Abstract
Treatment with monoclonal antibody (mAbs) is a viable therapeutic option in cancer. Recently, these mAbs such as cetuximab, herceptin, etc., have been used as targeting agents to selectively deliver chemotherapeutics to cancerous cells. However, mechanisms of nanoparticles-mAbs interactions with the target cells and its effect on intracellular trafficking and mechanism are currently unknown. In this paper, we demonstrate that the distinct patterning and dynamics of anti-EGFR (epidermal growth factor receptor) antibody cetuximab (C225)- induced EGFR internalization in pancreatic cancer cells with variable receptor expression is altered upon nanoconjugation. Nanoconjugation uniformly enhanced C225-induced EGFR endocytosis in PANC-1, AsPC-1, and MiaPaca-2 cells, influenced its compartmentalization and regulated the involvement of dynamin-2 in the endocytic processes. Receptor endocytosis and its intracellular trafficking were monitored by confocal microscopy and transmission electron microscopy. The role of dynamin-2 in EGFR endocytosis was determined after overexpressing either wild-type dynamin-2 or mutant dynamin-2 in pancreatic cancer cells followed by tracking the receptor-antibody complex internalization by confocal microscopy. Significantly, these findings demonstrate that the nanoconjugation cannot be construed as an innocuous reaction involved in attaching the targeting agent to the nanoparticle, instead it may distinctly alter the cellular processes at the molecular level, at least antibody induced receptor endocytosis. This information is critical for successful design of a nanoparticle-based targeted drug delivery system for future clinical translation.
Keywords: EGFR, nanoparticle, dynamin, pancreatic cancer
Nanotechnology provides opportunities for biomedical applications that may improve human health care in the future (1–13). Engineered nanoparticles are evolving as promising candidates for various biological applications due to their tunable biophysical and biochemical properties (14–16). However, fundamental studies to understand the mechanism of cell-nanomaterial interactions are still lacking. To advance the successful development of biomedical nanotechnology for clinical use, vivid understanding of such mechanisms is essential.
Epidermal growth factor receptor (EGFR) represents a unique target as over expression of EGFR has been implicated in the pathogenesis of many cancers (17, 18). Cetuximab (C225), a monoclonal anti-EGFR antibody, has been approved by the FDA for treatment of colon and head and neck cancers (19). Cetuximab is also in different phases of clinical trials for other cancers (20, 21). However, the mechanism and pattern of C225 induced endocytosis of EGFR in pancreatic cancer cells of variable EGFR expression is not fully understood (20, 21). Furthermore, the effect of nanoconjugation on the mechanism of C225 induced endocytosis remains to be investigated. Here, we demonstrate the mechanism and endocytic pattern of C225 and gold conjugated-C225 induced internalization of EGFR in pancreatic cancer cell lines characterized by differential expression of EGFR and thus varying in metastatic potential. We also investigate how conjugation of cetuximab to gold nanoparticle alters its cellular mode of function in metastatic vs. primary pancreatic cancer cell lines. We demonstrate that nanoconjugation uniformly promotes faster endocytosis of EGFR, influences its compartmentalization, and regulates the involvement of dynamin-2 (dyn-2) in the endocytic processes. These studies provide molecular insight into the mechanism of C225 induced endocytosis of EGFR and help to design the nanoconjugates for efficient targeting.
Results
Conjugation to Gold Nanoparticles Enhances C225 Induced Endocytosis of EGFR.
