Abstract
In the gustatory systems of mammals and flies, different populations of sensory cells recognize different taste modalities, such that there are cells that respond selectively to sugars and others to bitter compounds. This organization readily allows animals to distinguish compounds of different modalities but may limit the ability to distinguish compounds within one taste modality. Here, we developed a behavioral paradigm in Drosophila melanogaster to evaluate directly the tastes that a fly distinguishes. These studies reveal that flies do not discriminate among different sugars, or among different bitter compounds, based on chemical identity. Instead, flies show a limited ability to distinguish compounds within a modality based on intensity or palatability. Taste associative learning, similar to olfactory learning, requires the mushroom bodies, suggesting fundamental similarities in brain mechanisms underlying behavioral plasticity. Overall, these studies provide insight into the discriminative capacity of the Drosophila gustatory system and the modulation of taste behavior.
Keywords: chemosensory, gustatory, neurobiology
The gustatory system allows animals to detect chemical compounds in the environment and determine their value as potential food sources. To make this assessment, animals detect two different features of taste stimuli with the gustatory system: the concentration and the quality of a taste compound. In humans, taste concentration is perceived as intensity and taste quality as a component of flavor. Determining how these two features of taste stimuli are encoded by the nervous system and used to direct behavior is central for the neural basis of taste perception.
Mammals are thought to detect five different general taste qualities or modalities: sugars, bitter compounds, salt, acids, and amino acids (1). Each taste modality is detected by a unique taste cell population in the periphery, such that the activation of different taste cells provides a simple mechanism to encode modality. In addition, taste cells show dose-dependent activation, providing the potential to encode different concentrations.
Although taste cell activity readily allows for modality and concentration discrimination, does it allow for finer discrimination of individual taste compounds? Animals distinguish sugars from bitter compounds, but do they distinguish compounds within a single modality? In principle, different sugars could activate slightly different taste cell populations or activate the same population with different temporal properties, and these differences could be exploited to allow for the discrimination of individual compounds. Alternatively, different taste compounds could activate the same taste cell population with a different efficacy and lead to a perceived difference in sugar concentration or intensity rather than quality.
The Drosophila gustatory system provides an attractive model for studies of taste discrimination because it is an experimentally tractable system that retains similarities to mammalian taste. Like mammals, Drosophila distinguishes different taste concentrations, with dose-dependent preference and avoidance behaviors. These flies also distinguish a few taste qualities, including sugar and bitter tastes, and possess modality-specific, peripheral taste cells (i.e., sugar-selective and bitter-selective neurons) (2–4). Members of a large family of gustatory receptor genes (GRs) are expressed in taste sensory neurons and mediate the detection of sugars and bitter compounds (2, 3, 5–7). Expression patterns of GRs, based largely on transgenic Gal4 expression studies, suggest that different GRs are expressed in overlapping but nonidentical subsets of sugar- and bitter-sensing neurons (2, 3, 8). In addition, electrophysiological studies suggest heterogeneity among the responses of individual sugar- or bitter-sensing cells (9–11). Thus, there is evidence for diversity among the peripheral cell types that detect sugars or bitter compounds in Drosophila. This organization provides the potential for different taste cell populations to be activated in response to different compounds within a taste modality and the possibility for intramodality discrimination.
Here, we developed a behavioral paradigm for taste conditioning to examine the tastes that a fly can discriminate. Our results provide evidence for intensity-dependent discrimination but not for discrimination of individual compounds within a modality independent of concentration, highlighting similarities with mammalian taste and differences with fly olfaction.
Results
A Paradigm to Examine Taste Discrimination.
