Abstract
In most aphid species, facultative parthenogenetic reproduction allows rapid growth and formation of large single-genotype colonies. Upon predator attack, individual aphids emit an alarm pheromone to warn the colony of this danger. (E)-β-farnesene (EBF) is the predominant constituent of the alarm pheromone in Myzus persicae (green peach aphid) and many other aphid species. Continuous exposure to alarm pheromone in aphid colonies raised on transgenic Arabidopsis thaliana plants that produce EBF leads to habituation within three generations. Whereas naive aphids are repelled by EBF, habituated aphids show no avoidance response. Similarly, individual aphids from the habituated colony can revert back to being EBF-sensitive in three generations, indicating that this behavioral change is not caused by a genetic mutation. Instead, DNA microarray experiments comparing gene expression in naive and habituated aphids treated with EBF demonstrate an almost complete desensitization in the transcriptional response to EBF. Furthermore, EBF-habituated aphids show increased progeny production relative to EBF-responsive aphids, with or without EBF treatment. Although both naive and habituated aphids emit EBF upon damage, EBF-responsive aphids have a higher survival rate in the presence of a coccinellid predator (Hippodamia convergens), and thus outperform habituated aphids that do not show an avoidance response. These results provide evidence that aphid perception of conspecific alarm pheromone aids in predator avoidance and thereby bestows fitness benefits in survivorship and fecundity. Therefore, although habituated M. persicae produce more progeny, EBF-emitting transgenic plants may have practical applications in agriculture as a result of increased predation of habituated aphids.
Keywords: aphid, (E)-beta-farnesene, Hippodamia convergens, microarray, sesquiterpene
Rapid population growth of aphids is facilitated by their parthenogenetic lifestyle, which often results in the establishment of large single-genotype colonies in the field. In agricultural settings, aphid populations can be controlled with natural enemies such as coccinellid beetles and parasitoid wasps. Despite the effectiveness of biological control, behavioral responses to the threat of predation may allow aphids to persist as pests. Aphid avoidance of predators involves the production of an alarm pheromone. Across a remarkable diversity of aphids, the alarm pheromone contains a mixture of compounds with (E)-β-Farnesene (EBF) as the predominant component (1–3). Detection of EBF results in an array of aphid escape behaviors that, depending on the species and developmental stage, can include flying, walking away, and dropping off the plant.
EBF is a volatile sesquiterpene released from cornicles on the aphid's abdomen (4). When an aphid is attacked, it can release EBF in a range of concentrations, depending on the stress that is encountered, as well as the specific species, lineage, and developmental stage of the aphid itself (5–10). EBF-treated Acyrthosiphon pisum do not propagate this signal by releasing more EBF (11, 12), suggesting an uncoupling of alarm pheromone perception and biosynthesis. As EBF from wounded aphids can remain associated with the attacking predator, other aphids in the colony have a reliable signal for avoiding further predation (13). Even more so than, for instance, the alarm call of ground squirrels (14), EBF release by aphids increases the inclusive fitness of the attacked individual, because the other aphids in a colony are generally of the identical genotype.
Most aphid species can exhibit winged and nonwinged phenotypes, with the winged form being produced in response to environmental stresses, including crowding and low nutritional value of the host plant (reviewed in ref. 15), and the presence of natural enemies (16, 17). Treatment of early-instar A. pisum larvae with EBF results in wing formation in the next generation (18, 19), but it requires additional stimuli such as crowding. Alate aphids can move more rapidly to enemy-free space or more nutritious hosts, but this comes at a cost of a significantly slower development, lower fecundity, and a shorter life span (4).
A variety of plant species produce EBF, either constitutively or in response to herbivore damage (reviewed in ref. 20). Such insect-induced EBF production has been hypothesized to function either as a direct repellent (i.e., alarm pheromone function) or act as a kairomone for natural enemies of aphids (e.g., parasitoid wasps; reviewed in ref. 21). In fact, a study with transgenic Arabidopsis thaliana expressing peppermint EBF synthase revealed that both phenomena can take place. Myzus persicae (green peach aphids) were repelled by plants producing EBF, and the specialist parasitoid Diaeretella rapae was more attracted by transgenic than control plants (22).
It has been hypothesized that aphids, like many other animals that become habituated through repeated exposure to the same stimulus, can lose individual responsiveness to their alarm pheromone. Short-term habituation to EBF has been investigated in A. pisum upon repeated exposure to EBF. This caused reduced dropping behavior (20) and a lower effectiveness of insecticides (23). Moreover, several reports show that not all aphid species and lineages are equally responsive to EBF, suggesting a genetic basis for differences in this predator-avoidance reaction (5, 24–26).
Here, we present research showing how long-term EBF exposure affects M. persicae responses. Like many other aphids, this species uses EBF as the major constituent of its alarm pheromone (2). M. persicae that detect EBF rapidly withdraw their stylets from the plant sieve elements and move away from the emitting source. Maintenance of M. persicae colonies on A. thaliana that constitutively express EBF synthase (22) makes it possible to answer proximate and ultimate questions of alarm pheromone biology: How quickly do M. persicae individuals become habituated to their alarm pheromone? Do habituated aphids show altered transcriptional responses to EBF? Does habituation to EBF have fitness consequences in the presence or absence of natural enemies?
