimulus element B (e.g., smaller than 2 kHz or larger than 16 kHz), no responses at any recording sites were affected by the presentation of any preceding stimulus.
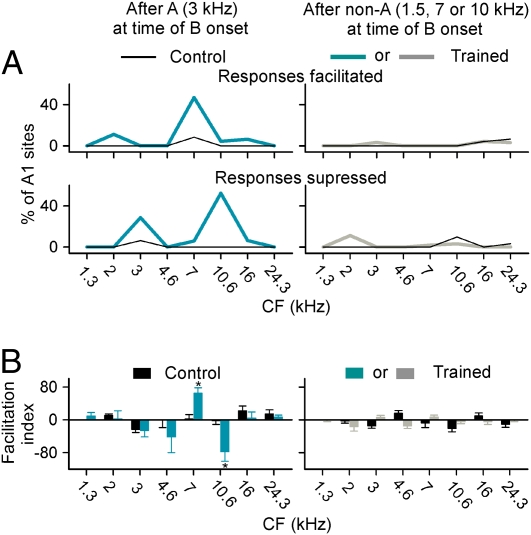
We calculated the percentage of cortical sites that exhibited significantly facilitated or suppressed responses (t test, P < 0.05) to CF tones when they were preceded by “maskers” of different frequencies by binning their CF values into eight 0.6-octave-wide categories. As shown in Fig. 4A, responses at more than 45% of cortical sites with CFs centered at 7 kHz were significantly facilitated in the time window of the expected occurrence of target stimulus B (7 kHz) if and only if it was preceded by target stimulus A (3 kHz). Responses at about 30% of sites for which CFs were centered at 3 kHz and at about 50% of sites with CFs centered near 10.6 kHz (frequency bands flanking the target stimulus band) were significantly reduced at this time epoch for the expected occurrence of target stimulus B, again, if and only if it was preceded by the presentation of target stimulus element A.
Fig. 4.
Tone sequence sensitivity of cortical neurons. (A) Percent recording sites with facilitated (Upper) or suppressed (Lower) responses to CF tone when it was preceded by a masker tone of 3 kHz or 1.5, 7, and 10 kHz for different CF categories. (B) Average facilitation indices of cortical responses to CF tone under different stimulus conditions. Error bars represent mean ± SEM. *P < 0.025.
For sites with CFs centered at all other frequency bands, responses to CF tones under all four documented two-stimulus paradigms (i.e., with initial stimuli presented at the target frequency of 3 kHz and at the three nontarget frequencies of 1.5, 7, and 10 kHz) did not differ from those recorded using the CF tone alone. Similarly, with rare exceptions, response magnitudes were stable when the same repeated measures were derived using the same paradigms in control rats.
To quantify the changes in cortical responses of trained rats under different stimulus conditions further, we calculated the facilitation index for responses to CF tones when they were preceded by a 1.5-, 3-, 7-, or 10-kHz stimulus for every cortical site (Fig. 4B). The facilitation index is 100 times the logarithm base 2 of the ratio of number of responses to a CF tone under a two-stimulus paradigm and number of responses to a CF tone only (5). This method of analysis again confirmed that the average index of cortical sites with CFs centered at 7 kHz was positive and significantly larger than in control rats in the expected time of occurrence of the 7-kHz target stimulus if and only if it was preceded by the first target element (3 kHz) (Fig. 4B Left; t test, P = 0.0092). Although the average index of cortical sites with CFs centered at 4.6 or 10.6 kHz was also larger than in rats under the same stimulus condition (t test, P = 0.31 and 0.024 for CF categories centered at 4.6 and 10.6 kHz, respectively; Fig. 4B Left), the negative values of the index in these bins manifest a suppression of the responses in this zone specifically at the moment of the expected occurrence of stimulus element B, given A. Once again, for sites with CFs centered at other frequency bands, indices were not significantly different from those of naive control rats under the same stimulus condition (Fig. 4B Left; t test, all P > 0.25). Again, the indices of cortical sites with various CFs were all similar to those recorded in control rats when they were defined after a preceding sound stimulus of 1.5, 7, or 10 kHz (Fig. 4B Right; t test, all P > 0.05).