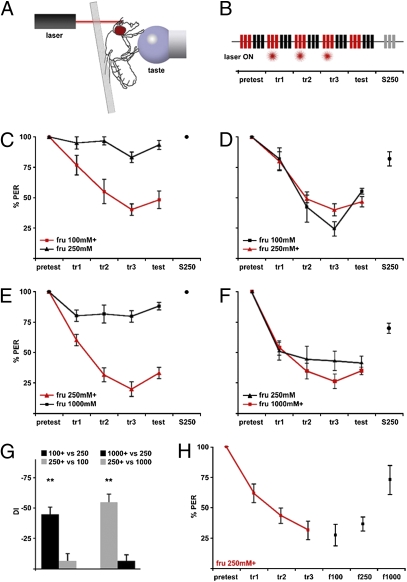
To test directly whether flies discriminate compounds within a taste modality, we performed behavioral experiments in which a fly was trained to associate a taste compound with an aversive stimulus, and the ability of other compounds to elicit aversion was examined for taste generalization or discrimination. The proboscis extension reflex (PER) is a rapid, robust, and quantitative taste behavior in the fly, in which stimulation on the leg with sugars elicits proboscis extension (12). To study taste discrimination, we modified the PER by pairing delivery of the taste stimulus with a noxious IR laser pulse (13) (Fig. 1A). Briefly, flies were tested for the PER to two taste compounds before, during, and after training in trial blocks of three stimulations per compound (Fig. 1B). To minimize variation, flies were used if they showed 100% PER pretraining. The training consisted of three blocks of each compound delivered to the leg, with one compound [the conditioned stimulus (CS)] paired with a brief laser pulse to the antennae [the unconditioned stimulus (US)]. In the test period, the fly received the two taste compounds without laser treatment to evaluate changes in behavior. A significant advantage of this paradigm, in contrast to mammalian models, is that detection of the taste compound is separated from ingestion. Thus, taste delivery on the leg provides no reward (sugar intake) or punishment (bitter intake) and does not alter the animal's satiety state or metabolism.
Fig. 1.
Flies discriminate higher sugar concentrations. (A) For taste conditioning, flies were mounted on a glass slide. Compounds (taste) were delivered onto tarsal segments. An IR beam (laser) was focused onto antennae. (B) Flies were tested for proboscis extension on stimulation (bars) with two taste compounds (red and black bars) after associating one compound with heat (laser). The protocol included a pretest, three training trials (tr1–3), and a test in blocks of three stimulations per compound. S250 equals 250 mM sucrose at the end of the experiment. (C–F) Plots of proboscis extension before (pretest), during (tr1–3), and after training (test). Responses for the compound paired with heat are shown in red (labeled + in legend), and those for the unpaired compound are shown in black. Each plot represents four to five experiments, with five flies per experiment. (C) Flies reduced the PER to 100 mM fructose (fru) but still extended to the unpunished 250 mM fructose. (D) Flies reduced the PER to 250 mM fructose paired with heat and the unpunished 100 mM fructose. (E) Flies conditioned to avoid 250 mM fructose still extended to 1 M fructose. (F) Flies showed conditioned aversion to 1 M fructose and the unpunished 250 mM fructose. (G) Plots of the DI for experiments shown in C–F [**P < 0.01, Student's t test (one population)]. (H) Fructose (250 mM) was paired with laser for three training blocks, and 100 mM (f100), 250 mM (f250), and 1,000 mM (f1,000) fructose were tested after training. The f100 and f250 responses are not statistically different; the f1,000 response is different from the f100 and f250 responses [P < 0.05, Student's t test (two populations)].
This paradigm allows us to test whether flies associate taste compounds with a noxious stimulus and whether this association is compound-specific. If flies associate the taste compound with a noxious stimulus, the PER should decrease during training and test periods. If the PER decreases only to the compound paired with the heat and not to the unpaired compound, this would argue that flies discriminate the two tested compounds. We tested whether flies discriminate different concentrations and compounds within a taste modality.
Flies Show Concentration-Dependent Taste Associations.
The simplest two taste stimuli that may be discriminated are two concentrations of a single compound. We tested flies’ ability to discriminate two fructose concentrations (100 and 250 mM) after pairing one concentration with heat. The Discrimination Index (DI) was calculated for the test response as the difference between the response to the compound associated with heat and the nonassociated compound (%PERtaste + US − %PERtaste − US). When 100 mM fructose was paired with heat, flies significantly reduced the PER to this fructose concentration yet still extended to the unpaired 250 mM fructose stimulation (Fig. 1 C and G). Thus, flies may be conditioned to decrease acceptance to fructose, and this conditioned behavior does not generalize to a higher fructose concentration.
