Abstract
Mutant larvae for the Drosophila gene lethal giant larva (lgl) develop neoplastic tumors in imaginal discs. However, lgl mutant clones do not form tumors when surrounded by wild-type tissue, suggesting the existence of a tumor-suppressing mechanism. We have investigated the tumorigenic potential of lgl mutant cells by generating wing compartments that are entirely mutant for lgl and also inducing clones of various genetic combinations of lgl− cells. We find that lgl− compartments can grow indefinitely but lgl− clones are eliminated by cell competition. lgl mutant cells may form tumors if they acquire constitutive activity of the Ras pathway (lgl− UAS-rasV12), which confers proliferation advantage through inhibition of the Hippo pathway. Yet, the majority of lgl− UAS-rasV12 clones are eliminated in spite of their high proliferation rate. The formation of a tumor requires in addition the formation of a microenvironment that allows mutant cells to evade cell competition.
Keywords: Drosophila imaginal discs, Hpo pathway, lgl
The formation of tumors normally occurs after a transformation of groups of cells that acquire a set of properties allowing them to proliferate in excess and to colonize normal tissues, superseding nontumor cells. In Drosophila there are a number of mutations known to cause excessive growth leading to the production of tumors. Among these mutations, there is the group of tumor suppressor genes scribble (scrib), disc large (dlg), and lethal giant larvae gene (lgl), whose function is necessary for normal cell polarity and asymmetric cell divisions (reviews in refs. 1–3). In mutant larvae for scrib and lgl the neuroblasts and imaginal cells develop massive neoplastic tumors that eventually kill the larvae. These tumors exhibit many of the properties of human tumors, including loss of tissue architecture and alterations of cell shape. Moreover, the human homologs of these genes are also associated with the formation of diverse types of cancers (4, 5).
These studies have identified genetic defects that may lead to tumor formation but are not informative about how tumors appear and progress within normal tissue. From this perspective, it is of interest to consider the behavior of cells mutant for scrib or lgl. Although mutant homozygous larvae for these genes develop extensive tumors in imaginal discs, clones of mutant cells surrounded by wild-type tissue do not produce tumors (6–9). Furthermore, Brumby and Richardson (6) and Igaki et al. (8) have shown that scrib tumorous cells are eliminated by JNK-dependent apoptosis. It appears that the potential of scrib and lgl mutant cells to form tumors depends on the cellular context: if they are surrounded by “like” cells they develop tumors, but if surrounded by normal cells they do not. This suggests the existence of a tissue-specific mechanism that recognizes individual features of cells and proceeds to the elimination of undesirable cells. This behavior resembles the phenomenon of cell competition (10–12); a compartment-specific short-range interaction between cells with different division rates that leads to JNK-mediated apoptosis of the slower dividing cells. A similar kind of interaction may also function to eliminate abnormal or malignant cells that may arise in development.
The observations that constitutive expression of the Ras pathway (6, 9) rescues the lethality of lgl or scrib clones and causes tumorous growth indicate that under certain conditions the tumor-suppressing mechanism can be evaded.
To address this issue we have analyzed the growth of discs and compartments mutant for lgl and also the behavior of clones of lgl mutant cells of various genetic combinations developing in normal (lgl/+) background. We find that lgl mutant clones are normally eliminated by a process akin to cell competition, but constitutive Ras activity (lgl− rasV12) confers on the clones the potential to survive and generate tumors. lgl− rasV12 clones acquire high growth rate through down-regulation of the Hippo (Hpo) pathway, but in spite of their growth advantage many of these clones are also eliminated. Our results indicate that clones of lgl mutant cells developing in normal tissue can form a tumor when (i) the Hpo pathway is inhibited or down-regulated, which confers lgl− cells a high proliferation rate, and (ii) the groups of fast proliferating cells are able to merge together to generate a microenvironment that allows the group to overcome cell competition and to continue growing.
Results and Discussion
Entire lgl− Discs or Compartments Can Grow Indefinitely, but Isolated lgl− Cells Are Eliminated by JNK-Mediated Apoptosis.