Temporal Selectivity of Second Stimulus Biasing.
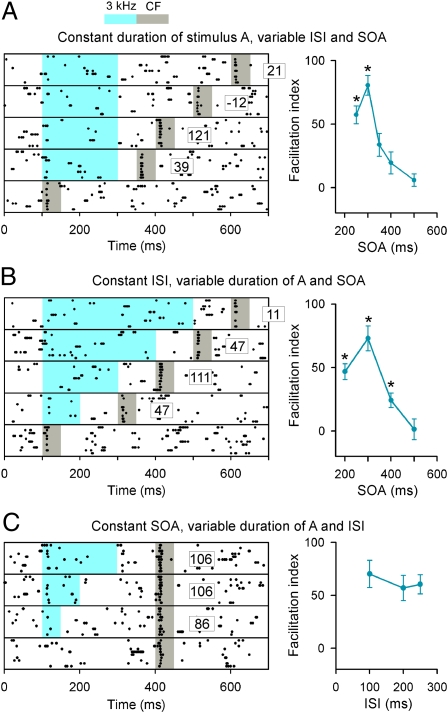
As noted earlier, a substantial number of cortical sites had facilitated responses when their CF stimulation was preceded by 3 kHz. In this neuronal population, we conducted a simple study designed to reveal the temporal specificity of the observed biasing effects. We began by systematically varying the stimulus onset asynchrony (SOA) following a constant duration stimulus A (3 kHz) (Fig. 5A Left). The biasing in response was strongest at an SOA of 300 ms, which specifically corresponded to the “expected” time of occurrence of the second target stimulus element (7 kHz). Note that the strength of biasing rapidly declined at longer SOAs. Pooled data analysis (Fig. 5A Right) showed that the response facilitation induced by the prior tone depended nonmonotonically on the SOA, with the strongest effect occurring at an SOA of 300 ms (repeated-measures ANOVA, P < 0.0001).
Fig. 5.
Temporal characteristics of response facilitation. (A) (Left) Raster plots of cortical responses to CF tone only or when it was preceded by a masker tone of 3 kHz at a different SOA by systematically varying the ISI. The facilitation index under each stimulus condition was shown aside. (Right) Plot of average facilitation indices against the SOA (17 recording sites). Error bars represent mean ± SEM. *Repeated-measures ANOVA, P < 0.05 compared with a value determined at a SOA of 500 ms. (B) (Left) Raster plots of cortical responses to CF tone using a two-tone stimulus at a different SOA by systematically varying the duration of the masker tone. (Right) Plot of average facilitation indices against the SOA (12 recording sites). *, repeated-measures ANOVA, P < 0.05 compared with a value determined at SOA of 500 ms. (C) (Left) Raster plots of cortical responses to CF tone using a two-tone stimulus with masker tones of different durations. (Right) Plot of average facilitation indices against the ISI (10 recording sites).
Because the interstimulus interval (ISI) separating our two target tones was also systematically altered with the SOA in this first series, we next systematically varied the SOA following 3-kHz stimuli of different variations (Fig. 5B Left). The facilitation function was similar, indicating that it again changed as a systematic function of SOA (repeated-measures ANOVA, P < 0.001) independent of the ISI (Fig. 5B Right).
To test further the conclusion that it is the absolute time between stimulus onsets that defines the point of maximum biasing, we next varied the ISI by systematically altering the duration of the 3-kHz stimulus while maintaining a constant SOA. Here, given the constant SOA, response facilitation did not differ as ISIs between stimuli and the durations of A were altered (Fig. 5C; ANOVA, P = 0.7).
These data show that response facilitation was maximal at the absolute time of expected occurrence of stimulus B, given target stimulus A, and independent, within these experimental limits, of the duration or the time of offset of stimulus A.