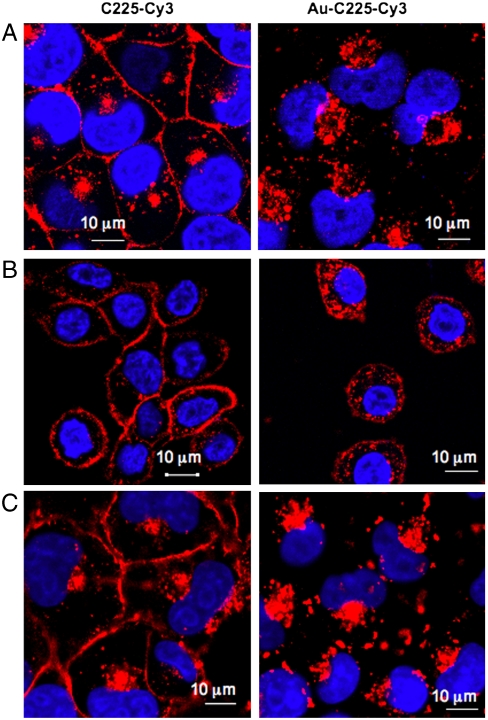
To test if conjugation to gold nanoparticle regulated the C225 induced endocytosis of EGFR in pancreatic cancer cells of variable EGFR expression, we tracked C225-Cy3 and Au-C225-Cy3 induced EGFR endocytosis by confocal microscopy. Previously we demonstrated the differential EGFR expression in PANC-1, MiaPaca2, and AsPC-1 cells (18). When cells were incubated with either C225-Cy3 or Au-C225-Cy3 at 4 °C for 30 min, the majority of the C225 was primarily localized at the plasma membrane, suggesting an energy dependent pathway for endocytosis (Figs. S1, S2, and S3) (5). A distinct difference in the endocytosis pattern of EGFR was observed in C225-Cy3 vs. Au-C225-Cy3 treated cells at 37 °C. In general, nanoconjugation promoted faster endocytosis of EGFR and the pattern of uptake was also quite different among various pancreatic cancer cells with variable EGFR expression. In PANC-1 cells (primary cell line with high EGFR expression), C225-Cy3 was predominantly localized at the membrane at 30 min, whereas Au-C225-Cy3 was predominantly visible in the perinuclear region. However, at 1 h, EGFR (Fig. 1A, right) was completely perinuclear in the Au-C225-Cy3 treatment group, whereas membrane staining could still be observed for C225-Cy3 (Fig. 1A, left). Similar results were obtained in MiaPaca2 cells (primary cell line with low EGFR expression). This data suggests that Au-C225-Cy3 promotes faster endocytosis of EGFR (Fig. 1C, right) as compared to C225-Cy3 (Fig. 1C, left). The pattern of EGFR endocytosis in the AsPC-1 cells (metastatic cell line) was distinct from PANC-1 cells even though they expressed similar levels of EGFR (18). In the case of AsPC-1 cell, Au-C225-Cy3 induced notable endocytosis within 1 h in the form of discrete puncta at the membrane and cytosol (Fig. 1B, right) as compared to PANC-1 and MiaPaca2 cells. In contrast, C225-Cy3 was unable to induce significant EGFR endocytosis even after 1 h of treatment in AsPC-1 cells. The number of puncta formation was also significantly higher for Au-C225-Cy3 comparing to C225-Cy3 (Fig. 1B, left). This increased number of puncta formation suggests enhanced clustering of EGFR induced by nanoconjugation and suggests faster endocytosis by nanoconjugated C225. Furthermore, we demonstrated previously that preincubation of various cancer cells with C225 for 30 min inhibited the uptake of Au-C225, indicating EGFR mediated uptake of Au-C225 by these cells (22). A detailed time course of C225-Cy3 and Au-C225-Cy3 induced EGFR internalization of the cell lines described above is provided in Fig. S1-PANC-1, Fig. S2-ASPC-1, Fig. S3 MiaPaca2. It is also important to note here that the information regarding endocytic sorting of antibody-receptor complex and the mechanism of endocytosis is still lacking (21, 23). We are demonstrating in this paper the mechanism and endocytic sorting of antibody-receptor complex in nanoconjugated and nonconjugated form. It is also important to note here that conjugating increasing amounts of C225 to gold nanoparticle did not change the pattern of endocytosis in both primary and metastatic cells, suggesting a lack of multivalency effect in such endocytic processes.
Fig. 1.
Conjugation with gold nanoparticle accelerates faster endocytosis of EGFR receptors by C225. Fluorescence image representing the binding of C225-Cy3 and Au-C225-Cy3 to EGFR in PANC-1, AsPC-1, and MiaPaca2 human pancreatic cancer cells. The nucleus is stained with DAPI (blue). Cells were treated separately with C225-Cy3 and Au-C225-Cy3 for different time points starting from 5 min–1 h at 37 °C. A significantly higher internalization of EGFR was observed in PANC-1 (1 h), AsPC-1 (1 h) and MiaPaca2 (1 h) cells A–C right, respectively) with Au-C225-Cy3 treatment as compared to C225-Cy3 alone (A–C left, respectively). All other time points are presented in Fig. S1-PANC-1, Fig. S2-AsPC-1, Fig. S3-MiaPaca2).
Taken together, these data suggest that EGFR endocytosis induced by C225 binding to the receptor is distinct in primary (PANC-1 and MiaPaca2) vs. metastatic pancreatic cancer cells (AsPC-1). In addition, conjugation to gold nanoparticle enhanced C225 induced endocytosis of EGFR and alters the endocytic patterning of the receptor primarily to the perinuclear region in the case of primary cell lines whereas in the punctate form throughout the cytosol in the case of metastatic cell line.