Results
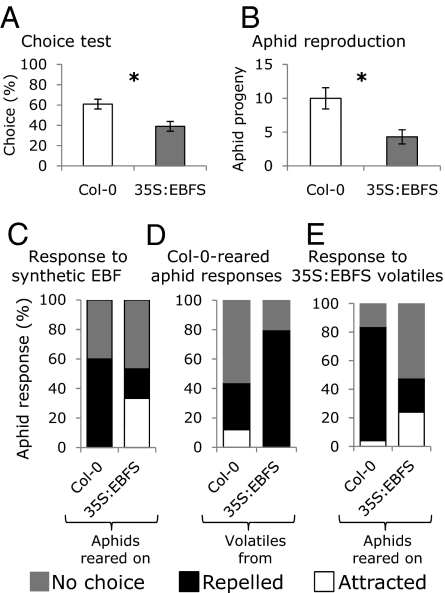
Experiments to test M. persicae responses to the EBF component of alarm pheromone were conducted with aphids reared on WT A. thaliana landrace Columbia-0 (Col-0), and otherwise isogenic A. thaliana constitutively expressing EBF synthase (35S:EBFS) (22) (Fig. S1A). In choice tests, naive aphids reared on WT A. thaliana preferred Col-0 A. thaliana plants over those emitting EBF (Fig. 1A and Fig. S1B). Moreover, in comparison with Col-0, aphid fecundity was reduced by approximately 60% on 35S:EBFS plants (Fig. 1B and Fig. S1C). Similar to previous experiments (22), naive aphids reared on Col-0 were repelled upon treatment with synthetic EBF relative to solvent-only controls (hexane; Fig. S2) or volatiles from 35S:EBFS plants (Fig. 1D). Aphids reared on 35S:EBFS were less likely to be repelled by EBF (Fig. 1 C and E).
Fig. 1.
Response of M. persicae to WT (Col-0), EBF-producing (35S:EBFS; line 11.4 in ref. 22) A. thaliana, and volatiles from these plants. (A) Choice tests; mean ± SEM of n = 25; *P < 0.05, generalized linear model with a binomial error structure. (B) Aphid progeny production; mean ± SEM of n = 10; *P < 0.05, generalized linear model with a Poisson error structure. (C) Aphids reared on Col-0 and EBF-producing (35S:EBFS) plants respond differently to 1 μg synthetic EBF (n = 15; P < 0.05, Fisher exact test). (D) Aphids reared on Col-0 are more repelled by 35S:EBFS than Col-0 volatiles (n = 25; P < 0.05, Fisher exact test). (E) EBFS-reared aphids are less repelled by 35S:EBFS volatiles than Col-0–reared aphids (n = 25; P < 0.05, Fisher exact test).
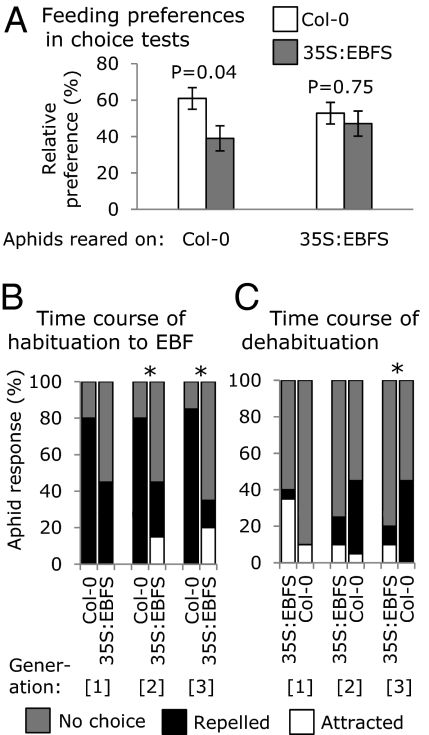
M. persicae from a colony that was reared for more than 10 generations on 35S:EBFS plants showed a completely different set of behaviors. They were neither repelled by synthetic EBF nor by volatiles from EBFS-expressing A. thaliana, suggesting that the aphids in this colony have become habituated to EBF (Fig. 1 C and E). In choice experiments, EBFS-reared aphids did not distinguish between Col-0 or 35S:EBFS leaves, whereas Col-0–reared aphids preferred Col-0 leaves over those from EBFS-expressing plants (Fig. 2A). EBF treatment causes transgenerational induction of wing formation in other aphid species (18), but this phenomenon was not observed with M. persicae in the current experiments. Although alates were occasionally found in both Col-0 and 35S:EBFS colonies, the 35S:EBFS colony did not have more alates, and neither colony showed wing formation in the next generation after short-term EBF exposure in an experiment to test this response.
Fig. 2.