In addition, we evaluated the temporal coordination of cortical responses for those cortical sites that had facilitated responses when their CF stimulation was preceded by 3 kHz by comparing response durations of peristimulus time histograms (PSTHs) recorded under each stimulus condition. When CF stimuli were preceded by the first target element (3 kHz), the durations of PSTHs were significantly shorter than when preceded by nontarget elements (6.9 ± 0.3 ms vs. 8.9 ± 0.3 ms, measured at 50% below the peak of PSTHs; t test, P < 0.0005), demonstrating an increased response coordination to the CF stimulus of a B-representing neuron if and only if it was preceded by the target stimulus element A.
Discussion
These studies show that when an animal is trained to identify the occurrence of a specific two-element sound sequence in a time frame in which strong syntactic biasing is known to occur in behavioral studies [e.g., with those successive sounds presented at the rate of natural animal vocalization or human speech sounds (phrases or syllables) at roughly three events per second], the primary auditory cortex was significantly and about equally positively biased in its representation of either of the two sounds. That static bias in the magnitude of these distributed responses might be interpreted as favoring the occurrence of either one of the two sounds of a target (rewarded) sound sequence. In a special class of control, we passively exposed other rats to the identical sound repertoire. No significant plastic changes in static or sequenced sound stimulus representations were recorded in these animals (Fig. S2).
In these respects, the static plasticity recorded for both tonal stimuli in our target stimulus pair was like that observed for training with simple acoustic stimuli in general. In a series of earlier studies, such training has resulted in an expansion of the representations of the specific target tonal stimuli in an operant or classic conditioning task (7–13). Similarly, when animals have been conditioned by the application of two or more rewarded tonal stimuli, narrowing of receptive field tuning has been the result (14). On the basis of these earlier studies, the sharpening of tuning recorded for the elements of this rewarded two-sound sequence in static A1 maps would be expected.
At the same time, a second specific syntactic effect for the occurrence of the second sound of the target sequence was recorded. Given the occurrence of the first event in the target sequence, biasing that facilitated responses to the second grew in the cortex to reach a peak at the approximate expected time of occurrence of the onset of the second target sequence event. If stimulus A occurred, neurons were more strongly excited by stimulus B, over a larger neuronal cell assembly, with a more coordinated distributed response and with greater specificity. In this context only, cortical networks greatly favored the representation of the second component of the rewarded stimulus pair, although they simultaneously disfavored the responses to representationally confusable sounds.
What is the basis of this biasing? What is its source? How is it controlled? We developed this simple model to begin to answer these questions. None are directly answered in this initial study. Innumerable studies have argued for the involvement of the frontal cortex in sequence learning and in prediction and syntax. We also know that A1 lesions eliminate a mammal's capacity for sequence learning (15–18). In ongoing studies, we have recorded unit and local field potential responses in direct and indirect frontal cortical targets of A1 in awake behaving rats, and the responses there are consistent with their contributing to A1 network modulation for syntactic rate biasing like that described here. These same areas are very effectively activated under anesthesia, as would be required for them to account for or contribute to the effects recorded in this current study. However, additional studies are required to determine whether these activities are necessary or sufficient to control the modulation recorded in A1 itself. Of course, it is also possible that much or most of this biasing arises locally in A1 or is contributed to by subcortical or other auditory cortical system effects. Studies that are now underway are designed to address these uncertainties.
Several earlier studies have revealed some aspects of the training-driven changes following conditioning with the sound sequences described here. Nakahara et al. (19) exposed infant rats to sound sequences within the critical period and showed that this exposure resulted in enduring sequence-specific activation of neurons recorded all across a broad cortical area representing sequence frequencies. Kilgard and Merzenich (5) paired sound sequences with electrical stimulation of the nucleus basalis in adult rats and showed that this pairing resulted in emergent sequence-specific responses that amplified the responses of later stimulus components of conditioned stimulus sequences. Yin and colleagues (20) operantly conditioned macaque monkeys to respond to sequences and then showed, in behaving animals, that many A1 and rostral area neurons in trained animals had strengthened or weakened responses to elements of trained sequences. Brosch and Schreiner (2, 3) and Brosch and Scheich (1) documented sequence-specific biases as a feature of neuronal responses in normal animals, although they could not determine if they represented some systematic aspect of A1 signal processing and/or were a result of learning-driven plasticity stemming from the earlier undocumented listening history of their studied animals.
