Abstract
Neural stem cells have a broad differentiation repertoire during embryonic development and can be reprogrammed to pluripotency comparatively easily. We report that adult neural stem cells can be reprogrammed at very high efficiency to monocytes, a differentiated fate of an unrelated somatic lineage, by ectopic expression of the Ets transcription factor PU.1. The reprogrammed cells display a marker profile and functional characteristics of monocytes and integrate into tissues after transplantation. The failure to reprogram lineage-committed neural cells to monocytes with PU.1 suggests that neural stem cells are uniquely amenable to reprogramming.
Keywords: PU.1, Ets transcription factor, transdifferentiation, microglia
Nuclear transfer, cell fusion, and reprogramming with defined factors have demonstrated that the differentiated state is not fixed, but is actively maintained and reversible (1 –4). Somatic cells can be directly reprogrammed to other differentiated fates (5, 6), as first demonstrated by the myogenic potential of ectopically expressed MyoD (7). Understanding the stability of the differentiated state and the possibility of altering this state is interesting both from a basic biological perspective and for its potential to generate new research tools and potential cells for therapy.
Neural stem cells have proven comparatively easy to reprogram to pluripotency, with fewer genes needed for full reprogramming (8 –10). This has been attributed to the endogenous expression of some of the genes required for reprogramming; however, it is also possible that neural stem cells are intrinsically more readily reprogrammable than other cells. During embryonic development, the neuroectoderm gives rise not only to neurons and glial cells, but also, via the neural crest, to various nonneural cell types, including mesenchymal stem cells, melanocytes, and cartilage and bone cells (11, 12). The degree of cellular commitment appears to be of pivotal importance for reprogramming efficiency (13), and the wide differentiation repertoire of neural stem cells might underlie their comparatively easy reprogramming.
To directly assess the reprogramming potential of adult neural stem cells, we asked whether they can be reprogrammed to monocytes, a differentiated fate not normally derived from the neuroectoderm. Their high motility and chemotactic attraction to sites of pathology make monocytes an interesting vehicle for therapeutic interventions in the central nervous system (CNS). We have an extensive understanding of the instructive mechanisms in hematopoietic lineage specification; one of the best-characterized genes in this respect is the Ets transcription factor PU.1. PU.1 is instructive for monocyte differentiation in the hematopoietic lineage (14), and ectopic expression of PU.1 in lymphocytes induces transdifferentiation to the myeloid lineage (15). However, PU.1 is insufficient for reprogramming of fibroblasts, more distantly related mesodermal cells, unless coexpressed with other transcriptional regulators (16).
Here we report that the ectopic expression of PU.1 in primary neural stem cells, but not in committed neural cells, results in efficient reprogramming to cells with a marker profile, morphology, and chemotactic properties characteristic of monocytes. This indicates that neural stem cells are uniquely amenable to reprogramming.
Results
Ectopic Expression of PU.1 Efficiently Reprograms Neural Stem Cells to Monocytes.
To directly assess the reprogramming potential of adult neural stem cells to monocytes, we transduced primary neural stem cells from the adult mouse forebrain with a lentiviral control vector containing only GFP (lenti-GFP; Fig. 1A) or a vector bicistronically expressing HA-tagged PU.1 and GFP (lenti-PU.1-GFP; Fig. 1B). The neural stem cells were cultured as neurospheres (17) and were dissociated 6 h before transduction. The cells were maintained under neurosphere conditions for 10 h after transduction and then plated in adherent tissue culture dishes, thereby inducing differentiation. The monocyte marker Iba1 was not detected in cells transduced with the control GFP virus (Fig. 1C). In contrast, cells transduced with PU.1-HA-GFP virus expressed the HA-tagged PU.1, and many cells were positive for the monocyte marker Iba1 (Fig. 1D) and lost expression of the neural markers GFAP and nestin.
Fig. 1.