Conjugation to Gold Nanoparticles Altered C225 Induced Intracellular Trafficking of EGFR.
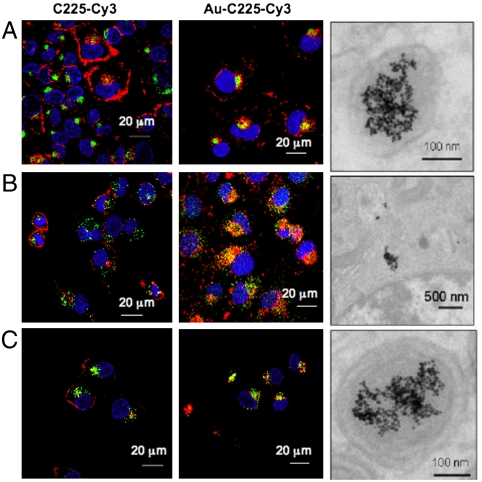
Next we wanted to investigate if nanoconjugation also altered the intracellular trafficking of EGFR. It is well documented that the ligand, EGF, induced internalization of EGFR leads to accumulation in the early endosomes, followed by the Golgi complex. Finally the receptors are either recycled back to the plasma membrane or degraded in the lysosomes (17, 24). However, the intracellular trafficking of C225 induced EGFR internalization (for that matter any antibody induced receptor endocytosis) is still not clear. To address this issue, we performed colocalization experiments to determine C225-Cy3 and Au-C225-Cy3 induced endocytosis in different organelles namely early endosomes (EEA), lysosomes, the Golgi complex, and transferrin, (representing the recycling compartment). In MiaPaca2 cells, significant colocalization of both C225-Cy3 and Au-C225-Cy3 occurred in the early endosomes (Table 1). However, in the case of Au-C225-Cy3, percent colocalization with the Golgi marker was at least twofold higher (24.2 ± 2.1) than the antibody itself (12.5 ± 1.6), with a p value of < 0.05 suggesting faster endocytosis (Table 1, Fig. 2A). Furthermore, colocalization with transferrin was significantly higher (52.4 ± 3.3) in Au-C225-Cy3 treated cells as compared to C225-Cy3 (36.0 ± 2.6) with a p value of < 0.05, suggesting increased accumulation of Au-C225-Cy3 in the recycling compartment, corroborating faster endocytosis of the receptor (Table 1, Fig. 2C). No difference in lysosomal colocalization was observed (Fig. S4). It was also not previously known whether any antibody-internalized receptor was trafficked to the lysosome (21, 23). Here, we demonstrate that a distinct amount of cetuximab induced internalized EGFR is trafficked to the lysosomes. Similar results were obtained in PANC-1 cells, Au-C225-Cy3 promoted significant higher localization (41.5 ± 4.8) to EEA as compared to C225-Cy3 (24.1 ± 3.5) (p < 0.05) (Table 1, Fig. 2B, Figs. S4, S5, S6, and S7). Since, C225 alone did not induce significant EGFR endocytosis in AsPC-1 cells at 1 h; we did not quantify the colocalization in this case. However, Au-C225-Cy3 treated AsPC-1 cells demonstrated notable localization of EGFR to early endosomes, Golgi complex, transferrin, and lysosomes (Table 1, Figs. S4, S5, S6, and S7). Since gold nanoparticles in the Au-C225 conjugate cannot be documented by confocal microscopy, transmission electron microscopy (TEM) was performed. Internalization of gold nanoparticles in PANC-1, AsPC-1, and MiaPaca2 cells in double layered-membrane bound vesicles is demonstrated (Fig. 2 A–C, right, respectively). These data suggest that conjugation of C225 to gold nanoparticles could enhance the internalization of EGFR in both metastatic and primary cancer cells. Colocalization with the transferrin compartment suggests involvement of the receptor mediated endocytic pathway by conjugation of antibody with gold nanoparticles (25, 26). A high degree of compartmentalization of receptor in specific organelles suggests the possibility of surface engineering of nanoparticle for specific intracellular targeting.
Table 1.