As reported long ago (13), lgl mutant larvae are unable to pupate and remain a long time in the culture medium to finally die as gigantic larvae when they are 12–13 d old. The CNS and the imaginal discs develop into extensive tumors that reach very large size (14), (Fig. S1C). We have studied the growth dynamics of the discs of mutant larvae (see Fig. S1 legend for details) and found that they grow actively as long as the larva is alive. Thus a principal feature of lgl mutant discs is that, unlike the wild type, they continue growing past the normal body and tissue size. We have reached a similar conclusion after studying the growth of posterior compartments that are entirely mutant for lgl. In larvae of genotype lgl− FRT40A/M(2)24F FRT40A; UAS-Flp hh-Gal4 (see Materials and Methods) most or all of the posterior (P) compartment becomes homozygous for lgl, whereas the anterior (A) compartment remain lgl+ and serves as control. We find that whereas the control lgl+ anterior compartment stops growth once it has reached final size, the lgl− compartment continues growing and reaches a large size (Fig. S1 D–F). This can be shown by the comparison of the P/A size ratios in larvae of different ages. The posterior lgl− compartment is initially somewhat smaller than a wild type, the P/A size ratio is 0.43 (n = 18) in 144-h larvae, whereas the normal value is 0.65 (15). However, by 168 h, the P/A ratio is 0.84 (n = 15) and in 216-h larvae, it is 1.25 (n = 10). These observations indicate that lgl mutant cells proliferate indefinitely, as they do not respond to the general mechanism that arrests growth when the final stereotyped size of compartments (15) has been reached. Some of the tumorous properties of lgl mutant cells may stem from this property.
We then examined the behavior of clones of lgl− cells growing in normal (lgl/+) discs. The clones were induced during the second larval period and the discs fixed at various times after clone initiation (see Materials and Methods). The results are illustrated in Fig. 1 A–C: lgl− clones develop until 72–96 h after induction and then most of them initiate apoptosis and disappear. They acquire activity of the JNK pathway (also reported by ref. 7), and this activity is required for clone elimination, because its suppression by overexpressing puc, a negative regulator of JNK (16), allows clone survival (Fig. 1 C–C′′ and Fig. S2 A–B′ and C).
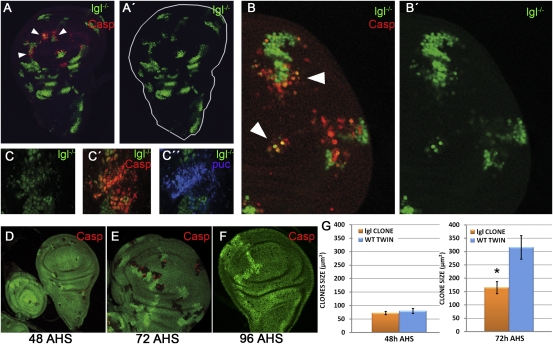
Fig. 1.
Behavior of mutant lgl clones developing in a normal (lgl−/+) disc. (A and A′) lgl− clones labeled green with GFP. Several clones in the wing pouch contain caspase activity (red). The A′ image shows the green channel to illustrate that the cells undergoing apoptosis are mutant for lgl. Note that clones in the hinge and the thorax do not show caspase activity. (B and B′) Several lgl clones (green) in the process of being eliminated. Caspase activity is in red. The dying cells are in the periphery of the top clone. (C–C′′) Dying clone (green) labeled with caspase (red) and puc-LacZ (blue). The latter is an indicator of JNK activity. (D–G) Results of an experiment in which lgl mutant cells are labeled by the loss of GFP (see main text). The twin control clones are labeled by 2x GFP. By 48 h after clone initiation the size of lgl and control twin clones is the same (D), but by 72 h (E) lgl− clones are smaller and also contain cells in apoptosis, normally at the borders (red). (F) By 96 h most of the lgl− clones have disappeared and only the control twins remain. (G) Comparison of the clone size between control and lgl− clones 48 and 72 h after clone initiation. Note that up to 48 h, control and lgl− clones are of the same size, but after 72 h, lgl− clones are significantly smaller than controls. Error bars indicate SE. *P < 0.0001.
We also performed an experiment to compare directly the division rate of lgl mutant cells with that of the lgl+ cells in the same disc. In discs of genotype ywhsFlp; lgl− FRT40A/ubi-GFP FRT40A the same recombination event will produce a lgl− clone with no GFP activity and a control twin lgl+ clone containing two doses of GFP. The results are shown in Fig. 1 D–G. In discs fixed 24 or 48 h after clone initiation lgl− clones are of the same size as the controls. However, after 72 h their mean size is significantly smaller and many of the clones contain cells in apoptosis. Finally, 96 h after initiation, most lgl− clones have disappeared (Fig. 1F). Consequently, our data do not indicate that lgl− cells proliferate at a lower rate than lgl+ cells. The small size of the 72 h lgl− clones is likely to be a consequence of the apoptosis detected in those clones (Fig. 1 E and F). Moreover, the proliferation rate of lgl− UAS-puc cells appears to be similar to that of surrounding lgl+ cells (Fig. S2 B and B′).