Ectopic expression of PU.1 in neural stem cells induces expression of the monocyte marker Iba1. (A–D) Adult neural stem cells were transduced with lentivurs expressing either GFP [lenti-GFP (A and C)] or GFP and HA-tagged PU.1 [lenti-PU.1-GFP (B and D)]. The monocyte marker Iba1 is detected in cells ectopically expressing PU.1 (D), but not in cells transduced with the control vector (C).
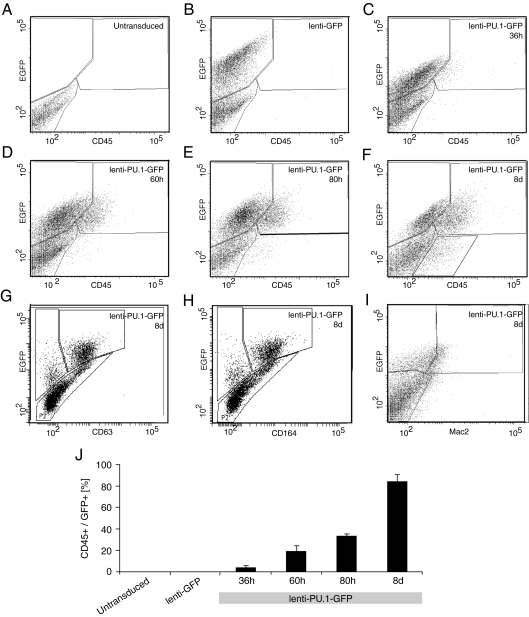
We assessed the dynamics of monocyte marker expression by flow cytometry (Fig. 2 A–J) after transduction with lenti-GFP (Fig. 2 B and J) or lenti-PU.1-GFP (Fig. 2 C–J). We observed a stable proportion (mean ± SEM, 38% ± 1.9%; n = 3) of cells expressing GFP up to 80 h, after which the expression decreased (19% ± 2.0%; n = 3; Fig. 2F) by day 8, due at least in part to silencing of the viral construct (Fig. 3). The hematopoietic marker CD45, which is present in monocytes but not in neural cells, was coexpressed with GFP in 4.1% ± 1.3% (n = 3) of the lenti-PU.1-GFP–transduced neural stem cell–derived cells at 36 h after transduction (Fig. 2C), and increased over time (Fig. 2 D–F) to 84% ± 6.0% (n = 3) of GFP-expressing cells by day 8 (Fig. 2F). Many lenti-PU.1-GFP–transduced neural stem cell–derived cells also displayed the monocyte markers CD63 (57%; Fig. 2G) and CD164 (36%; Fig. 2H) at 8 days after transduction. A smaller population was positive for the additional monocyte marker Mac2 (27%; Fig. 2I). All of the monocyte markers investigated were detected only in cells transduced with the lenti-PU.1-GFP and were not seen in nontransduced cells (Fig. 2 A and J) or in cells transduced with the control lenti-GFP virus (Fig. 2 B and J). The ectopic expression of PU.1 did not induce withdrawal from the cell cycle of the reprogrammed cells, which continued to incorporate BrdU (Fig. S1). Interestingly, cells with silenced viral expression maintained a monocyte marker profile, indicating that transient expression of PU.1 is sufficient for reprogramming (Fig. 3).
Fig. 2.
Reprogramming of adult neural stem cells to a monocytic phenotype by ectopic expression of PU.1. Flow cytometry analysis of expression of the hematopoietic marker CD45 (A–F, and J) and the monocyte markers CD63 (G), CD164 (H), and Mac2 (I). The data in J represent mean ± SEM.
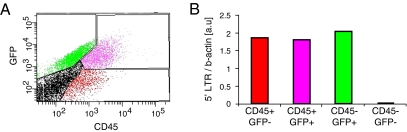
Fig. 3.