Quantification of colocalization in different organelles
| Organelles | Early endosome | Golgi | Transferrin | Lysosome | ||||
| Cell Types | C225 | Au-C225 | C225 | Au-C225 | C225 | Au-C225 | C225 | Au-C225 |
| PANC-1 | 24.1 ± 3.5 | 41.5 ± 4.8* | 23.1 ± 2.6 | 21.6 ± 1.8 | 35.8 ± 0.7 | 36.6 ± 2.6 | 32.1 ± 3.2 | 37.7 ± 3.6 |
| AsPC-1 | ND | 15.2 ± 1.3 | ND | 38.4 ± 3.3 | ND | 46.9 ± 1.4 | ND | 49.9 ± 2.3 |
| MiaPaca2 | 45.5 ± 4.9 | 47.1 ± 2.8 | 12.5 ± 1.6 | 24.2 ± 2.1* | 36.0 ± 2.6 | 52.4 ± 3.3* | 25.0 ± 2.8 | 29.9 ± 1.8 |
ND = Not Done
*p < 0.05
Fig. 2.
C225-Cy3 and Au-C225-Cy3-induced endocytosis of EGFR in different compartments. Figure demonstrates colocalization of C225-Cy3 (left) and Au-C225-Cy3 (right) in different compartments. Cells were incubated with either C225-Cy3 or Au-C225-Cy3 for 1 h followed by colocalization with different reagents. A and C demonstrate colocalization of C225-Cy3 and Au-C225-Cy3 to the Golgi complex and transferrin in the case of MiaPaca2 cells. B demonstrates localization to EEA when PANC-1 cells were treated with C225-Cy3 and Au-C225-Cy-3 for 1 h. Internalization of gold nanoparticles in PANC-1, AsPC-1, and MiaPaca2 cells in double layered-membrane bound vesicles is observed by TEM and demonstrated in A–C, right, respectively.
Conjugation to Gold Nanoparticles Altered the Mechanism of C225 Induced Endocytosis of EGFR.
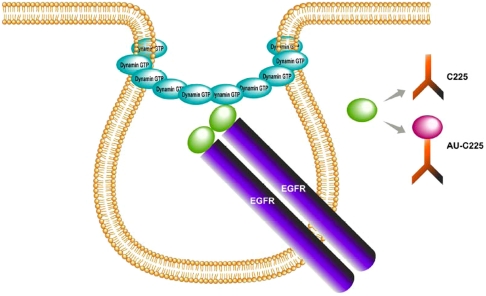
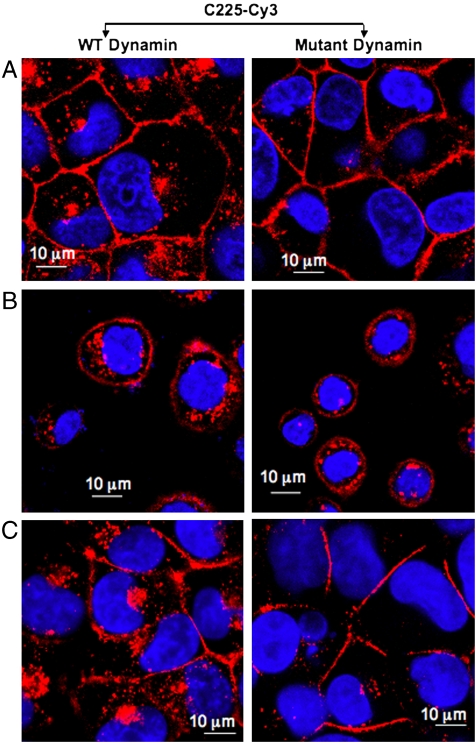
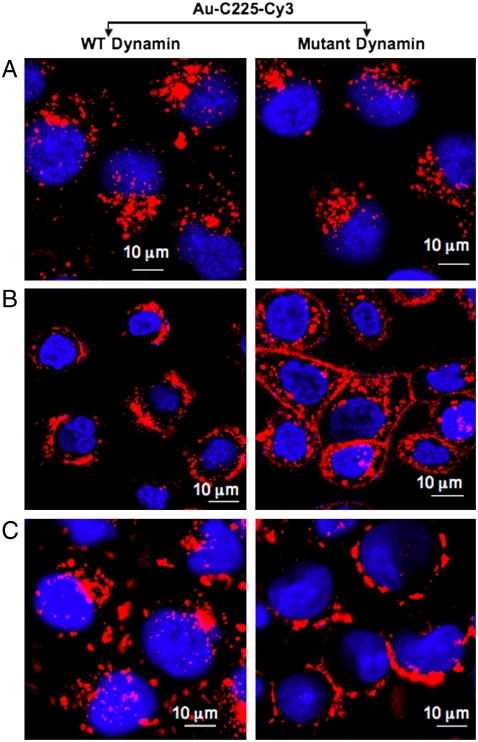
To determine if nanoconjugation modulated the mechanism of C225 induced endocytosis of EGFR, we tested the role of dyn-2 in this process. Dyn-2 is a signal transducing GTPase that has been implicated in EGF-induced endocytosis of EGFR (26, 27). Accumulating evidence suggests the significant involvement of dyn-2 in generation, constriction, membrane ruffling, and fission of endocytotic vesicle stalks and is involved in both clathrin-dependent and independent pathways (Scheme 1) (28). However, the role of dyn-2 in C225 induced endocytosis of EGFR has not been elucidated. To test the involvement of dyn-2 in C225 induced endocytosis of EGFR, wild-type or mutant dyn-2 (K44A lacking GTPase activity) were expressed in PANC-1, AsPC-1, and MiaPaca2 cells respectively, followed by treatment with C225-Cy3 or Au-C225-Cy3. Expression of WT or K44A dyn-2 mutant was confirmed by western blot analysis (Fig. S8). In PANC-1 cells, expression of mutant dyn-2 inhibited C225-Cy3 induced endocytosis of EGFR (evident from the persistent Cy3 florescence at the membrane) as compared to PANC-1 cells expressing WT dyn-2. However, upon nanoconjugation (Au-C225-Cy3), enhanced endocytosis was observed in the same cells despite expression of mutant dyn-2. However, in MiaPaca2 cells expression of mutant dyn-2 inhibited both C225-Cy3 and Au-C225-Cy3 induced endocytosis of EGFR.
Scheme 1.
Role of dynamin in EGFR endocytosis; highlights a possible role of dynamin in the formation of membrane constriction in EGFR endocytosis induced either by C225 or Au-C225.