Human studies have documented complex receptive biasing in behavioral and imaging studies in our great sensory/perceptual systems (vision/reading, audition/aural language, and somesthesia) (21–24). Studies in monkeys and humans have recorded fluctuations in ongoing activity levels that at least partially reflect these sequence-based (“predictive”) facilitatory and suppressive modulatory effects (23, 25). Those studies support the view that cortical areas at all system levels are subject to continuous “top-down” biasing as a function of ongoing prediction. These current studies should further help to reveal neurological phenomenology and mechanisms contributing to these fundamental neurological processes.
Materials and Methods
All experiment procedures used in this study were approved by the Animal Care and Use Committee at the University of California, San Francisco.
Subjects.
Eighteen female Sprague–Dawley rats aged 2 mo were used in the study. These rats were randomly divided into three groups: (i) control rats (n = 8) housed in a normal environment until A1 responses were documented ~3 mo later, (ii) experimental rats (n = 6) trained to identify a target auditory stimulus from a set of distractors as described below for ~3 mo, and (iii) passively exposed rats (n = 4) passively exposed to stimuli identical to those delivered to the experimental rats across the same epoch but with free access to food.
Behavioral Training.
To receive food rewards, rats assigned to the experimental group were trained to identify a two-element auditory stimulus target presented with nine nontarget pairs (Fig. 1A). During training, only one of these tone pairs was presented in each trial. Rats were rewarded for making a go response within a limited time window after the target stimulus was presented. Training was conducted in an acoustically transparent operant training chamber [20 × 20 × 18 cm (length × width × height)] enclosed within a sound-attenuated chamber. Stimuli were calibrated using a Brüel & Kjær microphone and sound level meter as well as a ubiquitous spectrum analyzer. An input and output system (photobeam detector, food dispenser, sound card, and house light; Med Associates) was used to control behavioral training.
As in earlier studies (7, 11, 26), a single trial describes the length of time between the onsets of two successive tone pairs. The intertrial interval was selected at random from a range of 3–9 s. The rats’ behavior was reduced to a “go” or “no-go” response. Rats were in the go state when the photobeam was interrupted (i.e., nose-poke response). All other states were considered no-go. For a given trial, rats could elicit one of five reinforcement states. The first four states were given by the combinations of responses (go or no-go) and stimulus properties (target or nontarget). Go responses within 3 s of a target were scored as a “hit”; a failure to respond within this time window was scored as a “miss.” A go response within 3 s of a nontarget stimulus was scored as a “false-positive” response, and the absence of a response was scored as a “withhold” response. The fifth state, “false alarm,” was defined as a go response that occurred 3 s or more after stimulus presentation. A hit triggered the delivery of a 45-mg food pellet (BioServe). A miss, false-positive response, or false alarm initiated a 5-s “time-out” period; during this period, the house lights were turned off and no stimuli were presented. A withhold did not produce a reward or a time-out.
Rats were initially pretrained to make a nose-poke response after presentation of a target stimulus only (i.e., 100% target; Fig. 1B, dashed horizontal line). During the actual training stage (Fig. 1B, solid horizontal line), rats were conditioned to make discriminative responses to the target stimulus from a set of distractors, with a 10% target presentation rate. At the conclusion of each training day, a target response rate (number of hits/number of target trials) and a nontarget response rate (number of false-positive responses/number of nontarget trials) were calculated. A response rate for each nontarget was also calculated by dividing the number of false-positive responses by the number of nontarget trials.
Electrophysiological Recording Procedure.