Transient expression of PU.1 in neural stem cells is sufficient for reprogramming. (A) Over time, an increasing number of neural stem cell–derived cells transduce with lenti-PU.1-GFP lack GFP expression, but are still expressing CD45 (CD45+/GFP−) at 8 days after transduction (Fig. 1). This population of cells could have arisen in at least two ways; either transduced cells silenced the lentiviral expression of PU.1-HA-GFP but the initial expression of PU.1 was sufficient to activate an endogenous genetic monocyte program, or, alternatively, nontransduced cells were induced to express CD45 by paracrine signals from transduced cells. (B) We analyzed genomic DNA for the presence of integrated viral genetic material. The presence of the viral 5′ LTR sequence was found at similar levels in flow cytometry–isolated CD45+/GFP− cells as in CD45+/GFP+ and CD45−/GFP+ cells. In contrast, 5′ LTR sequences were detected at levels only marginally above background in CD45−/GFP− cells. This indicates that the CD45+/GFP− cells were initially transduced and the new monocytic marker profile in these cells was established and stabilized, even though the viral expression of PU.1 was later silenced.
Reprogramming by PU.1 to Monocytes Is Specific to Neural Stem Cells.
Neural stem cell cultures may be a heterogeneous population with cells at different maturational stages. Nestin is a commonly used marker for neural stem/progenitor cells (18, 19). To specifically investigate whether nestin-expressing neural stem/progenitor cells can give rise to monocytes following PU.1 expression, we transfected cells with a modified vector in which the nestin second intron enhancer controlled PU.1 expression (nestin-PU.1; Fig. S2A). Nestin is expressed in some nonneural cells, but the nestin second intron enhancer drives expression specifically in neural stem/progenitor cells (20, 21). We detected reprogramming of primary neural stem cells with the nestin-PU.1 vector into CD45+ cells, suggesting that PU.1 expression can convert nestin-positive stem/progenitor cells into monocyte-like cells (Fig. S2B).
To investigate whether more differentiated neural cells can be reprogrammed into monocytes, we plated dissociated neurosphere cells on laminin and poly-D-lysine without growth factors, which induced differentiation and loss of neural stem cells, and transduced the cells with lenti-PU.1-GFP virus 24 h later (Fig. S2 C and D). We found no cells with the monocyte marker Iba1 under these conditions (Fig. S2C). Instead, most cells with PU.1 expression were positive for the astrocytic/glial marker GFAP (Fig. S2D). When neurosphere cells were transduced with lenti-PU.1-GFP virus 4 days before induction of differentiation, the vast majority of PU.1 expressing cells differentiated into Iba1-expressing monocytes, and Iba1+ cells did not coexpress GFAP (Fig. S2E). These findings indicate that reprogramming to monocytes by ectopic expression of PU.1 is a unique feature of undifferentiated neural stem/progenitor cells in these cultures.
Functional Characterization of Neural Stem Cell–Derived Monocytes.
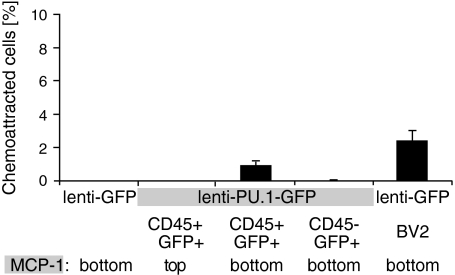
We next asked whether the reprogrammed neural stem cell–derived cells had acquired functional properties of monocytes. A hallmark of monocytes is their directed migration in response to chemotactic cues, such as monocyte chemoattractant protein 1 (MCP-1) (22). We isolated CD45+/GFP+– and CD45−/GFP+–transduced neurosphere-derived cells by flow cytometry and assessed their chemoattraction to MCP-1 in a transwell chemotaxis assay. We observed no chemotactic response of cells transduced with control lenti-GFP virus (0.0% ± 0.0%, n = 3; Fig. 4). In contrast, the sorted CD45+/GFP+ neural stem cell–derived monocytes displayed a robust chemotactic response, similar to that of the monocytic cell line BV2 (0.9% ± 0.2% and 2.4% ± 0.6%, respectively; n = 3 or 4; Fig. 4). As a control, the addition of MCP-1 in the upper chamber together with CD45+/GFP+ cells did not affect migration (0.0% ± 0.0%, n = 3; Fig. 4), indicating that MCP-1 induced a true chemotactic response in these cells.