Since C225-Cy3 induced only a marginal induction of EGFR endocytosis in AsPC-1 cells at 1 h, we extended the incubation time and treated AsPC-1 cells with either C225-Cy3 or Au-C225-Cy3 for 2 h to determine the role of dynamin. Expression of either WT or mutant dyn-2 did not affect C225-induced endocytosis of EGFR, confirming that C225-induced EGFR endocytosis is dyn-2 independent in AsPC-1 cells. However, increased inhibition of EGFR endocytosis was observed when AsPC-1 cells, expressing mutant dyn-2 was treated with Au-C225-Cy3. These results clearly demonstrate the alteration of C225-induced endocytosis from dyn-2 independent to dyn-2 dependent pathway in this cell upon nanoconjugation. Therefore, in AsPC-1 cells characterized by their high metastatic potential, nanoconjugation shifted the mechanism of C225-Cy3 induced EGFR endocytosis from dynamin independent to dynamin dependent pathway. These results clearly demonstrate that nanoconjugate specifically modulates the involvement of dyn-2 in pancreatic cancer cells with high EGFR expression. In PANC-1 cells with high EGFR expression, where C225-Cy3 induced EGFR endocytosis was dyn-2 dependent, switched it to dyn-2 independent pathway upon nanoconjugation. On the otherhand, in AsPC-1 cells with similar level of EGFR expression as PANC-1 cells, where C225-Cy3 induced EGFR endocytosis was dyn-2 independent, switched it to dyn-2 dependent pathways by Au-C225-Cy3. However, in MiaPaca2 cells with low EGFR expression, both C225-Cy3 and Au-C225-Cy3 induced EGFR endocytosis remained dyn-2 dependent. Thus, the regulation of dynamin in EGFR endocytosis upon nanoconjugation may provide valuable insight in to the mechanism of C225 function.
Discussion
Cells use endocytosis as a tool to internalize a variety of molecules (cargo) to communicate with the extracellular environments. These endocytic processes are extremely complex and tightly regulated (29). Cetuximab is approved by the FDA for the treatment of EGFR-positive colorectal cancer. However, the mechanism of communication between cetuximab and EGFR expressing cancer cells has not been elucidated. Furthermore, it has also been unknown whether nanoconjugation could lead to the alternation of the mechanism of endocytosis of a targeting agent. Here, we demonstrate that nanoconjugation plays an important role in regulating the mechanism of C225; it uniformly promotes faster EGFR endocytosis across different pancreatic cancer cell lines of metastatic and primary origin. This finding is of clinical significance since attenuation of membrane presentation of the receptor implies reduced signaling through its corresponding ligand. Furthermore, the pattern of EGFR endocytosis is distinct in metastatic vs. primary cell lines. Whether such a distinct patterning of EGFR in highly aggressive and metastatic cell line is responsible for metastatic potential is not known but this is an important finding that requires further investigation.