The conclusion from these experiments is that there is a mechanism to induce apoptosis in lgl mutant cells when they are in the same population with normal cells. It is worth mentioning that preventing apoptosis of lgl− clones, as in the lgl− UAS-puc experiment, does not make those clones tumorigenic, suggesting a further requirement.
lgl− rasV12 Clones Acquire Proliferation Advantage Through Inhibition of the Hippo Pathway.
Contrasting with the observation that lgl mutant clones are eliminated and do not form tumors, it has been shown that lgl− clones survive if they contain constitutive activity of the Ras pathway (9), provided by the rasV12 construct (17). Moreover, they give rise to neoplastic tumors in the imaginal discs. A similar observation was made for scrib− rasV12 clones (6, 9). In those experiments the mutant clones were generated by overall flipase activity in the eye disc, which induces FRT-mediated recombination in the great majority of the eye cells. This method ensures the production of many mutant clones that cover a large part of the disc, but does not discriminate the behavior of individual clones.
We followed a different experimental strategy, as we wanted to check the ability of individual lgl− rasV12 clones to induce tumors in the wing disc. As a control, we first generated clones that contain rasV12 that are wild type for lgl. The characteristics of these clones have been reported in previous publications (18–20). In agreement with previous reports we find that rasV12 clones form small outgrowths and also acquire elevated levels of dMyc (the Drosophila homolog of the mammalian proto-oncogene Myc) (Fig. S3).
To study the behavior of individual lgl− rasV12 clones we have used an hsFlp construct under conditions (see Materials and Methods) to generate few clones per disc. Heat shocks (HS) of either 7 or 15 min were administered to second instar (48–72 h of age) larvae and the discs were fixed either 72 or 96 h after HS.
An important feature of the lgl− rasV12 clones is that their growth rate is much higher than that of surrounding cells, as indicated by BrdU incorporation (Fig. 2 A and A′). This is in contrast with control rasV12 clones, which grow at a normal rate (Fig. 2 B and B′), and the lgl− clones mentioned above. We examined the possibility that the excess of growth of lgl− rasV12 clones is due to an inhibition or down-regulation of the Hippo (Hpo) pathway. This pathway is a negative regulator of cell proliferation and its inhibition causes large overgrowths in the imaginal discs (21, 22). A key element in the pathway is the transcriptional coactivator Yorkie (Yki), which is normally present in the cytoplasm in phosphorylated form. The inactivation of the pathway results in the production of the nonphosphorylated form of Yki, which translocates to the nucleus (21, 23). Thus Yki nuclear translocation is an indication of inhibition of the Hpo pathway. We find that in either lgl− or rasV12 clones Yki localizes to the cytoplasm (Fig. S4 A–B′), whereas in lgl− rasV12 clones it is predominantly nuclear (Fig. 2 D–E′′′ and Fig. S5 A–D′). Another marker of the Hpo pathway is the expression of the target gene diap1, which is normally down-regulated (23). The high expression levels of diap1 in lgl− rasV12 clones (Fig. 2 C and C′) also indicates suppression of Hpo activity. We have also examined the cellular distribution of Yki in scrib− UAS-rasV12 clones and found it localizes in the nuclei (Fig. S5 E–E′′).
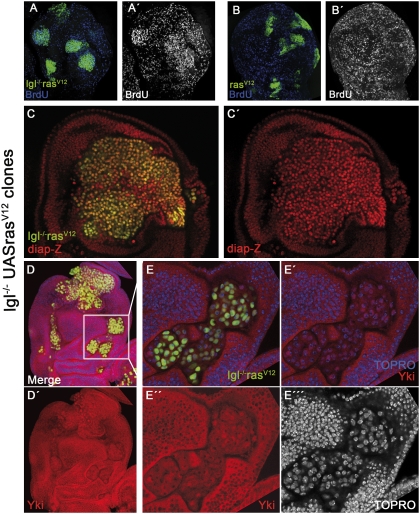
Fig. 2.