In the reciprocal experiment, when 250 mM fructose was paired with heat, flies decreased acceptance to 250 mM fructose, again demonstrating conditioned behavior (Fig. 1 D and G). However, the PER was also reduced to 100 mM fructose. The fact that flies trained to avoid 250 mM fructose now also avoided 100 mM fructose suggests that they generalize experiences with higher concentrations to lower concentrations.
An alternative possibility is that flies in which 250 mM fructose was paired with an aversive stimulus simply become unresponsive to taste compounds. Therefore, flies were trained with 250 and 1,000 mM fructose. When 250 mM fructose was paired with heat, flies displayed a reduced PER to 250 mM fructose but a normal PER to 1,000 mM fructose (Fig. 1 E and G), demonstrating that the flies are taste-responsive and that the conditioned behavior does not generalize to the higher concentration. When 1,000 mM fructose was punished, flies reduced the PER to both concentrations (Fig. 1 F and G). These data suggest that flies have a simple taste-learning rule: Avoid the punished substance and lower concentrations but accept higher concentrations.
To test directly whether flies become conditioned to avoid the punished concentration as well as lower but not higher concentrations, flies were trained with 250 mM fructose paired with heat in three training blocks and then tested with 100, 250, and 1,000 mM fructose. Trained flies reduced the PER to 100 and 250 mM fructose but still extended to 1,000 mM fructose (Fig. 1H). These experiments reveal that flies show concentration-dependent taste associations, with concentrations higher than the punished concentration deemed acceptable and lower concentrations deemed unacceptable.
Evaluation of Innate Preferences of Different Sugars.
Drosophila melanogaster recognizes several sugars, including fructose, glucose, and maltose, and flies’ innate preferences to these compounds differ (2, 3, 6, 8, 14, 15). Do flies discriminate different sugars based on perceived concentration differences (i.e., one compound has a higher stimulation efficacy) or based on qualitative differences of individual compounds (i.e., trehalose is processed differently from maltose)? To examine whether the quality of a taste compound is discriminated in addition to its concentration, it was necessary to evaluate concentration-dependent preferences for different sugars.
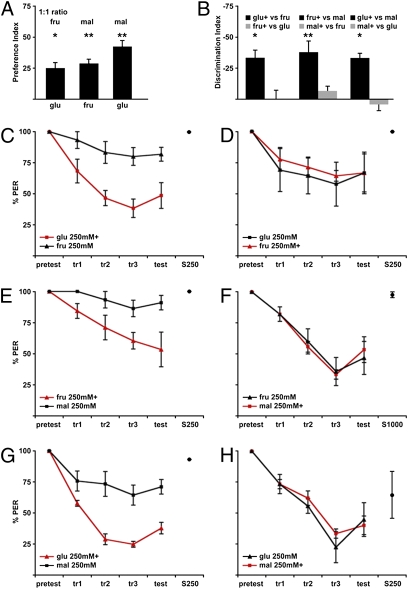
We determined the relative preferences for fructose, glucose, and maltose. In initial experiments, the PER was used to evaluate innate preference. A dose–response curve for fructose, glucose, and maltose revealed that the probability of extension was similar for the different sugars (Fig. S1). As a more sensitive evaluation of relative preferences, we carried out a two-choice drinking assay, which has the advantage of allowing a direct comparison of the ingestion of two taste solutions presented simultaneously (16). This assay measures relative ingestion as an indirect measure of relative preference; however, previous studies have shown that there is a strong correlation between frequency of proboscis extension and ingestion (17). At 100 mM concentrations, flies preferred maltose over fructose and fructose over glucose (Fig. 2A). Informed about the relative preferences for different sugars, we examined the ability of the fly to discriminate different compounds.
Fig. 2.