Electrophysiological recording of cortical responses was conducted at ~5 mo of age for all rats of each group. Rats were initially anesthetized with an i.p. injection of sodium pentobarbital (50 mg/kg of body weight, followed by 10- to 15-mg/kg supplements as needed). Respiratory rate, heart rate, and corneal and hind-paw withdrawal reflexes were monitored to ensure that a moderately deep anesthetic plane was maintained as uniformly as possible throughout the recording procedure. After the rat reached a surgical plane of anesthesia, a tracheotomy was performed and a cisternal drain was introduced so as to minimize bronchial secretions and brain edema. The skull was secured in a headholder leaving the ears unobstructed. After reflecting the right temporalis muscle, the auditory cortex was exposed and the dura was resected. The cortex was maintained under a thin layer of viscous silicone oil to prevent desiccation. The animal's body temperature was maintained near 37.7 °C with a rectal probe and homeothermic blanket system (Harvard Apparatus). Saline and Ringer's solution were administered periodically throughout the experiment to ensure adequate hydration.
Cortical responses were recorded with parylene-coated tungsten microelectrodes (1–2 MΩ at 1 kHz; FHC) in a shielded double-walled sound chamber. Recording sites were chosen to sample from the auditory cortex evenly while avoiding blood vessels and were marked on a magnified digital image of the cortical surface vasculature. At each recording site, the microelectrode was lowered orthogonally into the cortex to a depth of ~500 μm (layers 4 and 5), where vigorous stimulus-driven responses were recorded. Acoustic stimuli were generated using a TDT System III (Tucker–Davis Technologies) and delivered to the left ear through a calibrated STAX earphone (STAX Ltd.) with a sound tube positioned inside the external auditory meatus. A software package (SigCal, SigGen, and Brainware; Tucker–Davis Technologies) was used to calibrate the earphone, generate acoustic stimuli, monitor cortical response properties online, and store data for offline analysis.
Cortical Mapping and Data Analysis.
Frequency tuning curves were reconstructed by presenting pure tones of 50 frequencies (1–30 kHz, 25-ms duration, 5-ms ramps) at eight sound intensities (0- to 70-dB sound pressure level in 10-dB increments) to the contralateral ear in a random interleaved sequence at a rate of 2 pulses per second. The CF of a cortical site was defined as the frequency at the tip of the V-shaped tuning curve. For flat-peaked tuning curves, the CF was defined as the midpoint of the plateau at threshold. For tuning curves with multiple peaks, the CF was defined as the frequency at the most sensitive tip (i.e., with the lowest threshold). BW20s were measured for all sites.
As previously described (6, 26), the overall boundaries of A1 were functionally determined using nonresponsive sites and responsive sites that did not have a well-defined pure tone-evoked response area. To generate A1 maps, Voronoi tessellation (a Matlab routine; MathWorks) was performed to create tessellated polygons, with electrode penetration sites at their centers. Each polygon was assigned the characteristics (e.g., CF) of the corresponding penetration site. In this way, every point on the surface of the auditory cortex was linked to the characteristics experimentally derived from a sampled cortical site that was closest to this point.
To examine changes in the frequency representation of A1 induced by training, cortical CF maps and frequency response specificity (i.e., BW20) were also documented by using a two-stimulus paradigm in which pure tones of different frequencies and intensities (i.e., probe tones) were preceded by a masker tone (200-ms tone of 1.5, 3, 7, or 10 kHz).
Statistical significance was assessed using ANOVA or a two-tailed t test. Data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank T. Babcock for assistance with animal behavioral training. This work was supported by US National Institutes of Health grants, the Sandler Fund, the Coleman Fund, Fundamental Research Funds for the Central Universities in China, the Program for New Century Excellent Talents in University, the Shanghai Rising-Star Program (Grant 09QH1400900), and the Canadian Institutes of Health Research. R.P. is a recipient of a Long-Term Fellowship from the Human Frontier Science Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009433107/-/DCSupplemental.