Fig. 4.
Chemotaxis by neural stem cell–derived monocytes. Neural stem cell–derived CD45+/GFP+ lenti-PU.1-GFP–transduced cells or BV2 monocytes, respectively, placed in the top well migrate toward monocyte chemoattractant protein-1 (MCP-1) in the bottom well in a transwell assay. This is considered a chemotactic response rather than a result of increased motility, because placing MCP-1 in the top well with the CD45+/GFP+ lenti-PU.1-GFP–transduced cells fails to induce migration to the bottom well. Neural stem cells transduced with control lenti-GFP or cells transduced with lenti-PU.1-GFP that do not express CD45 demonstrate no chemotactic response to MCP-1. The data represent mean ± SEM.
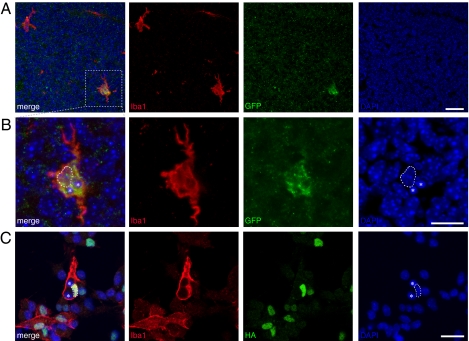
To further test the phenotypic stability and functional properties of the reprogrammed monocyte-like cells, we transplanted flow cytometry–sorted GFP+/CD45+ cells into the lateral ventricles of mouse embryos at gestational day 15. We observed GFP+ cells expressing the monocyte marker Iba1 and displaying a typical morphology of microglia (i.e., the monocytes resident in the brain) in three out of four transplanted animals at 4 days after transplantation (Fig. 5A). The vast majority (81%) of Iba1+/GFP+ cells (96% of the GFP+ cells were Iba1+) migrated away from the ventricular region and were scattered throughout the parenchyma of the mouse brain (Fig. 5A). The cells had migrated 52–250 μm from the ventricle (mean ± SEM, 160 ± 7.6 μm; n = 37 cells). Some of these cells appeared to have phagocytosed cellular debris (Fig. 5B), another characteristic of monocytes. The incorporation and migration of the transplanted cells in the developing mouse brain, together with their morphology and marker profile, support their reprogramming to a monocytic phenotype. We also detected indications of phagocytosis by the PU.1-transduced neural stem cell–derived cells in vitro; neurosphere cells transduced with lenti-PU.1-GFP virus and induced to differentiate into Iba1-expressing monocytes contained what appeared to be engulfed nuclei in culture (Fig. 5C).
Fig. 5.
PU.1 reprogrammed neural stem cell–derived cells display monocyte traits. (A and B) Neural stem cell–derived CD45+/GFP+ lenti-PU.1-GFP–transduced cells were transplanted into the lateral ventricle in E15 mouse embryos. A GFP-labeled cell (shown in the box in A and in higher magnification in B) expresses the monocyte marker Iba1 and displays a morphology indistinguishable from that of a resident microglial cell at 4 d after transplantation. The GFP-labeled cell contains two vacuoles with DAPI+-condensed chromatin (asterisks), indicating phagocytosis of cellular debris. The dashed lined delineates the cell's own nucleus. (C) Iba1-expressing PU.1-transduced neurosphere cells in vitro were found to have phagacytotic capacity and to contain condensed DAPI+ cellular debris (asterisks). The dashed lined delineates the cell's own nucleus. (Scale bar: 25 μm.)
Discussion
We have found that neural stem cells can be reprogrammed with high efficiency to cells with a marker profile, morphology, and chemotactic response of microglia/monocytes by the ectopic expression of a single gene, PU.1. This property appears to be unique to neural stem cells within the neural lineage; cells with an active nestin promoter within neurosphere cultures demonstrated this capacity, whereas neural stem cells exposed to differentiating conditions for 24 h were not reprogrammed to monocytes.