Limited discrimination of different sugars. (A) Two-choice drinking assay was used to monitor the innate preference of fructose (fru), glucose (glu), and maltose (mal). The graph shows the Preference Index for different sugar pairs (100 mM, 1:1 ratio). Each bar represents one sugar pair (i.e., the first bar shows the choice between glucose and fructose, with a preference toward fructose) (n = 3–4 experiments, ~40 flies per experiment) [*P < 0.05, **P < 0.01, Student's t test (one population)]. (B) Plots of the DI for experiments shown in C–H (n = 3–4 experiments, 5 flies per experiment) [*P < 0.05, **P < 0.01, Student's t test (one population)]. (C–H) Plots of proboscis extension before, during, and after training for different sugar pairs. Each plot represents three to four experiments, with five flies per experiment. (C) Flies showed conditioned aversion to 250 mM glucose but still extended to the unpunished 250 mM fructose. (D) Flies reduced the PER to 250 mM fructose as well as to the unpunished 250 mM glucose. With 250 mM fructose and 250 mM maltose, flies showed selective aversion to 250 mM fructose paired with heat (E) and generalized aversion when 250 mM maltose was paired with heat (F). With 250 mM glucose and 250 mM maltose, flies showed selective aversion to 250 mM glucose paired with heat (G) and generalized aversion when 250 mM maltose was paired with heat (H).
No Evidence for Quality Discrimination of Different Sugars.
To examine whether flies can distinguish sugars based on chemical quality, taste discrimination experiments were performed with different sugar pairs. If flies can discriminate different taste compounds independent of concentration, the pairing of one sugar with a noxious stimulus should not affect proboscis extension to the unpaired sugar. Alternatively, if flies can discriminate taste intensity but not taste quality, pairing of one sugar with a noxious stimulus should produce discrimination of higher perceived concentrations but not lower perceived concentrations, similar to the concentration-dependent association studies.
Different sugar pairs were tested in the taste association paradigm. When 250 mM fructose and 250 mM glucose were used, flies reduced the PER to glucose paired with heat but not to the unpunished fructose (Fig. 2 B and C). However, when 250 mM fructose was paired with heat, the PER was reduced for both sugars (Fig. 2 B and D). Because fructose is preferred to glucose, these results are consistent with the notion that flies generalize aversion to lower intensities but not higher intensities. Similarly, pairings of 250 mM fructose vs. 250 mM maltose (Fig. 2 B, E, and F) and 250 mM glucose vs. 250 mM maltose (Fig. 2 B, G, and H) produced results most consistent with intensity-dependent discrimination and an innate preference of maltose over fructose and fructose over glucose. Flies showed discrimination only when the less preferred sugar was punished but an equal reduction in the PER when the more preferred sugar was paired with heat.
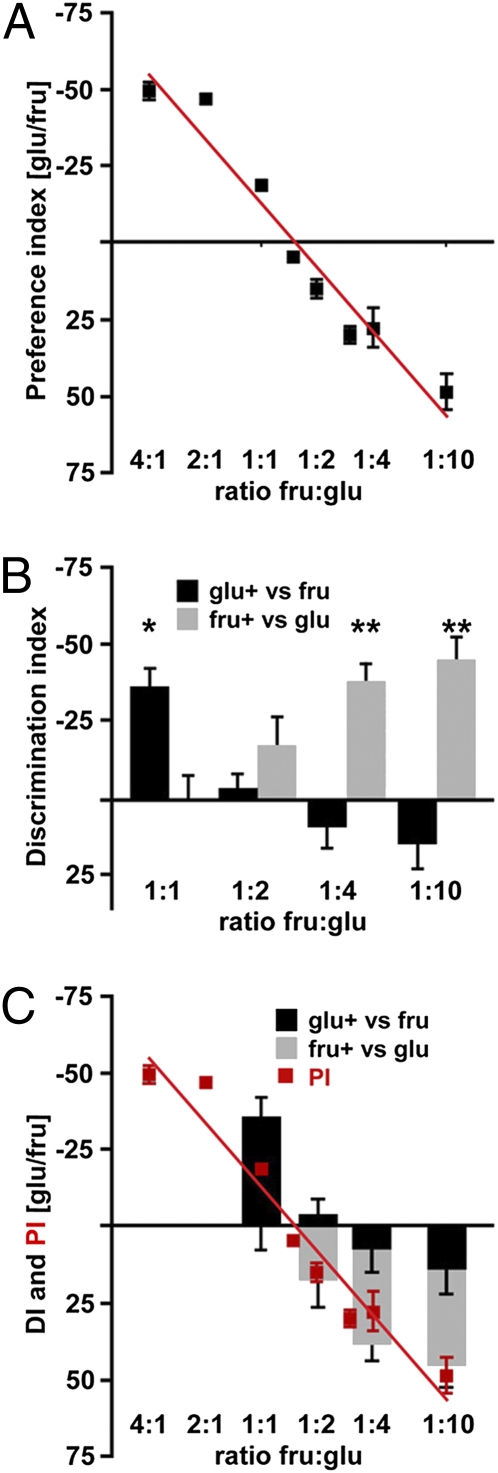
To examine the relationship between concentration-dependent preference and discriminative associations more extensively, different concentration ratios of fructose and glucose were paired in the two-choice preference assay and in the association paradigm. As expected, a plot of the ingestion of the two sugars vs. concentration ratio revealed concentration-dependent preferences, with an equal preference for glucose and fructose at a ratio of ≈1:1.5 fructose/glucose (Fig. 3A). Discrimination was also examined over a range of concentrations (Fig. 3B). The results illustrate that the ability to discriminate fructose from glucose is concentration-dependent, with flies showing selective aversion to glucose at 1:1 fructose/glucose