It is also important to understand whether nanoconjugation alters the mechanism and hence compartmentalization of antibody induced internalization of EGFR and their subsequent functional consequences. Current studies have speculated the involvement of clathrin- and caveolin-dependent vesicle formation in nanoparticle mediated cellular endocytosis (30). It has been reported that size is one of the key factors that determine the cellular uptake of the nano-composites (5). However, the upstream event of nanoparticle entry to the cellular compartment and the molecular machinery that drives the dynamics of the internalization to cancer cells is not known. Evidence suggests an important role of dyn-2 in many cellular processes such as EGFR and other receptor endocytosis (28, 29, 31). EGF stimulated EGFRs have been found to involve both clathrin-dependent and independent pathways (32). In our study, we have addressed whether dyn-2, implicated in both clathrin mediated endocytosis (CME) and clathrin independent endocytosis (CI), is involved in nanoconjugated-C225 induced EGFR endocytosis in pancreatic cancer cells. Furthermore, dynamin is also evolving as a new therapeutic target in various diseases. In both PANC-1 and MiaPaca2 cells mutant dyn-2 significantly inhibited free C225 induced endocytosis of EGFR, suggesting the involvement of CME pathways. However, nanoconjugation resulted in increased endocytosis of EGFR despite expression of mutant dyn-2, implicating a possible role of dyn-2 independent, CI pathways in nanoconjugated form. These results suggest that C225 induced endocytosis of EGFR may require partial involvement of both the CI and CME pathways. Further investigation is required to substantiate the role of CI and dyn-2 independent pathways by probing other players such as CDC42, ARF, or RHO GTPase (29, 33, 34). On the other hand, in AsPC-1 cells, nanoconjugation altered the mechanism of C225 induced endocytosis of EGFR from dyn-2 independent to dyn-2 dependent pathway, implicating a possible shift from CI to CME pathways. However, additional studies are required to identify other factors and molecular players involved during nanoparticle mediated receptor endocytosis. This understanding will help us to engineer and design suitable nanomachinery to regulate intracellular pathways.
In summary, we have demonstrated that membrane receptor endocytosis is distinct in metastatic vs. primary human pancreatic cancer cell lines. Nanoconjugation induces faster endocytosis of EGFR, influences the compartmentalization, and alters the mechanism of endocytosis. Thus, nanoconjugation cannot be construed as an innocuous reaction involved in attaching the targeting agent to the nanoparticle, instead it may distinctly alter the cellular processes at the molecular level. The mechanism of such alteration of endocytic pathways upon nanoconjugation is unique and warrants further investigation. It is possible that nanoconjugation enables C225 to induce receptor clustering to a greater extent than the free form (35) which results in faster endocytosis leading to attenuation of membrane presentation of the receptor. These findings provide the mechanism through which nanoconjugation alters C225 function and may have significant clinical implications in the treatment of EGFR-positive human cancers. Furthermore, this finding will also help to expedite the design of nanofabrication for efficient targeting and therapy.
Methods
Reagents.
Antibody was principally purchased from Cell Signaling and Santa Cruz, EEA1 (Cell Signaling, cat #2411), Dynamin (Santa Cruiz, sc-11362) Lysotracker (Invitrogen, L7526), Golgi antibody (TNF46, Sigma), Cy3 labeling kit (GE healthcare, PA33001), Cetuximab (Bristol Mayer Squib), Transferrin antibody with Alex Flour 488, Para formaldehyde (Electron Microscopy Science, 15710), BSA (Fischer Scientific, S-5058), and ammonium chloride (Sigma, A0171). Mutant (K44A) and WT dyn-2 adenovirus was a kind gift from Mark McNiven, Mayo Clinic.
Cell Culture.
The pancreatic cancer cell lines AsPC-1, PANC-1, and MiaPaca-2 were purchased from American Type Culture Collection and cultured using RPMI medium 1640 and DMEM with L-glutamine (Cellgro Mediatech, Inc.) supplemented with 10% FBS and 1% antibiotics (penicillin-streptomycin).
Synthesis of Gold Nanoparticles.
Gold nanoparticles (AuNPs) were synthesized from tetrachlorauric acid by wet chemical methods using sodium borohydride as a reducing agent as previously described in ref. 36. Briefly, an aqueous stock solution of tetrachloroauric acid (HAuCl4) was reduced with an aqueous solution of sodium borohydride (NaBH4) under vigorous stirring (18). Stirring continued for 12 h to obtain AuNPs used in this study. AuNPs thus formed were characterized using UV-Vis and TEM confirming ∼5 nm size spherical nanoparticles formed by this method.