Feeding preferences of Col-0- and 35S:EBFS-reared aphids, and time course of habituation and dehabituation. (A) Aphids reared on Col-0 and 35S:EBFS were given a choice between Col-0 and 35S:EBFS leaves. Mean ± SEM of n = 14; P values from a generalized linear model with a binomial error structure. (B) Nonhabituated M. persicae previously reared on Col-0 were placed on Col-0 and 35S:EBFS, and responses to synthetic EBF were determined after one, two, and three generations. (C) Habituated M. persicae previously reared on 35S:EBFS were placed on Col-0 and 35S:EBFS, and responses to synthetic EBF were determined after one, two, and three generations (n = 20; *P < 0.05, pair-wise comparison of Col-0 vs. 35S:EBFS with Fisher exact test).
EBF habituation could result from several factors with genetic, epigenetic, or neurobiological origins. To address the basis of habituation, we conducted experiments to determine how quickly aphids become habituated and whether habituated aphids can revert to the EBF-responsive state. A reduced avoidance response was observed in the second and third generation of previously naive aphids on 35S:EBFS plants (Fig. 2B). Conversely, aphid lines derived from the habituated population revert back to being EBF-sensitive after only three generations on WT Col-0, showing that EBF habituation in M. persicae is not caused by a stable genetic mutation (Fig. 2C).
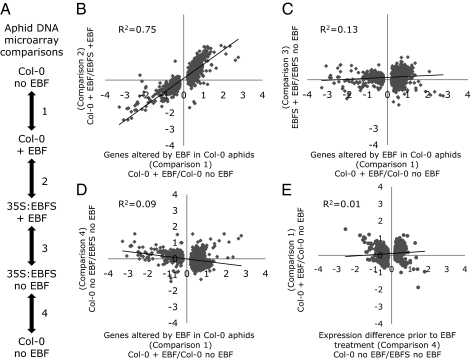
To provide insight into the underlying molecular mechanisms of EBF perception and signal transduction, four-way comparisons of M. persicae transcriptional responses to EBF treatment were conducted (Fig. 3A). Comparison 1 in Fig. 3A shows that, among 10,478 unique genes on the M. persicae DNA microarray (27), 849 were significantly up-regulated and 712 were significantly down-regulated 30 min after treating Col-0–reared aphids with EBF (x axis in Fig. 3B; Dataset S1). These 1,561 genes are expressed at a similar level in Col-0–reared aphids without EBF and 35S:EBFS-reared aphids with EBF (as indicated by the approximate 45° slope of the trend line in Fig. 3B), showing that EBF treatment has little effect on transcription of these genes in 35S:EFB-raised aphids. Another microarray experiment (comparison 2 in Fig. 3A) directly addresses the question whether EBF-regulated transcripts are differentially responsive in EBF-sensitive and habituated aphids. In this case, the vast majority (96%) of transcripts that were significantly regulated by EBF in Col-0–reared aphids are either not regulated or showed attenuated responses in 35S:EBFS aphids (Fig. 3C and Dataset S1). Transcriptional changes observed in 35S:EBFS-reared aphids treated with EBF did not correlate with those induced by EBF in Col-0–reared aphids. Furthermore, the complete desensitization of the transcriptional regulation was not a result of inherently higher or lower expression of the regulated genes in Col-0–reared aphids (Fig. 3D and Dataset S1). The different slopes of the trend lines in Fig. 3 B and D (P < 0.0001, linear regression) show that short-term EBF-induced differences in gene expression between Col-0- and 35S:EBFS-reared aphids (y axis in Fig. 3B) are not present before the treatment (y axis in Fig. 3D). Expression of genes that are significantly altered by long-term EBF habituation (comparison 4, 421 up and 451 down) shows a low correlation to that of these same genes in Col-0–reared aphids treated with a single EBF dose (Fig. 3E). Therefore, the M. persicae transcriptional responses caused by long-term EBF habituation are very different from those caused by the acute EBF alarm response.
Fig. 3.
Microarray analysis of M. persicae gene expression responses to 30 min of EBF exposure. All data are log2-transformed. (A) Outline of four DNA microarray comparisons of transcription in aphids reared on Col-0 or 35S:EBFS, with and without EBF treatment. (B) Genes significantly regulated upon EBF treatment of Col-0–reared aphids (n = 1,561; P < 0.05) are expressed similarly in Col-0–reared aphids without EBF and 35S:EBFS-reared aphids with EBF. (C) Most genes that are significantly regulated by EBF treatment of Col-0–reared aphids show attenuated responses in EBF treatment of 35S:EBFS-reared aphids. (D) Genes significantly regulated upon EBF treatment of Col-0–reared aphids show smaller basal gene expression differences when comparing Col-0- and 35S:EBFS-reared aphids. Only the 1,561 genes with EBF-induced expression changes in Col-0–reared aphids are shown in B–D. The gaps in the center reflect the remaining 9,864 genes on the microarrays whose expression was not significantly altered in comparison 1. (E) Genes significantly regulated (n = 872; P < 0.05) when comparing untreated Col-0- and 35S:EBFS-reared aphids (comparison 4) to those induced by EBF in Col-0–reared aphids (comparison 1). Note that this is a different subset of the total genes on the array than those displayed in B–D.