and selective aversion to fructose when glucose concentration exceeds 1:4 fructose/glucose. The switching point of ≈1:2, where flies do not discriminate glucose from fructose, is similar to the ratio for equivalent innate preference for glucose and fructose (Fig. 3C). Thus, the ability to discriminate fructose from glucose in this assay can be entirely accounted for by intensity rather than by the perception of different compounds within a modality. These studies are most consistent with the notion that flies distinguish sweet and sweeter but not the identity of individual sugars independent of intensity.
Fig. 3.
Discrimination of fructose and glucose varies with concentration. (A) Two-choice drinking assay monitored the innate preference of fructose (fru) and glucose (glu). The graph shows the Preference Index for different concentration ratios (n = 4–8 experiments, ~40 flies per experiment). (B) Taste discrimination varies with concentration. Taste conditioning experiments were performed and the DI was determined for several concentration ratios. Black bars depict DI for glucose paired with heat, and gray bars denote DI for fructose paired with heat (n = 3–4 experiments, 5 flies per experiment) [*P < 0.05, **P < 0.01, Student's t test (one population)]. (C) DI in B was plotted as negative when the score reflected a selective PER decrease after punishing glucose and as positive when the score reflected a selective PER decrease from punishing fructose. Overlain is the Preference Index (PI) in red (same as in A).
No Evidence for Quality Discrimination of Different Bitter Compounds.
Flies, like humans, recognize a great diversity of bitter compounds that have unrelated molecular structures and use many different receptor genes for bitter taste detection, which might afford discrimination of bitter compounds based on molecular features rather than concentration (2, 3, 7, 18). Therefore, the conditioning paradigm was used to examine the ability to discriminate bitter compounds.
We first evaluated the effect of concentration by performing conditioning experiments with different concentrations of the same bitter compound. Because bitter compounds do not elicit proboscis extension, 100 mM sucrose was included and flies were selected that showed 100% PER pretraining. In control experiments, there was no reduction in the PER when bitter compounds were applied in the paradigm without laser treatment (Fig. S2). When 2 mM quinine was paired with heat, flies avoided 2 mM quinine but still extended to 0.5 mM quinine (Fig. S3). When 0.5 mM quinine was punished, flies avoided both 0.5 and 2 mM quinine (Fig. S3). Similarly, 2 and 4 mM quinine produced concentration-dependent associations (Fig. S3). This demonstrates that flies show concentration-dependent bitter associations, with associations generalizing to a less preferred but not to a more preferred concentration. For bitter compounds, the more preferred concentration is the lower concentration, whereas for sugars, it is the higher concentration. Thus, flies apparently evaluate palatability rather than concentration per se in taste conditioning.
As a test of whether flies evaluate palatability, we trained flies to avoid 25 mM sucrose and then examined their response to 100 mM sucrose with or without 0.5 mM denatonium. This denatonium concentration does not affect the response of flies without conditioning (Fig. S2). Flies trained to avoid 25 mM sucrose still extended to 100 mM sucrose but did not extend when denatonium was included (Fig. S3). Thus, the fly's probability of extension changed not based simply on sugar concentration but rather on the palatability of the mixture relative to the punished concentration. This experiment argues that flies evaluate relative palatability rather than concentration.