Proliferation rate of lgl− rasV12 cells and down-regulation of the Hpo pathway. (A and A′) Cell proliferation rate in lgl− rasV12 clones is higher than in surrounding tissue, as indicated by BrdU incorporation. In contrast, rasV12 clones (B and B′) grow normally. (C and C′) Disc with a large tumor showing elevated levels of diap1 (red) in the tumor cells. (D and D′) Disc containing several small lgl− rasV12 clones. The disc is triply stained for GFP, Yki (red), and TOPRO (blue), the latter labeling cell nuclei. The panels in E–E′′′ are high magnifications of the clones in the Inset in D. Note in E′′ and E′′′ that Yki and TOPRO are coextensive in the clone cells. The small nuclear regions with very high TOPRO label (E′–E′′′) correspond to heterochromatin and Yki is not present there (E′′).
Many Fast-Dividing lgl− rasV12 and lgl− UAS-Yki Clones Are Also Eliminated.
An unexpected result was that in spite of their high growth rate many lgl− rasV12 clones were very small and frequently contained cells in apoptosis (Fig. 3 A–D′). Although it is more notable in small clones, large lgl− rasV12 patches also present apoptosis at their borders (Fig. 3 D and D′). In some cases all or nearly all of the clone cells become apoptotic (Fig. 3A), in turn suggesting that a number of clones may have been eliminated. To check this possibility and to estimate the percentage of eliminated clones, we measured the frequency of lgl− rasV12 clones after 7-min HS in dronc+ and dronc− discs (see Materials and Methods). In the latter, the virtual absence of apoptosis ensures that the great majority of the clones would survive. In the dronc+ experiment the clone frequency was 1.9 per disc (n = 25 discs), whereas in dronc− it rises to 4.3 (n = 24 discs), which is significantly different (P < 0.0005). This result indicates that more than half of the lgl− rasV12 clones are eliminated from the disc.
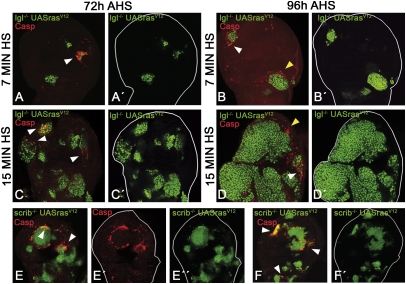
Fig. 3.
Behavior of lgl− rasV12 and scrib− rasV12 clones. (A, A′, B, and B′) Typical discs from larvae given a 7-min heat shock (HS) and fixed 72 (A) or 96 h (B) after HS. Note the small size of the lgl− rasV12 clones and also that one of them is almost entirely apoptotic (caspase in red) and will be eliminated. The disc in B also shows small clones. One of them contains apoptotic cells in the border (arrowhead). The larger clone at the Bottom (yellow arrowhead) is surrounded by a group of apoptotic cells, but in this case they do not belong to the clone. (C, C′, D, and D′) Discs from the 15-min HS experiment. (C and C′). The 72-h after-heat-shock (AHS) disc contains several lgl− rasV12 clones, some of which appear to be close to fuse. Nevertheless some of their cells are in apoptosis (arrowheads, caspase in red) and probably some clones, like the one in the Top, will be eliminated. (D and D′) 96-h AHS disc containing a large tumor covering the disc almost entirely. Even though the lgl− rasV12 tumor is overgrowing, some of its cells undergo apoptosis (arrowhead, caspase in red). There are also lgl+ cells close to the border of the tumor that are in apoptosis (yellow arrowhead). (E–E′′, F, and F′) Two discs containing several scrib− rasV12 clones in which numerous cells in the border are dying. In some cases (E′) the apoptotic cells form a near complete ring. In other cases (F, Top Left, arrows) the entire clone is in apoptosis.
Thus the confrontation of lgl− with lgl+ cells usually results in the elimination of the mutant cells, even if they divide faster than lgl+ cells. We have explored this issue further by generating clones of lgl− cells containing overexpression of Yki, which is known to produce large overgrowths (23). We find that lgl− UAS-Yki cells grow faster than surrounding lgl+ cells (Fig. S3C) and develop massive overgrowths, very similar to those formed by lgl− rasV12 clones. This is a significant observation because it shows that overexpression of Yki (that is, down-regulation of Hpo) is sufficient to allow lgl mutant cells to develop tumors, highlighting the relevant role of the Hpo pathway as a tumor suppressor factor. Yet isolated lgl− UAS-Yki clones, or cells located in the clone borders, are often subjected to apoptosis (Fig. 4).