Two genes previously associated with EBF perception (OBP3; ref. 28; contig 3757; Dataset S1) and synthesis [IPPS/FPS (29–31); contig 912; Dataset S1] were not significantly altered by EBF treatment in either aphid population (comparisons 1 and 3 in Fig. 3A), nor were there significant differences in the expression of these genes in aphid populations before adding EBF (comparison 4 in Fig. 3A). IPPS/FPS activity also produces precursors for other sesquiterpenes, including the acyclic juvenile hormones (JHs; e.g., farnesoic acid and methyl farnesoate), which have important functions in insect development (32) and are structurally similar to EBF (Fig. S3). Interestingly, several genes encoding enzymes involved in JH-related processes were significantly down-regulated by EBF in Col-0–reared aphids. For example, JH acid methyltransferase (contig 3986), a key regulator involved in activation of JH from their precursors and several members of the multiprotein family of JH-binding proteins (i.e., Drosophila takeout; contigs 164, 2,127, and 1,067) are among the most highly suppressed genes in Col-0–reared aphids treated with EBF (Dataset S1). Other genes suppressed by EBF include those involved in dopamine regulation (dopamine N acetyltransferase, contig 654), chemosensory responses (OS-D-like protein, contig 34), odorant-binding (smell impaired 21F, contig 1,338), and pheromone binding (contig 391).
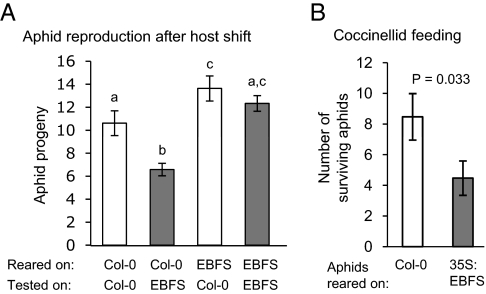
Further experiments were conducted to determine whether EBF habituation has significant fitness consequences for aphids. Whereas Col-0–reared aphids experience a significant reduction in fecundity when transferred onto EBF-producing plants, this did not happen in the converse experiment with habituated aphids transferred to Col-0 plants (Fig. 4A). Progeny production was higher in EBF-habituated aphids feeding on Col-0, suggesting that EBF responses have a fitness cost in the absence of predation (Fig. 4A). However, EBF-habituated aphids produce the same amount of EBF as naive aphids (0.07 ± 0.02 ng and 0.09 ± 0.02 ng EBF per aphid, respectively; P = 0.44, generalized linear model with a Poisson error structure; n = 5; Fig. S4), indicating that EBF production and perception are not coregulated. Experiments with a coccinellid predator (Hippodamia convergens, convergent lady beetle) were conducted to determine whether EBF habituation has fitness consequences in the presence of natural enemies. When aphids feeding from Col-0 plants were exposed to 24 h of coccinellid predation, survival was higher among Col-0–reared aphids than 35S:EBFS-reared aphids (Fig. 4B).
Fig. 4.
Fitness consequences of EBF habituation in M. persicae. (A) Host switch experiments show that EBF-habituated aphids produce more progeny than nonhabituated aphids. Mean ± SEM of n = 11–15; different letters above the bars indicate significant differences (P < 0.05, generalized linear model with a Poisson error distribution). (B) EBF-habituated aphids raised on 35S:EBFS plants suffer more predation from coccinellid beetles than nonhabituated aphids raised on Col-0. Mean ± SE of n = 34; P value determined using a generalized linear model with a Poisson error distribution.
Discussion
Single-genotype aphid colonies rely on visual, tactile, and chemical communication to sense conspecifics in their environment. Alarm pheromone, which for most aphid species consists predominantly of EBF, is a central component of this intraspecies communication (1, 2). Other alarm pheromone components have been studied less extensively, and it remains to be determined whether aphids can become habituated to these compounds. In contrast to previous short-term habituation experiments (20, 23), our study of M. persicae raised for several generations on transgenic 35S:EBFS A. thaliana has produced an EBF-habituated colony that can be used to study ecological dynamics. It is likely that the production of alarm pheromone incurs direct biosynthetic costs or indirect opportunity costs if aphids responding to false alarms spend less time feeding or use more energy as a result of EBF perception. Aphid development and total offspring numbers can also be negatively impacted by EBF perception (13). Consistent with this observation, EBF-habituated aphids, which still synthesize EBF but are less repelled by this compound (Fig. 1 C and E and Fig. S4), have increased fecundity on WT Col-0 plants compared with naive aphids (Fig. 4A). Under certain environmental circumstances, the need for aphid alarm pheromone is likely to be minimal. For instance, reduced predation risk caused by ant tending can lead to reduced EBF responsiveness in aphids (16, 33). M. persicae is not tended by ants, but there may be other environmental conditions that affect EBF-responsiveness within this species.