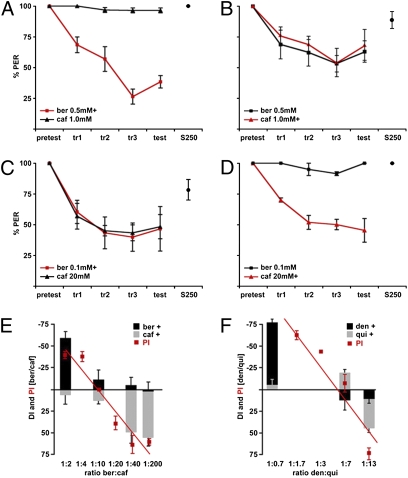
Do flies discriminate different bitter compounds independent of concentration? To test this, conditioning experiments were done with two bitter compounds, berberine and caffeine, at different concentrations. At concentrations of 0.5 mM berberine and 1 mM caffeine (1:2 ratio), flies showed selective aversion to berberine paired with heat but generalized aversion when caffeine was punished (Fig. 4 A and B). At 0.5 mM berberine and 5 mM caffeine (1:10 ratio), flies avoided both berberine and caffeine equally, regardless of which compound was punished (Fig. S4). At 0.1 mM berberine and 20 mM caffeine (1:200 ratio), flies selectively avoided caffeine paired with heat and avoided both compounds when berberine was punished (Fig. 4 C and D). These experiments reveal intensity-dependent taste associations with bitter compounds, similar to what was seen with sugars.
Fig. 4.
Limited discrimination of different bitter compounds. (A) Flies reduced proboscis extension to 0.5 mM berberine (ber) but still extended to the unpunished 1 mM caffeine (caf). (B) Flies reduced the PER to both the punished 1 mM caffeine and the unpunished 0.5 mM berberine. (C) Flies reduced the PER to both the punished 0.1 mM berberine and the unpunished 20 mM caffeine. (D) Flies trained to avoid 20 mM caffeine still extended to 0.1 mM berberine. (E) DI was determined for the taste conditioning experiments. The DI was plotted as negative when the PER selectively decreased after punishing berberine and as positive when the PER decreased from punishing caffeine. Overlain is the Preference Index (PI, in red), as determined by the two-choice drinking assay (n = 6–27 experiments, ~40 flies per experiment). (F) Taste conditioning experiments were performed for quinine (qui) and denatonium (den) pairs at three different concentration ratios (1:0.7, 1:7, and 1:13). Conditioning experiments were not performed for concentration ratios of 1:1.7 and 1:3. The DI was plotted as negative when punishing denatonium and as positive when punishing quinine (n = 4 experiments, 5 flies per experiment). The Preference Index (PI, in red) is overlain (n = 9–21 experiments, ~40 flies per experiment).
We next compared taste discrimination with innate aversion of different bitter compounds. Avoidance of bitter compounds was examined using two-choice taste assays for pairs of berberine and caffeine and denatonium and quinine. For berberine and caffeine, comparing discrimination and innate preference (Fig. 4E) reveals that the reversal point for both selective aversion and innate avoidance is ≈1:10. For denatonium and quinine, equivalent preference occurred at ~1:7 denatonium/quinine (Fig. 4F). Similarly, discrimination between denatonium and quinine shows a concentration-dependent switch at ~1:7 (Fig. 4F). These results demonstrate a switching of selective discrimination from one bitter compound to the other in a concentration-dependent manner, arguing that flies discriminate different bitter compounds based on relative intensities and not chemical identity.
Taste Conditioning Is Mediated by the Mushroom Bodies.
To gain insight into brain regions underlying taste conditioning, we examined whether this associative learning paradigm utilizes the mushroom bodies (MBs) implicated in other forms of learning in insects (19–22).