Synthesis and Characterization of Gold Nanoconjugates Containing Anti-EGFR Antibody Labeled with Cy3 dye.
The monoclonal antibody C225 was conjugated with monoclonal antibody labeling dye Cy3 according to manufacturers protocol (GE healthcare, PA33001). In brief, the antibody (2 mg/mL) was diluted (1∶1) with PBS buffer and then incubated with Cy3 dye for 30 min in the presence of coupling buffer. Free dye was separated through a gel filtration column provided in the kit. Cy3-labeled C225 (C225-Cy3) thus obtained was used to synthesize Au-C225-Cy3 by a simple mixing technique by adding 2 μg/mL of C225-Cy3 to AuNP solution for 1 h. Au-C225-Cy3 was separated from unbound C225-Cy3 by ultracentrifugation as previously described in ref. 18. The ultracentrifugation was repeated twice after washing and the loose pellet resuspended in distilled water for use (see legend of Fig. S8).
Immunofluorescence Microscopy.
Cells (2 × 104/well) were seeded in four well chamber slides. After 24 h, each well was treated either with C225-Cy3 or Au-C225-Cy3 in a concentration of 2 μg/mL at 37 °C. Endocytosis was followed over time (5 min–1 h) by confocal microscopy. In separate experiments cells were also incubated with C225-Cy3 and Au-C225-Cy3 in cold for 30 min. At the end of the experiments each well in the chamber slide was washed with cold PBS (three times). Cells were fixed in 2% paraformaldehyde in PBS at room temperature for 15 min. At the end of fixation wells were washed with PBS and then mounted in a mounting media containing the nuclear stain DAPI (Vectashield). For colocalization experiments, cells were fixed and then blocked with 2% BSA in PBS for 2 h followed by incubation with respective antibodies with appropriate dilution as suggested in the company manual. After incubation at 4 °C, cells were washed three times with PBS. To detect the primary antibody that bind specifically to organelles (Golgi or early endosome), a secondary antibody of goat anti-rabbit IgG1 conjugated with Alexa fluor 488 were added to the chamber. Pictures were taken at 63X/1.2 W with scan zoom 1.0 and 2.0.
To determine the role of dynamin, cells were seeded in four well chamber slides at 2 × 104 per well per mL. The next day cells were infected with adenovirus that expresses either the WT or the K44A GTPase dynamin mutant. The medium was changed after 48 h and treated as above.
Quantification of Compartmentalization.
Image quantification was done using KS400 image analysis software (Carl Zeiss Micro Imaging). For quantification of colocalization a minimum threshold of red and green channels was selected. Images were analyzed from different areas of the plate and percentage of colocalization was calculated from the amount of colocalized areas from the total green or red area. The data represent the calculation of the snaps taken from five different areas (n = 5) and having at least 20–40 number of cells with data presented as mean ± SEM.
Transmission Electron Microscopy.
Pancreatic cancer cells were seeded and grown in 100 mm tissue culture plates. At ∼70% confluence, cells were treated with Au-C225 (Au∶C225 ∼ 0.5) for 2 h followed by thorough washing of the cells with 1X PBS and then added to Trumps fixative for microtome sectioning for TEM microscopy as previously reported in ref. 37.
Statistical Analysis.
Statistical analysis was done by a two-tailed student t-test and P < 0.05 is considered as significant.
Supplementary Material
Fig. 3.
Role of dynamin in C225-Cy3 induced internalization of EGFR. Figure demonstrates the role of dynamin in C225-Cy3 induced internalization of EGFR. Different human pancreatic cancer cells were infected with adenovirus that expresses either the WT or the K44A GTPase dynamin mutant followed by treatment with C225-Cy3 for 1 h. Internalization was monitored using confocal microscopy. A–C demonstrate the role of dynamin in C225-Cy3 induced internalization of EGFR in PANC-1, AsPC-1, and MiaPaca2 cells, respectively.
Fig. 4.
Role of dynamin in Au-C225-Cy3 induced internalization of EGFR. Figure demonstrates the role of dynamin in Au-C225-Cy3 induced internalization of EGFR. Different pancreatic cancer cells were infected with adenovirus that expresses either the WT or the K44A GTPase dynamin mutant followed by treatment with Au-C225-Cy3 for 1 h. Internalization was monitored using confocal microscopy. A–C demonstrate the role of dynamin in Au-C225-Cy3 induced internalization of EGFR in PANC-1, AsPC-1, and MiaPaca2 cells, respectively.