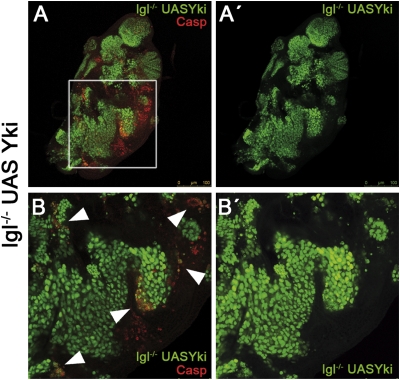
Fig. 4.
Behavior of lgl− UAS-Yki clones. (A and A′) Wing disc almost filled with lgl− UAS-Yki clones. Note the areas of apoptosis (red) close to the borders of the tumors. (B and B′). High magnification of a region of the disc in A. Note (arrowheads) the incidence of apoptosis in lgl− UAS-Yki cells located in the tumor borders or in clones that are isolated.
Because this result was unanticipated in view of previous reports (6, 9), we examined the behavior of individual clones of other genotypes also known to produce tumors. It has been shown that scrib− UAS-rasV12 clones can give rise to neoplastic overgrowths (6). Following a protocol similar to that used to induce lgl− rasV12 clones, we find that many individual scrib− UAS-rasV12 clones exhibit apoptosis in cells located in their borders and in some cases the whole clone becomes apoptotic (Fig. 3 E–F′). This suggests that the apoptosis observed in lgl− rasV12 cells may be a general feature of tumorous cells growing in a normal tissue.
Tumorous lgl− Clones Are Eliminated by a Mechanism Akin to Cell Competition Irrespective of Their Cell Division Rate.
Because the viability of the lgl mutant tissue appears to be normal, the elimination of lgl mutant cells when they are in the same population with lgl+ cells suggests that it is due to cell competition. This is based on several observations: (i) the apoptotic cells are always inside the clones, indicating that only lgl− cells are being eliminated; (ii) the cells in apoptosis appear preferentially at the clone borders (Fig. 1 B, B′, and E and Figs. 3 and 4), suggesting that it is caused by short-range interactions with lgl+ cells located in the vicinity; (iii) the lgl− cells are eliminated by JNK-mediated apoptosis, a typical feature of cell competition (6, 8, 12); and (iv) the elimination of lgl mutant cells is compartment specific; it does not occur across compartment borders, as indicated by our experiment in which we generate a posterior compartment made exclusively of lgl mutant cells (Fig. S1). The close proximity of lgl− and lgl+ cells at the A/P border does not result in apoptosis of lgl− cells.
The one difference between the cell elimination process we demonstrate here and classical cell competition is that the latter has been associated with slow division rate (10, 12). Our experiments indicate that lgl− cells proliferate approximately at the same rate as normal cells, and most importantly, that lgl− rasV12 and lgl− UAS-Yki cells proliferate more rapidly that lgl+ cells and yet they are frequently eliminated.
In our view, cell competition can be defined in a more general way as a close-range interaction between two types of viable cells that leads to apoptosis of one type. This would include the classical example of slow dividing cells (10, 12) and also other similar interactions. Milan et al. (24) reported that clones of cells overexpressing spalt (sal) survive in the normal sal domain but are eliminated by apoptosis outside the domain. It appears that cells outside the sal domain recognize those overexpressing sal as “different” and drive them to apoptosis. Similarly, ventral or dorsal disc cells were eliminated if they appear in the inappropriate compartment. Comparable observations have been made for other changes of identity (25, see review in ref. 26). These observations suggest the existence of an intrinsic mechanism to remove cells that respond abnormally to developmental cues (see ref. 27).
We propose that a similar process plays a role in the case of clones of mutant lgl− cells growing in lgl+ discs. The lgl− clones are identified as being developmentally different through their interactions with neighbor lgl+ cells. This leads to JNK induction and subsequently apoptosis in the mutant cells. It is a forceful mechanism able to remove fast proliferating cells like lgl− rasV12 and lgl− UAS-Yki cells. Along a similar line of thought, Igaki et al. (8), have proposed the existence of a mechanism to remove oncogenic cells on the basis of the endocytic activation, via Eiger, of the apoptotic JNK pathway.
lgl rasV12 Cells Need to Form a Microenvironment to Evade Cell Competition and Form a Tumor.
Finally, we have examined the ability of lgl− rasV12cells to form tumors. We consider that a disc contains a tumor when the lgl− rasV12 cells occupy at least 50% of the disc and it also shows outgrowths and gross morphological alterations associated with lgl− rasV12 cells.