Continuous alarm pheromone perception in aphids reared on EBFS-expressing A. thaliana would exert a selective pressure and therefore could have resulted in a mutation in a crucial step in EBF perception or a subsequent component of the signal cascade. However, this is not likely to be the case in the current experiments. Even after several months on 35S:EBFS plants, the aphid colony reverted to EBF sensitivity in only a few generations on WT Col-0 (Fig. 2C). The observed delay in acquiring EBF resistance or sensitivity (Fig. 2 B and C) may be a result of the telescoping generations found in aphids. An adult aphid contains not only developing parthenogenetic daughters, but also granddaughters that could be directly or indirectly affected by EBF exposure (34).
Thirty minutes after exposure to synthetic EBF, 15% of the genes in naive aphids showed significant expression changes (x axis, Fig. 3B). Although our DNA microarray does not contain all expressed aphid genes, the 10,478 unigenes are likely a good representation of the more abundant M. persicae transcripts. Therefore, the immediate M. persicae response to EBF is not simply to walk away, but also includes a massive change in gene expression. These transcriptional changes may also lead to the longer-term changes in aphid development and reproduction that have been observed in other studies of aphids (15, 35). Given the large number of gene expression changes in this study and the unknown function of many aphid genes, it is premature to speculate about the role of specific genes in altering aphid behavior. However, it is interesting that EBF-habituated aphids show an almost complete desensitization in the transcriptional response, with only 4% of the previously identified responsive genes showing significant changes in expression (Fig. 3C and Dataset S1). Moreover, if similar directional changes in gene expression were observed, these were generally more pronounced in EBF-responsive aphids (Fig. 3B). The differences in gene expression between EBF-responsive and habituated aphids are not caused by intrinsic expression differences before EBF treatment (comparison 4; or comparison of Fig. 3 B and D). Therefore, most of the expression changes seen after 30 min of EBF exposure are likely transient in nature and different from the altered gene expression in long-term EBF-habituated M. persicae.
Expression of a predicted IPPS/FPS (contig 912), which encodes a known enzyme in EBF biosynthesis, was not altered by EBF treatment. This is consistent with the observation that aphids do not propagate the alarm pheromone signal with further EBF release (11, 12). Given that enzymes involved in activation of JH are suppressed upon EBF treatment (Dataset S1), it is tempting to speculate that these transcriptional changes influence long-term changes in aphid development (ref. 15 and references therein). However, although transgenerational wing formation has been observed in other aphid species (9, 16, 36), our experiments do not provide evidence of this effect in M. persicae. Predator-triggered transgenerational alate formation is a clone-specific response in other aphid species (9). As we are unaware of similar experiments conducted with other M. persicae lineages, we can only confirm that our specific isolate does not show this response under our experimental conditions.
It has been proposed that EBFS expression in crop plants could be used to increase aphid resistance (22). This hypothesis is consistent with the observation that naive aphids avoid EBF-producing A. thaliana and reproduce less well in no-choice experiments (Fig. 1 A and B) (22). However, as these effects disappear when aphids have become habituated to EBF over the course of a few generations (Figs. 1, 2, and 4A), there is unlikely to be a direct agricultural benefit from plant-based EBF synthesis alone. However, the increased predation of EBF-habituated M. persicae by coccinellid beetles (Fig. 4B) indicates that the coupling of transgenic EBFS-expressing crops with aphid predators would be a promising control strategy. Some plants, e.g., Solanum berthaultii, produce EBF at levels comparable to those in the transgenic A. thaliana (22, 37). However, it remains to be determined whether this can result in aphid habituation to EBF, rather than just deterrence, in a more complex natural setting.
Some coccinellids, including H. convergens, and parasitoid wasps use EBF as a kairomone for locating aphid prey (5, 22, 38–41), and might be less effective hunting in a field of EBF-producing plants. It is unknown whether parasitoids and predators become habituated to EBF upon constant stimulation, or whether they would alter their behavior toward EBF as a reliable kairomone if no suitable host/prey is found. However, other volatiles that are produced by plants in response to aphid feeding (42, 43) can permit prey location, even in the absence of EBF as a reliable cue. Plants that naturally produce EBF may receive a similar indirect defensive benefit if aphid herbivores become habituated to their alarm pheromone and are thereby more likely to be eaten by predators.
Methods
Plant and Aphid Rearing.
WT A. thaliana landrace Col-0 was obtained from the Arabidopsis Biological Resource Center (www.arabidopsis.org) and transgenic A. thaliana expressing EBFS from the cauliflower mosaic virus 35S promoter were supplied by J. Pickett (Rothamsted Research, Harpenden, UK). Plants were grown in Conviron growth chambers in 20 × 40-cm nursery flats using Cornell Mix [by weight, 56% peat moss, 35% vermiculite, 4% lime, 4% Osmocoat slow-release fertilizer (Scotts), and 1% Unimix (Peters)] at 23 °C, 60% relative humidity, with a light intensity of 180 μmol m−2 s−1 photosynthetic photon flux density and a 16:8-h light:dark photoperiod.