Acknowledgments.
Supported by National Institutes of Health (NIH) Grant CA135011, CA136494 and University of Texas MD Anderson Cancer Grant UTMD-1 (to P.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006507107/-/DCSupplemental.
References
- 1.Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol. 2004;22:47–52. doi: 10.1038/nbt927. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 3.Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliver Rev. 2009;62:346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang B, Mackey MA, El-Sayed MA. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J Am Chem Soc. 2010;132:1517–1519. doi: 10.1021/ja9102698. [DOI] [PubMed] [Google Scholar]
- 5.Jiang W, Kim BY, Rutka JT, Chan WC. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 6.Boal AK, Rotello VM. Radial control of recognition and redox processes with multivalent nanoparticle hosts. J Am Chem Soc. 2002;124:5019–5024. doi: 10.1021/ja016894k. [DOI] [PubMed] [Google Scholar]
- 7.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 8.Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh PS, Verma A, Rotello VM. Binding and templation of nanoparticle receptors to peptide alpha-helices through surface recognition. Chem Commun. 2007:2796–2798. doi: 10.1039/b705554d. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch LR, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirkin CA, Taton TA. Semiconductors meet biology. Nature. 2000;405:626–627. doi: 10.1038/35015190. [DOI] [PubMed] [Google Scholar]
- 12.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 13.Whitesides GM. The “right” size in nanobiotechnology. Nat Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 14.Li S-D, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 15.Veronese FM, et al. PEGâ’doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjugate Chem. 2005;16:775–784. doi: 10.1021/bc040241m. [DOI] [PubMed] [Google Scholar]
- 16.Kukowska-Latallo JF, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 17.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314:3093–3106. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patra CR, et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68:1970–1978. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 19.Masui H, et al. Growth inhibition of human tumor cells in athymic mice by antiepidermal growth factor receptor monoclonal antibodies. Cancer Res. 1984;44:1002–1007. [PubMed] [Google Scholar]
- 20.Carpenter G, Liao HJ. Trafficking of receptor tyrosine kinases to the nucleus. Exp Cell Res. 2009;315:1556–1566. doi: 10.1016/j.yexcr.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao HJ, Carpenter G. Cetuximab/C225-induced intracellular trafficking of epidermal growth factor receptor. Cancer Res. 2009;69:6179–6183. doi: 10.1158/0008-5472.CAN-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curley SA, et al. Noninvasive radiofrequency field-induced hyperthermic cytotoxicity in human cancer cells using cetuximab-targeted gold nanoparticles. Journal of Experimental Therapeutics and Oncology. 2008;7:313–326. [PubMed] [Google Scholar]
- 23.Jaramillo ML, et al. Effect of the anti-receptor ligand-blocking 225 monoclonal antibody on EGF receptor endocytosis and sorting. Exp Cell Res. 2006;312:2778–2790. doi: 10.1016/j.yexcr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66:3603–3610. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- 25.Gao H, Shi W, Freund LB. Mechanics of receptor-mediated endocytosis. Proc Natl Acad Sci USA. 2005;102:9469–9474. doi: 10.1073/pnas.0503879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 27.Thompson HM, McNiven MA. Discovery of a new “dynasore”. Nat Chem Biol. 2006;2:355–356. doi: 10.1038/nchembio0706-355. [DOI] [PubMed] [Google Scholar]
- 28.Rappoport JZ, Heyman KP, Kemal S, Simon SM. Dynamics of dynamin during clathrin mediated endocytosis in PC12 cells. PLoS ONE. 2008;3:e2416. doi: 10.1371/journal.pone.0002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface (Translated from eng) Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. (in eng) [DOI] [PubMed] [Google Scholar]
- 31.Lee CS, et al. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat Cell Biol. 2006;8:477–484. doi: 10.1038/ncb1401. [DOI] [PubMed] [Google Scholar]
- 32.Sigismund S, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Nat’l Acad Sci USA. 2005;102:2760–2765. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkham M, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber AB, Libermann TA, Lax I, Yarden Y, Schlessinger J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J Biol Chem. 1983;258:846–853. [PubMed] [Google Scholar]
- 36.Bhattacharya R, et al. Gold nanoparticles inhibit the proliferation of multiple myeloma cells. Adv Mater. 2007;19:711–716. [Google Scholar]
- 37.Bhattacharya R, et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomedicine: Nanotechnology, Biology, and Medicine. 2007;3:224–238. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.