As mentioned above, lgl rasV12clones were induced by Flp-induced mitotic recombination under heat shock control (see Materials and Methods). Surprisingly, there is a large difference regarding the frequency of tumors in the 7- and 15-min HS experiments. Whereas in the 15-min experiment, 90% of the discs (n = 91) contain extensive tumors (Fig. 3D), only 8% of the discs (n = 100) develop tumors in the 7-min HS experiment.
The low number of tumors in the 7-min experiment can be explained by the results above, indicating that about half of the lgl− rasV12 clones in the 7-min experiment are eliminated by apoptosis. To check this idea we have compared the frequency of lgl− rasV12 tumors in dronc+ and dronc− background. The results are the following: in the 7-min experiment tumor frequency in dronc+ background after 96 h is 8%, whereas in dronc− (n = 65) it is 33%. Thus the conclusion is that only a small subset of lgl− rasV12 clones is able to develop tumors and that apoptosis is playing a major tumor-suppressing role. Interestingly, a similar comparison in dronc+ and dronc− discs in the 15-min HS experiment yield close values of tumor frequency of 91 and 100%, respectively (see below).
The 10-fold difference in tumor frequency between the 7- and 15-min experiments indicates that the higher clone density after 15 min of HS facilitates the formation of tumors. This idea in turn suggests that many tumors may result from merging of individual clones. This is supported by the examination of discs from the 15-min HS experiment, like the one in Fig. 2 C and C′; it contains several relatively small clones some of which are adjacent and will probably merge into individual patches to form a tumor. Other clones are likely to disappear as they contain many apoptotic cells.
The comparison of patch density in discs fixed 72 and 96 h after HS also suggests a merging process: in the 15-min HS experiment patch frequency per disc decreases from 12.3 (n = 20) in the 72-h discs to 5.1 (n = 18) in the 96-h discs. In contrast, in the 7-min HS experiment there is no significant difference in patch frequency in 72-h (1.9, n = 25) and 96-h discs (2.2, n = 18). These results indicate that the higher clone density in the 15-min HS experiment facilitates clone fusion, visualized by the diminution of patch frequency.
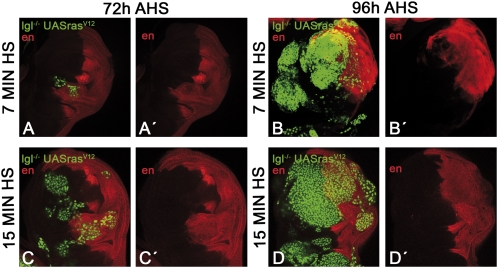
The idea that clone merging facilitates tumor formation is also supported by an experiment in which we discriminated the compartmental origin (anterior or posterior) of the lgl− rasV12 clones using engrailed (en) expression as a marker. Of a sample of 29 discs containing massive tumors covering most of the disc, 26 contained anterior and posterior cells (Fig. 5 B and D), whereas 3 were composed exclusively of anterior cells. Even the rare cases of tumors in the 7-min experiment contain cells from anterior and posterior origin (Fig. 5 B and B′). Thus, the great majority or all of the tumors are of mixed origin.
Fig. 5.
Merging of lgl− rasV12 clones in tumors. The expression of engrailed (en, in red) marks the anterior or posterior compartment origin of the clones. The Top row shows two discs from the 7-min HS experiment. The disc in A and A′ is the typical case fixed 72 h after clone initiation, in which there are very few clones and these are small. The disc in B and B′ was fixed after 96 h and represents the unusual case in which the disc develops a tumor after 7 min of HS. Note that the tumor contains cells of anterior and posterior origin and therefore must have been formed by at least two clones. The Bottom row shows typical cases from the 15-min HS experiment. The 72-h AHS disc (C and C′) contains several clones, some anterior and some posterior. Note the closeness of some clones, suggesting they are about to merge. The 96-h AHS disc (D and D′) has a large tumor that contains cells of anterior and posterior origin.