PU.1 has been well studied in the context of its instructive role in monocyte differentiation in the hematopoietic lineage (14). Ectopic expression of PU.1 in lymphocytes induces transdifferentiation to the myeloid lineage (15). This property appears to be specific to the hematopoietic lineage, however; PU.1 is insufficient to reprogram fibroblasts, which are more distantly related mesodermal cells, unless coexpressed with other transcriptional regulators (16). Although neural stem cells are even more distantly related to the hematopoietic lineage from a developmental perspective, they can be reprogrammed to monocytes by the expression of PU.1, indicating that they are more amenable to reprogramming.
In the hematopoietic system, the efficiency with which induced pluripotent stem cells can be generated is inversely correlated with the degree of differentiation (13). The same property appears to apply to neural stem cells, as demonstrated by the high efficiency of PU.1 in reprogramming neural stem cells, in contrast to the complete failure to reprogram committed neural cells.
There are several indications of neural stem cell plasticity, which might explain the relative ease with which they can be reprogrammed both to pluripotent cells (8 –10) and, as described herein, to an unrelated linage fate. For example, the neural crest gives rise to many diverse cell types during development (11, 12), including some indistinguishable from mesoderm-derived cells, and adult neural stem cells retain this potential (23). Furthermore, neural stem cells have been suggested to give rise to cell types of all germ layers after exposure to embryonic-inductive signals (24), although whether cell fusion may contribute to this has not been established (25). Neural stem cells also appear to be a default fate of embryonic stem cells (26). The broad differentiation repertoire of neural stem cells suggests that they may have an open chromatin structure, which may underlie our finding that they are amenable to reprogramming.
Gene therapy holds the hope of providing novel therapies for neurologic diseases. A possible problem when transferring therapeutic genes is the difficulty of achieving broad dissemination within the CNS in, for example, metabolic disorders. Monocytes are easily collected from peripheral blood, and there is little reason to consider generating monocytes from other sources for therapeutic purposes. However, it may be attractive to consider reprogramming of endogenous neural stem cells in situ in the brain into monocytes, which could serve as vehicles for therapeutic genes. Thus, the migratory capacity and chemotaxis of microglia/monocytes toward areas of pathology could help distribute the therapeutic genes within the tissue.
In addition, microglia/monocytes in the CNS exhibit a wealth of properties and differentiation stages, and there is evidence that they influence development and the course of certain neurologic diseases. For example, it was recently shown that transgenic mice with a HOXb-8 gene mutation displayed a behavior similar to obsessive-compulsive disorder, which could be abolished by introducing wild-type monocytes by bone marrow transplantation (27). Accordingly, further studies on manipulating neural stem cells in vivo to generate monocytes of various differentiation stages in a regulated fashion might prove rewarding in the search for novel therapeutic approaches for CNS-associated diseases.
Materials and Methods
Neurosphere Cultures.
Cultures were established as described previously (28). Lateral ventricle wall tissue from adult male C57BL/6 mice was dissected and dissociated using papain (0.2 mg/mL; Worthington). Neurospheres were cultured in low-attachment Petri dishes (Corning) using DMEM/F12 medium supplemented with EGF, bFGF (both 10 ng/mL), and B27. Cells were passaged every fourth day by dissociating neurospheres into single cells with 0.02% EDTA (Sigma-Aldrich).
Viral and Plasmid Vectors.
For lenti-PU.1-HA-GFP, the PU.1 cDNA fused to the heamagglutin tag (HA-tag) was kindly provided by Harinder Singh (University of Chicago, Chicago, IL) and cloned into the lentiviral vector pRRL-SIN-PPT-hPGK-GFP-WPRE (29) containing the human phosphoglycerate kinase promoter (hPGK). The GFP sequence was exchanged for ires2-GFP to allow for bicistronic expression of PU.1-HA and GFP (Fig. 1). The lentiviral vector pRRL-SIN-PPT-hPGK-GFP-WPRE was used as control (lenti-GFP; Fig. 1).