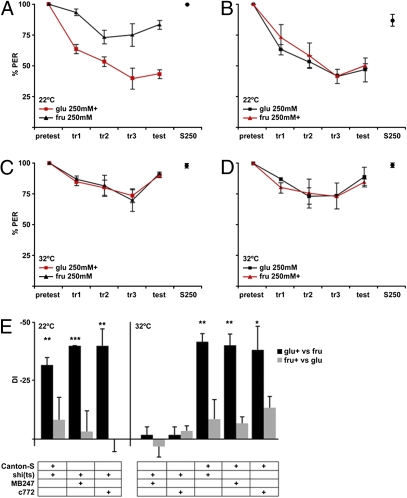
Taste conditioning experiments were performed on flies in which the output of the MBs was conditionally blocked, using MB-Gal4 lines to express temperature-sensitive shibire, a dominant-negative dynamin [UAS-shi(ts)] (23). Two MB-Gal4 lines, c772 and MB247, were used because conditional silencing experiments with these lines impairs olfactory associative learning (24, 25). MB247 is expressed fairly specifically in the MBs, whereas c772 is more broadly expressed (26). At permissive temperature (22 °C), all flies tested on 250 mM glucose and 250 mM fructose showed conditioned aversion to taste compounds, with selective aversion to glucose associated with heat (Fig. 5 A and E) and generalized aversion when fructose was punished (Fig. 5 B and E). At restrictive temperature (32 °C), parental lines performed similarly to permissive temperature (Fig. 5E). However, two MB-Gal4 lines, c772 and MB247, expressing shi(ts) did not show conditioned aversion at 32 °C (Fig. 5 C–E). During the training period, these flies showed a slight decrease in the response, suggesting that they were not indifferent to the laser punishment. During the test, these flies did not show decreased proboscis extension to the punished (or unpunished) sugar; they continued to extend to both sugars. These data reveal that the decreased responsiveness to taste compounds paired with heat is specific and requires the MBs. This argues that there must be a neural pathway from the MBs that impinges on taste behavior and that the MBs participate in multiple sensory associations.
Fig. 5.
MBs are required for taste associations. (A and B) Flies with MB247-Gal4 and temperature-sensitive shibire [UAS-shi(ts)] display taste associations at permissive temperature (22 °C). Similar to WT flies, they show selective aversion when 250 mM glucose (glu) is paired with laser (A) and generalize aversion when 250 mM fructose (fru) is paired with laser (B). (C and D) At restrictive temperature (32 °C), MB247-Gal4 flies with UAS-shi(ts) fail to associate taste compounds with heat. The test response after training is not different for the punished or unpunished compound when either 250 mM glucose (C) or 250 mM fructose (D) is paired with laser heat. (E) Summary of DI for taste conditioning experiments with 250 mM glucose and 250 mM fructose for various genotypes (n = 4 experiments, 5 flies per experiment) [*P < 0.05, **P < 0.01, ***P < 0.001, Student's t test (one population)].
Discussion
Taste behaviors are robust and innate, with sugars mediating acceptance and bitters mediating avoidance. Here, we show that taste behaviors may be modified by conditioning, because sugars (or sugars plus bitter compounds) become less acceptable when paired with a noxious stimulus. This conditioning assay provided the opportunity to examine the tastes that a fly discriminates.
The ability to associate a taste compound with a unique behavior allowed us to test whether learned associations are based on intensity or quality. In the simplest scenario, when two concentrations of the same sugar were tested, flies learned to avoid the concentration paired with heat. This association generalized to lower concentrations (less palatable) but not to higher concentrations (more palatable). Experiments testing different sugar pairs or bitter pairs showed similar results, with flies generalizing associations to less palatable compounds but not to more palatable compounds. These results argue that flies discriminate compounds based on intensity but not on chemical identity. Although we cannot rule out discrimination based on chemical structure with other taste compounds or a different behavioral assay, our data support the notion that the salient feature flies discriminate is palatability or “sweeter.”
The limited discrimination of the fly taste system is quite different from the discrimination seen in the fly olfactory system. Several olfactory associative learning paradigms have demonstrated exquisite discrimination of different odors (reviewed in 19–22). Flies associate an odor paired with a reward or a punishment, and, in general, this association is specific to the paired odor. Both the identity of the individual compound and the concentration may be used to distinguish an odor, with concentration discrimination occurring in situations in which quality information is not accessible to the fly (27, 28). These studies argue that the olfactory system distinguishes both intensity and quality for thousands of odors.