In our view, the formation of a tumor by lgl− rasV12 cells requires the generation of a microenvironment that prevents the elimination of the tumor cells. We have identified two factors that contribute to this microenvironment. One is an increase in proliferation rate, which in the case of lgl− rasV12 cells is achieved through down-regulation of the Hpo pathway. However, although it facilitates the confrontation with lgl+ cells, alone is not normally sufficient, as we find that fast-dividing lgl− rasV12 or lgl− UAS-Yki cells are often outcompeted. A similar process appears to occur in scrib− rasV12 clones, which are outcompeted (Fig. 3 E and F) even though they also show nuclear localization of Yki (Fig. S5 E–E′′)
The second factor is clone merging. Because the interactions between lgl+ and lgl− acts only at short range (see Figs. 1, 3, and 4), the fusion of individual clones helps to generate an internal environment within the patch that is not accessible to cell competition. Although lgl− rasV12 cells in the border of the patch (Fig. 3D) can be eliminated, those in the center will be protected and because of their high proliferation rate will form large masses of overgrowing tissue. Our observation that most tumors are of mixed anterior and posterior lineages (Fig. 5) supports this view strongly. The fact that the inhibition of apoptosis in the 15-min experiment does not affect tumor frequency also supports this view. The high density of clones facilitates merging and functions as a tumor-promoting mechanism.
In conclusion, we envisage two major steps in the process of tumor formation in Drosophila: (i) the transformation of a normal cell into a tumor cell and (ii) the ability of the tumor cells to generate a microenvironment to confront the cell competition mechanism designed to eliminate them.
The first step may be caused by different genetic defects, including lgl, scrib, dlg, and possibly mutations in other tumor-suppressing genes. In the case of the lgl mutations, we believe that a critical feature is the property of lgl mutant tissue of not responding to the size control process: even though lgl mutant cells proliferate at a normal rate or perhaps even slightly lower, lgl mutant discs or compartments can grow indefinitely. This feature provides a tumorous property, but alone would not be tumorigenic in a normal growing disc.
The fulfillment of the second step is facilitated by the acquisition by the tumor cells of a high division rate, through the inhibition of the Hpo pathway or other causes. However, a high division rate appears not to be sufficient to escape cell competition. It also requires, at least in the case of lgl− rasV12 and probably scrib− rasV12 cells, a process of merging of a sufficient number of cells. Whether the cell merging process is an absolute requirement is an open question. It is conceivable that a very large difference in division rate between tumor and nontumor cells might be sufficient for individual clones to generate the necessary microenvironment to escape cell competition.
We wish to point out that in humans and other species tumors normally appear in the form of groups of malignant cells that are mixed with normal ones. The progression of the tumor requires that the malignant cells compete successfully with the normal surrounding ones. It is possible that in normal life the appearance of tumor cells may be a frequent occurrence, but they are normally eliminated by a process similar to that described here. Only after acquisition of some additional property, higher division rate and/or ability to generate a protective environment, they can successfully confront the tumor suppressing mechanism.
Materials and Methods
Mutant Stocks.
The fly stocks used were obtained from the Bloomington Stock Center except where indicated. We used two different lgl alleles, lgl4 (a gift of Peter Bryant, University of California, Irvine, CA) and lglM, an allele that appeared fortuitously in our laboratory. We have also used the chromosome Df(2L)net62, a physical deletion of lgl (28). The lgl4 allele has been characterized as a null (29). We consider lglM as a null, on the basis of staining with anti-Lgl antibody and its interactions with the lgl4 allele and the deletion Df(2L)net62. In the experiments we used the lgl4 and lglM alleles as convenient and refer to them as lgl−. The scrib1 mutation is described in ref. 6. The droncI29 alelle (30) was also used to induce lgl mutant clones in a background resistant to apoptosis.
Generating lgl and scrib Mutant Clones.
To induce lgl− clones by the MARCM system we used the stock yw hsFlp122 tub-Gal4 UAS-GFP; tubGal80 FRT40A/Cyo. Larvae were subjected to a 15- or 7-min heat pulse at 37 °C, 48–72 h after egg laying (AEL). We also used several UAS stocks to generate different genetic combinations of lgl mutant clones: UAS-puc/TM6B, UAS-yki/TM6B (a gift from Ken Irvine, Rutgers University, NJ), UAS-RasV12/TM6B and pucE69 (puc-lacZ)/TM6B.
To generate scrib− rasV12 clones we used the scrib1 FRT82B and the Gal80 FRT82B stocks with a protocol similar to that used to induce lgl− rasV12 clones. In this case the heat shock time was 20 min.
To induce lgl mutant clones at the same time as control twin clones, we used the ywhsFlp; ub-GFP FRT40A stock and the lgl4 allele.
To make entire compartments lgl− we used the stock yw hsFlp122; M(2L)24F ub-GFP FRT40A/Cyo; hh-Flp/TM6B and the lgl4 allele. All crosses were performed at 25 °C.