Lentiviral particles were produced as described previously (30). In brief, 293T cells were cotransfected by calcium phosphate precipitation with the required transfer vector plasmid, the pMD.Lg/pRRE.D64VInt packaging plasmid, the pMD2.VSV-G envelope–encoding plasmid, and pRSV-Rev. Vector particles were concentrated by ultracentrifugation, and stocks were titered by endpoint expression titer in 293T cells whenever possible (using anti-GFP and anti-HA antibodies, respectively) and/or quantified for viral particle content by an HIV-1 group-specific antigen p24 immunocapture assay (Perkin-Elmer).
The nestin-PU.1 construct was generated by exchanging the hPGK promoter in lenti-PU.1-HA-GFP with the nestin second intron enhancer and removing the ires2-GFP sequence.
Neurosphere Transduction and Transfection.
Neurospheres, which had undergone between three and six passages, were dissociated with 0.02% EDTA solution (Sigma-Aldrich) and resuspended in cultured medium. Virus was added to the medium 4–6 h later, after which cells were placed in an incubator. After 24 h, the cells were transferred to adherent Petri dishes (Corning) with DMEM/F12 supplemented with B27. For transfection, neurospheres were dissociated as described above and 2 h later transfected with FuGENE (Roche) at a 3:2 ratio following the manufacturer's recommendations.
Immunocytochemistry.
Cultured cells were fixed with 4% formaldehyde in PBS for 10 min, followed by three washes with PBS and blocking in 10% donkey serum in PBS with 0.3% Triton-X 100 for 1h at room temperature. The following primary antibodies were used: Iba1 (Wako), CD45 (BD PharMingen), CD63 (BD PharMingen), CD11b (BD PharMingen), CD164 (BD PharMingen), Mac2 (BD PharMingen), HA (Abcam), and GFAP (Sigma-Aldrich). Primary antibodies were detected using the fluorophore-conjugated secondary antibodies Cy3, Cy5 (Jackson Laboratory), and Alexa Fluor 488 (Molecular Probes). Images were taken using a Zeiss confocal microscope.
BrdU Labeling.
BrdU (10 μg/mL; Sigma-Aldrich) was added to the tissue culture medium, and after 12 h, cells were fixed with 4% formaldehyde in PBS for 10 min. After extensive washing in PBS, the cells were stained with DAPI for 10 min. The DNA was denatured with 2 N HCl for 30 min, followed by treatment with 0.1 M sodium borate buffer (pH 8.5) for 10 min. Immunostaining with primary (BrdU 1:250; Dako) and secondary antibodies were performed as described above.
Genomic Analysis of Viral Integration.
Eight days after transduction with lenti-PU.1-GFP, cell populations were isolated by flow cytometry and genomic DNA was extracted with the Wizard genomic extraction kit (Promega). qPCR analysis using 5′LTR primers (31) was performed for three replicates for each population.
Flow Cytometry.
Neurospheres were dissociated with 0.02% EDTA. Cells were resuspended in L15 media with 20 mM Hepes (Sigma-Aldrich) and B27 (Invitrogen) and incubated with antibodies against CD45, CD11b, CD63, or CD164 (1:100; BD) for 1 h on ice. The cells were washed and resuspended in L15 medium with 20 mM Hepes and B27 and analyzed with a FACSAria flow cytometer (BD). The cells were then sorted in L15 media with 20 mM Hepes and B27 and pelleted at 300 × g for 5 min.
Transendothelial Chemotaxis Assay.
This assay was performed as described previously (32). In brief, cells were placed in Costar Transwell culture inserts (Corning) with an 8-μm pore size. The chemoattractant protein MCP-1 (0.02 ng/μL; Sigma-Aldrich) was added to the bottom chamber. In control experiments, MCP-1 was placed in the top chamber. The assay plates were incubated for 12 h at 37 °C. The cells in each well bottom were counted by fluorescent microscopy. Four 10 × 10 grids (0.1 mm per grid) were counted per well. All samples were tested as duplicates, and each experiment was repeated a minimum of two times.