M. persicae colonies were established with offspring from a single parthenogenetic female of a lineage collected from tobacco by S. Gray (US Department of Agriculture, Ithaca, NY). M. persicae were reared on WT A. thaliana Col-0 or 35S:EBFS plants for at least 6 mo before experimentation with a 16-h day (150 mmol m−2 s−1 at 24 °C) and an 8-h night (19 °C) at 50% relative humidity. For no-choice growth experiments, 3-wk-old plants were infested with one adult aphid and the number of progeny was determined after 7 d of aphid feeding. For choice tests, fully expanded leaves from Col-0 or 35S:EBFS plants with their petioles were inserted into a 1.5-mL microcentrifuge tube containing 500 μL of water and were placed in a Petri dish. Twenty adult aphids were allowed to choose between plant genotypes for 24 h, after which the aphid numbers on each genotype were determined.
Aphid behavior upon exposure to synthetic EBF (Chromadex), 100 ng to 100 μg in 5 μL hexane, and volatiles from Col-0 and 35S:EBFS A. thaliana was observed. An individual fourth instar Col-0- or EBFS-reared larva was placed on a filter paper in the center of a previously unused, sealed 50-mL plastic tube (Fisher Scientific) and its response was monitored visually (Fig. S2A). EBF and hexane solvent-only control samples were introduced with a syringe at one end of the tube. Leaf volatiles were created by crushing a single leaf in a 1-mL syringe (Becton Dickinson) pushing the volatiles into aphid behavior chamber. M. persicae behavior was scored as walking away, attraction to the odor source, or no response. Among all aphids tested, those that were responsive usually acted within the first 30 s after the treatment was administered, a measure of the upper end for the range of the time required for the EBF volatile front to move from the inlet to the center of the tube. Aphids not showing any movement within 2 min were scored as unresponsive.
Wing formation assays were performed similarly to the manner of Kunert et al. (18). In brief, 50 adult Col-0- and EBFS-reared aphids were treated with 1 μg EBF in hexane or hexane alone for 30 min by applying a 5-μL droplet to a filter paper in a 50-mL tube. Subsequently, individual aphids were put on Col-0 plants to give birth to the next generation. Adult aphids were removed after 48 h and wing formation was assessed in the next generation.
To investigate the time course of habituation, fourth-instar individuals from synchronized populations arising from 10 adults from the Col-0 colony were placed on 3-wk-old Col-0- and EBFS-expressing plants. The behavioral responses to 1 μg EBF were assessed in three subsequent generations. Conversely, to test for loss of habituation, EBFS-reared aphids were synchronized and placed on Col-0- and EBFS-expressing plants, and the behavioral response of individual aphids was assessed upon treatment with 1 μg EBF in three subsequent generations.
To determine whether habituated aphids have a higher fitness (fecundity) in the absence of natural enemies, progeny produced over a period of 7 d by a single fourth-instar aphid (Col-0- or EBFS-reared) on Col-0 and 35S:EBFS plants were counted. The other component of aphid fitness (survivorship) was measured in the presence of a predator; one H. convergens adult was allowed to attack 30 fourth-instar M. persicae larva from the Col-0– or EBFS-reared colony confined to a Col-0 plant in a cage. After 24 h of predation, the surviving aphids were counted.
Aphid Transcriptome Analysis.
For DNA microarray experiments, fourth-instar M. persicae in a closed container were exposed to 1 μg EBF in 5 μL hexane or hexane alone for 30 min. Synthesized 60-mer DNA oligonucleotide microarrays for measuring M. persicae gene expression changes were obtained from Agilent (27) and were hybridized and analyzed as described in SI Methods.
EBF Measurements.
Aphid-emitted EBF was measured using a zNOSE 4200 Ultra Fast GC Analyzer (Electronic Sensor Technology) according to the protocol developed by Schwartzberg et al. (44), with minor changes. In brief, one adult Col-0- or EBFS-reared aphid was placed in a 4-mL glass GC-MS vial (Chrom Tech), the stainless steel inlet needle of the zNOSE was inserted through the septum of the vial, and EBF was measured before and after crushing the aphid with a needle. Ambient air replaced the air pulled into the zNOSE during sampling. The surface acoustic wave detector was held at 60 °C. Known concentrations of a synthetic EBF standard, ranging from 0.1 to 10 ng, were sampled for quantification purposes and to verify the identity of the observed peak.
Supplementary Material
Acknowledgments
We thank J. Pickett (Rothamsted Research, Harpenden, UK) for the 35S:EBFS lines; W. Wang, J. Kresovich, and J. Zhao for assisting with microarray experiments; J. Booth and J. Ramsey for help with statistical analysis; and A. Agrawal and J. Thaler for useful comments on an earlier version of the manuscript. This work was funded by National Science Foundation Grant IOS-0718733 and US Department of Agriculture National Research Initiative Grant 2005-35604-15446.
Footnotes
The authors declare no conflict of interest.
Data deposition: The DNA microarray data sets reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo. For a list of accession numbers, see Dataset S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001539107/-/DCSupplemental.