References
- 1.Brosch M, Scheich H. Tone-sequence analysis in the auditory cortex of awake macaque monkeys. Exp Brain Res. 2008;184:349–361. doi: 10.1007/s00221-007-1109-7. [DOI] [PubMed] [Google Scholar]
- 2.Brosch M, Schreiner CE. Time course of forward masking tuning curves in cat primary auditory cortex. J Neurophysiol. 1997;77:923–943. doi: 10.1152/jn.1997.77.2.923. [DOI] [PubMed] [Google Scholar]
- 3.Brosch M, Schreiner CE. Sequence sensitivity of neurons in cat primary auditory cortex. Cereb Cortex. 2000;10:1155–1167. doi: 10.1093/cercor/10.12.1155. [DOI] [PubMed] [Google Scholar]
- 4.Brosch M, Schulz A, Scheich H. Processing of sound sequences in macaque auditory cortex: Response enhancement. J Neurophysiol. 1999;82:1542–1559. doi: 10.1152/jn.1999.82.3.1542. [DOI] [PubMed] [Google Scholar]
- 5.Kilgard MP, Merzenich MM. Order-sensitive plasticity in adult primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:3205–3209. doi: 10.1073/pnas.261705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X, Merzenich MM. Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc Natl Acad Sci USA. 2007;104:15935–15940. doi: 10.1073/pnas.0707348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond DM, Weinberger NM. Role of context in the expression of learning-induced plasticity of single neurons in auditory cortex. Behav Neurosci. 1989;103:471–494. doi: 10.1037//0735-7044.103.3.471. [DOI] [PubMed] [Google Scholar]
- 11.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merzenich MM, Recanzone GH, Jenkins WM, Nudo RJ. How the brain functionally rewires itself. In: Arbib M, Robinson JA, editors. Natural and Artificial Parallel Computations. Cambridge, MA: MIT Press; 1990. pp. 177–210. [Google Scholar]
- 15.Cowey A, Weiskrantz L. Auditory sequence discrimination in Macaca mulatta: The role of the superior temporal cortex. Neuropsychologia. 1976;14:1–10. doi: 10.1016/0028-3932(76)90002-6. [DOI] [PubMed] [Google Scholar]
- 16.Heffner HE, Heffner RS. Temporal lobe lesions and perception of species-specific vocalizations by macaques. Science. 1984;226:75–76. doi: 10.1126/science.6474192. [DOI] [PubMed] [Google Scholar]
- 17.Heffner HE, Heffner RS. Effect of restricted cortical lesions on absolute thresholds and aphasia-like deficits in Japanese macaques. Behav Neurosci. 1989;103:158–169. doi: 10.1037//0735-7044.103.1.158. [DOI] [PubMed] [Google Scholar]
- 18.Strominger NL, Oesterreich RE, Neff WD. Sequential auditory and visual discriminations after temporal lobe ablation in monkeys. Physiol Behav. 1980;24:1149–1156. doi: 10.1016/0031-9384(80)90062-1. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara H, Zhang LI, Merzenich MM. Specialization of primary auditory cortex processing by sound exposure in the “critical period”. Proc Natl Acad Sci USA. 2004;101:7170–7174. doi: 10.1073/pnas.0401196101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin P, Mishkin M, Sutter M, Fritz JB. Early stages of melody processing: Stimulus-sequence and task-dependent neuronal activity in monkey auditory cortical fields A1 and R. J Neurophysiol. 2008;100:3009–3029. doi: 10.1152/jn.00828.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson PL, Meltzoff AN, Decety J. Neural circuits involved in imitation and perspective-taking. NeuroImage. 2006;31:429–439. doi: 10.1016/j.neuroimage.2005.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 23.Rossi AF, Pessoa L, Desimone R, Ungerleider LG. The prefrontal cortex and the executive control of attention. Exp Brain Res. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiethoff S, et al. Cerebral processing of emotional prosody—Influence of acoustic parameters and arousal. NeuroImage. 2008;39:885–893. doi: 10.1016/j.neuroimage.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 25.Alho K. Selective attention in auditory processing as reflected by event-related brain potentials. Psychophysiology. 1992;29:247–263. doi: 10.1111/j.1469-8986.1992.tb01695.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou X, Merzenich MM. Developmentally degraded cortical temporal processing restored by training. Nat Neurosci. 2009;12:26–28. doi: 10.1038/nn.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.