Cell Transplantation.
Transplantation of GFP+/CD45+ neurosphere-derived cells was performed as described previously (33, 34). In brief, pregnant mice (n = 3) were anesthetized with isoflurane. A midline incision was made to access the embryos, and ~4,000 cells were injected into the lateral ventricle of E14–E15 embryos. Four days later, the embryos were collected and the brains were fixed in 4% formaldehyde (Sigma-Aldrich) at 4 °C overnight, cryoprotected in 30% sucrose at 4 °C overnight, and cryosectioned at 20 μm. Immunohistochemistry was performed as described above.
Supplementary Material
Acknowledgments
We thank Marcelo Toro, Christian Göritz, and Malin Eriksson for their valuable advice and help. This study was supported by grants from the Swedish Research Council, the Swedish Cancer Society, the Swedish Agency for Innovation Systems, the Karolinska Institute, Tobias Stiftelsen, Knut och Alice Wallenbergs Stiftelse, and the Foundation for Strategic Research. M.F. was supported by a fellowship from the Swedish Brain Foundation (Hjärnfonden), M.C. and D.M. were supported by fellowships from the Knut and Alice Wallenberg Foundation. F.B.-H. was supported by fellowships from the Canadian Institutes of Health Research and the Christopher and Dana Reeve Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009412107/-/DCSupplemental.
References
- 1.Gurdon JB. Adult frogs derived from the nuclei of single somatic cells. Dev Biol. 1962;4:256–273. doi: 10.1016/0012-1606(62)90043-x. [DOI] [PubMed] [Google Scholar]
- 2.Blau HM, et al. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–712. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986;47:649–656. doi: 10.1016/0092-8674(86)90507-6. [DOI] [PubMed] [Google Scholar]
- 8.Duinsbergen D, Eriksson M, ’t Hoen PA, Frisén J, Mikkers H. Induced pluripotency with endogenous and inducible genes. Exp Cell Res. 2008;314:3255–3263. doi: 10.1016/j.yexcr.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Kim JB, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 10.Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Takashima Y, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–1388. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 13.Eminli S, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 15.Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Feng R, et al. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds BA, Rietze RL. Neural stem cells and neurospheres: Reevaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 18.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 19.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman L, et al. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron. 1994;12:11–24. doi: 10.1016/0896-6273(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 21.Carlén M, Meletis K, Barnabé-Heider F, Frisén J. Genetic visualization of neurogenesis. Exp Cell Res. 2006;312:2851–2859. doi: 10.1016/j.yexcr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein 1 (MCP-1): An overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sailer MH, et al. BMP2 and FGF2 cooperate to induce neural-crest–like fates from fetal and adult CNS stem cells. J Cell Sci. 2005;118:5849–5860. doi: 10.1242/jcs.02708. [DOI] [PubMed] [Google Scholar]
- 24.Clarke D, Frisén J. Differentiation potential of adult stem cells. Curr Opin Genet Dev. 2001;11:575–580. doi: 10.1016/s0959-437x(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 25.Frisén J. Stem cell plasticity? Neuron. 2002;35:415–418. doi: 10.1016/s0896-6273(02)00798-5. [DOI] [PubMed] [Google Scholar]
- 26.Tropepe V, et al. Direct neural fate specification from embryonic stem cells: A primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 27.Chen S-K, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Consiglio A, et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci USA. 2004;101:14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson CB, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 30.Bonci DC, et al. “Advanced” generation lentiviruses as efficient vectors for cardiomyocyte gene transduction in vitro and in vivo. Gene Ther. 2003;10:630–636. doi: 10.1038/sj.gt.3301936. [DOI] [PubMed] [Google Scholar]
- 31.Fleury SS, et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–2382. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- 32.Carr MWRS, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtsuka TI, et al. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBOJ. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnabé-Heider FW, et al. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48:253–265. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.