References
- 1.Bowers WS, Nault LR, Webb RE, Dutky SR. Aphid alarm pheromone: Isolation, identification, synthesis. Science. 1972;177:1121–1122. doi: 10.1126/science.177.4054.1121. [DOI] [PubMed] [Google Scholar]
- 2.Pickett JA, Griffiths DC. Composition of aphid alarm pheromones. J Chem Ecol. 1980;6:349–360. [Google Scholar]
- 3.Pickett JA, Wadhams LJ, Woodcock CM, Hardie J. The chemical ecology of aphids. Annu Rev Entomol. 1992;37:67–90. [Google Scholar]
- 4.Dixon AFG. Aphid Ecology. 2nd Ed. London: Chapman and Hall; 1998. [Google Scholar]
- 5.Foster SP, Denholm I, Thompson R, Poppy GM, Powell W. Reduced response of insecticide-resistant aphids and attraction of parasitoids to aphid alarm pheromone: A potential fitness trade-off. Bull Entomol Res. 2005;95:37–46. doi: 10.1079/ber2004336. [DOI] [PubMed] [Google Scholar]
- 6.Mondor EB, Baird DS, Slessor KN, Roitberg BD. Ontogeny of alarm pheromone secretion in pea aphid, Acyrthosiphon pisum. J Chem Ecol. 2000;26:2875–2882. [Google Scholar]
- 7.Montgomery ME, Nault LR. Aphid alarm pheromones - dispersion of Hyadaphis erysimi and Myzus persicae (Hemiptera-Aphididae) Ann Entomol Soc Am. 1977;70:669–672. [Google Scholar]
- 8.Pickett JA, Glinwood RT. Chemical Ecology. In: van Emden H, Harrington R, editors. Aphids as Crop Pests. Wallingford, UK: CAB International; 2007. pp. 235–260. [Google Scholar]
- 9.Schwartzberg EG, Kunert G, Roese USR, Gershenzon J, Weisser WW. Alarm pheromone emission by pea aphid, Acyrthosiphon pisum, clones under predation by lacewing larvae. Entomol Exp Appl. 2008;128:403–409. [Google Scholar]
- 10.Xiangyu J-G, et al. Behavioural response of aphids to the alarm pheromone component (E)-β-farnesene in the field. Physiol Entomol. 2002;27:307–311. [Google Scholar]
- 11.Hatano E, et al. Do aphid colonies amplify their emission of alarm pheromone? J Chem Ecol. 2008;34:1149–1152. doi: 10.1007/s10886-008-9527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verheggen FJ, Mescher MC, Haubruge E, De Moraes CM, Schwartzberg EG. Emission of alarm pheromone in aphids: A non-contagious phenomenon. J Chem Ecol. 2008;34:1146–1148. doi: 10.1007/s10886-008-9528-x. [DOI] [PubMed] [Google Scholar]
- 13.Mondor EB, Roitberg BD. Age-dependent fitness costs of alarm signaling in aphids. Can J Zool. 2003;81:757–762. [Google Scholar]
- 14.Sherman PW. Nepotism and the evolution of alarm calls. Science. 1977;197:1246–1253. doi: 10.1126/science.197.4310.1246. [DOI] [PubMed] [Google Scholar]
- 15.Braendle C, Davis GK, Brisson JA, Stern DL. Wing dimorphism in aphids. Heredity. 2006;97:192–199. doi: 10.1038/sj.hdy.6800863. [DOI] [PubMed] [Google Scholar]
- 16.Dixon AFG, Agarwala BK. Ladybird-induced lifehistory changes in aphids. Proc Biol Sci. 1999;266:1549–1553. [Google Scholar]
- 17.Kunert G, Schmoock-Ortlepp K, Reissmann U, Creutzburg S, Weisser WW. The influence of natural enemies on wing induction in Aphis fabae and Megoura viciae (Hemiptera: Aphididae) Bull Entomol Res. 2008;98:59–62. doi: 10.1017/S0007485307005391. [DOI] [PubMed] [Google Scholar]
- 18.Kunert G, Otto S, Röse USR, Gershenzon J, Weisser WW. Alarm pheromone mediates production of winged dispersal morphs in aphids. Ecol Lett. 2005;8:596–603. [Google Scholar]
- 19.Podjasek JO, Bosnjak LM, Brooker DJ, Mondor EB. Alarm pheromone induces a transgenerational wing polyphenism in the pea aphid, Acyrthosiphon pisum. Can J Zool. 2005;83:1138–1141. [Google Scholar]
- 20.Petrescu AS, Mondor EB, Roitberg BD. Subversion of alarm communication: Do plants habituate aphids to their own alarm signals? Can J Zool. 2001;79:737–740. [Google Scholar]
- 21.Hatano E, Kunert G, Michaud JP, Weisser WW. Chemical cues mediating aphid location by natural enemies. Eur J Entomol. 2009;105:797–806. [Google Scholar]
- 22.Beale MH, et al. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior. Proc Natl Acad Sci USA. 2006;103:10509–10513. doi: 10.1073/pnas.0603998103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Agamy FM, Haynes KF. Susceptibility of the pea aphid (Homoptera: Aphididae) to an insecticide and a predator in the presence of synthetic aphid alarm pheromone. Ann Entomol Soc Am. 1992;85:794–798. [Google Scholar]