The size of the clones was measured with Wright Cell Imaging Facility ImageJ software.
Inmunohistochemistry.
Imaginal discs were dissected in PBS and fixed in paraformaldehyde 4% for 30 min at room temperature. The washing and the dilution of the antibodies were performed in PBT. The antibodies used were rabbit anti-cleaved Casp3, 1:50 (Cell Signaling Technology), mouse anti-βGal, 1:50 (Hibridoma Bank), rabbit anti-Yki, 1:200 (a gift of K. Irvine), rabbit anti-PH3, 1–400 (Cell Signaling Technology), rabbit anti-P35, 1:5,000.
(Stratagene), guinea pig anti-dMyc, 1:1,000. Secondary antibodies were purchased from Invitrogen. For the BrdU detection we used the BrdU labeling kit from Sigma.
Samples were mounted in Vectashield and imaged in Leica TCS SPE and Zeiss LSM510 confocal microscopes.
Supplementary Material
Acknowledgments
We thank Ernesto Sánchez-Herrero for critical comments on the manuscript, Kenneth Irvine for providing the anti-Yki antibody, Luna Ballesteros for help in the UAS-Yki experiment, Salvador C. Herrera for help with the figures, and Angélica Cantarero and Rosa González for general assistance. This work has been supported by grants from the Ministerio de Ciencia e Innovación (Consolider and BFU-02427), from the Comunidad de Madrid, and from an institutional grant from the Fundación Ramón Areces.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009376107/-/DCSupplemental.
References
- 1.Bilder D. Epithelial polarity and proliferation control: Links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 2.Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- 3.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Humbert PO, et al. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 5.Kuphal S, et al. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene. 2006;25:103–110. doi: 10.1038/sj.onc.1209008. [DOI] [PubMed] [Google Scholar]
- 6.Brumby AM, Richardson HE. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Froldi F, et al. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 2010;8:33. doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igaki T, Pastor-Pareja JC, Aonuma H, Miura M, Xu T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev Cell. 2009;16:458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 10.Morata G, Ripoll P. Minutes: Mutants of Drosophila autonomously affecting cell division rate. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 11.Simpson P, Morata G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev Biol. 1981;85:299–308. doi: 10.1016/0012-1606(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 12.Moreno E, Basler K, Morata G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature. 2002;416:755–759. doi: 10.1038/416755a. [DOI] [PubMed] [Google Scholar]
- 13.Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- 14.Woods DF, Bryant PJ. Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev Biol. 1989;134:222–235. doi: 10.1016/0012-1606(89)90092-4. [DOI] [PubMed] [Google Scholar]
- 15.Martín FA, Morata G. Compartments and the control of growth in the Drosophila wing imaginal disc. Development. 2006;133:4421–4426. doi: 10.1242/dev.02618. [DOI] [PubMed] [Google Scholar]
- 16.Martín-Blanco E, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 18.Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 20.Prober DA, Edgar BA. Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 2002;16:2286–2299. doi: 10.1101/gad.991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Milán M, Pérez L, Cohen SM. Short-range cell interactions and cell survival in the Drosophila wing. Dev Cell. 2002;2:797–805. doi: 10.1016/s1534-5807(02)00169-7. [DOI] [PubMed] [Google Scholar]
- 25.Adachi-Yamada T, O'Connor MB. Morphogenetic apoptosis: A mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251:74–90. doi: 10.1006/dbio.2002.0821. [DOI] [PubMed] [Google Scholar]
- 26.Igaki T. Correcting developmental errors by apoptosis: Lessons from Drosophila JNK signaling. Apoptosis. 2009;14:1021–1028. doi: 10.1007/s10495-009-0361-7. [DOI] [PubMed] [Google Scholar]
- 27.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 28.Korochkina LS, Golubovsky MD. Cytogenetic analysis of induced mutations on the left end of the second chromosome of D. melanogaster. Drosoph Inf Serv. 1978;53:197–200. [Google Scholar]
- 29.Mechler BM, McGinnis W, Gehring WJ. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 1985;4:1551–1557. doi: 10.1002/j.1460-2075.1985.tb03816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu D, Li Y, Arcaro M, Lackey M, Bergmann A. The CARD-carrying caspase Dronc is essential for most, but not all, developmental cell death in Drosophila. Development. 2005;132:2125–2134. doi: 10.1242/dev.01790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.