- 24.Braendle C, Weisser WW. Variation in escape behavior of red and green clones of the pea aphid. J Insect Behav. 2001;14:497–509. [Google Scholar]
- 25.Losey JE, Denno RF. Positive predator-predator interactions: Enhanced predation rates and synergistic suppression of aphid populations. Ecology. 1998;79:2143–2152. [Google Scholar]
- 26.Muller FP. Differential alarm pheromone responses between strains of the aphid Acyrthosiphon pisum. Entomol Exp Appl. 1983;34:347–348. [Google Scholar]
- 27.Ramsey JS, et al. Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics. 2007;8:423. doi: 10.1186/1471-2164-8-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao H, et al. Discrimination of alarm pheromone (E)-beta-farnesene by aphid odorant-binding proteins. Insect Biochem Mol Biol. 2009;39:414–419. doi: 10.1016/j.ibmb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Lewis MJ, Prosser IM, Mohib A, Field LM. Cloning and characterisation of a prenyltransferase from the aphid Myzus persicae with potential involvement in alarm pheromone biosynthesis. Insect Mol Biol. 2008;17:437–443. doi: 10.1111/j.1365-2583.2008.00815.x. [DOI] [PubMed] [Google Scholar]
- 30.Vandermoten S, et al. Characterization of a novel aphid prenyltransferase displaying dual geranyl/farnesyl diphosphate synthase activity. FEBS Lett. 2008;582:1928–1934. doi: 10.1016/j.febslet.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YL, Li ZX. Two different farnesyl diphosphate synthase genes exist in the genome of the green peach aphid, Myzus persicae. Genome. 2008;51:501–510. doi: 10.1139/G08-037. [DOI] [PubMed] [Google Scholar]
- 32.Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondor EB, Rosenheim JA, Addicott JF. Mutualist-induced transgenerational polyphenisms in cotton aphid populations. Funct Ecol. 2008;22:157–162. [Google Scholar]
- 34.Mousseau TA, Dingle H. Maternal effects in insect life histories. Annu Rev Entomol. 1991;36:511–534. [Google Scholar]
- 35.Schwartzberg EG, Kunert G, Westerlund SA, Hoffmann KH, Weisser WW. Juvenile hormone titres and winged offspring production do not correlate in the pea aphid, Acyrthosiphon pisum. J Insect Physiol. 2008;54:1332–1336. doi: 10.1016/j.jinsphys.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Kunert G, Weisser WW. The interplay between density- and trait-mediated effects in predator-prey interactions: A case study in aphid wing polymorphism. Oecologia. 2003;135:304–312. doi: 10.1007/s00442-003-1185-8. [DOI] [PubMed] [Google Scholar]
- 37.Gibson JA, Pickett JA. Wild potato repels aphids by release of aphid alarm pheromone. Nature. 1983;302:608–609. [Google Scholar]
- 38.Acar EB, Medina JC, Lee ML, Booth GM. Olfactory behavior of convergent lady beetles (Coleoptera: Coccinellidae) to alarm pheromone of green peach aphid (Hemiptera: Aphididae) Can Entomol. 2001;133:389–397. [Google Scholar]
- 39.Al Abassi S, et al. Response of the seven-spot ladybird to an aphid alarm pheromone and an alarm pheromone inhibitor is mediated by paired olfactory cells. J Chem Ecol. 2000;26:1765–1771. [Google Scholar]
- 40.Francis F, Lognay G, Haubruge E. Olfactory responses to aphid and host plant volatile releases: (E)-beta-farnesene an effective kairomone for the predator Adalia bipunctata. J Chem Ecol. 2004;30:741–755. doi: 10.1023/b:joec.0000028429.13413.a2. [DOI] [PubMed] [Google Scholar]
- 41.Micha SG, Wyss U. The importance of plant odours for host searching of Aphidius uzbekistanicus (Hymenoptera, Aphidiidae), a parasitoid of the grain aphid (Sitobion avenae) Gesunde Pflanzen. 1995;47:300–307. [Google Scholar]
- 42.Du YJ, et al. Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol. 1998;24:1355–1368. [Google Scholar]
- 43.Pareja M, Moraes MCB, Clark SJ, Birkett MA, Powell W. Response of the aphid parasitoid Aphidius funebris to volatiles from undamaged and aphid-infested Centaurea nigra. J Chem Ecol. 2007;33:695–710. doi: 10.1007/s10886-007-9260-y. [DOI] [PubMed] [Google Scholar]
- 44.Schwartzberg EG, et al. Real-time analysis of alarm pheromone emission by the pea aphid (Acyrthosiphon pisum) under predation. J Chem Ecol. 2008;34:76–81. doi: 10.1007/s10886-007-9397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.