The fly taste system instead resembles the mammalian taste system, with the capacity to discriminate a few taste modalities but not compounds within a modality. In mammals, quality- and intensity-based discrimination has been described (29, 30), but experiments in which taste compounds were compared over a concentration range generally argue against intramodality discrimination (31–33). Why would taste systems evolve that allow animals to detect many compounds but maintain a limited ability to discriminate among them? The primary function of taste is to ensure that animals eat foods that are caloric and avoid foods that are toxic. To achieve this, animals need not discriminate between different compounds but rather simply accept carbohydrates and reject toxins. Thus, animals categorize taste compounds into a few modalities that direct appropriate behavior, but this categorization limits the ability to distinguish individual compounds.
As a first step toward a neural basis for taste associations, we tested the function of the MBs because they are critical for olfactory associations in Drosophila and process information from multiple senses in other insects (19–22, 34, 35). Our studies clearly demonstrate that the MBs are required for conditioned taste aversion and reinforce the notion that the MBs are critical sites for plasticity. How the MBs impinge on taste detection or taste behavior is unknown. However, these studies argue that neural pathways exist from the MBs to taste behaviors and open the door for future studies of taste neural circuitry. In addition, taste associative learning provides a powerful system for comparative studies with other Drosophila learning models.
Materials and Methods
Experimental Animals.
Experiments were performed on Canton-S flies (Wuerzburg). Two MB-Gal4 lines, MB247 (36) and c772 (37), and temperature-sensitive shibire [UAS-shi(ts)1] (23) were used.
Taste Conditioning Paradigm.
Approximately 100 7- to 14-d-old flies were starved in vials with wet paper for 24 h for sugar discrimination and 48 h for bitter discrimination. The survival of Canton-S flies vs. starvation period is shown in Fig. S5. Flies were mounted on a glass slide, with head and legs unconstrained, and placed into a humidified box overnight.
The taste conditioning paradigm used the PER and its suppression attributable to laser heat. Laser heat has been used as an effective punishment in visual learning paradigms (38), because flies avoid noxious heat stimuli (39). The laser was custom-built with an IR laser module (808 nm, 3.2-V dc, 150 mW; AixiZ Service and International LLC). A voltage regulator was used to adjust the power output, with 1.2 V to position the beam and 2.35 V for punishment. The laser beam was targeted onto antennae.
Conditioning experiments were carried out on single flies. Taste compounds were delivered to leg tarsi. Before experiments, flies were satiated with water. Only flies that responded 100% pretest and to the first exposure during the training were included. Flies were trained in three blocks (training 1–3), each consisting of three exposures to taste A (CS+, paired with laser) and three exposures to taste B (CS−, no laser). A laser pulse was delivered for 0.5 s during every presentation of taste A. After training, flies were tested for the PER for tastes A and B without the laser (test). The experiments illustrated in Fig. S3 C and D and Fig. 4F (1:7 ratio) were performed blinded to the compound delivered, with results similar to those of other experiments. Other experiments were not performed blinded.
Two-Choice Taste Preference Assay.
To test naive preference, we modified a volumetric drinking assay (16). Flies were allowed to drink two solutions presented in capillaries attached to an empty vial. Approximately 30–60 flies were starved for 24 h (sugars) or 48 h (bitter compounds) and then put into the vial for up to 12 h. The volume consumed was measured as the length of liquid missing from the capillary minus the length missing attributable to evaporation. Bitter substances were diluted in 100 mM sucrose. Taste compounds were mixed with Allura red food dye (red no. 40; FD&C) in a 5-mg/100-mL dilution for better visibility.
Data Calculations.
The DI was calculated as the difference between the PER for CS+ and CS− during the test. This is equivalent to the half-score in olfactory conditioning experiments (40). This calculation is more appropriate than the Learning Index when the response is different depending on the compound that is punished (27, 40). The Preference Index was calculated as: (volume consumed from capillary 1 − volume consumed from capillary 2)/total volume consumed.
Statistical Analysis.
Values for all assays are reported as mean ± SEM. One-population or two-population Student's t tests were performed assuming equal variance. Conditioning experiments were repeated at least three times on 3 different d with five flies per experiment.
Supplementary Material
Acknowledgments
We thank the Scott laboratory, Heisenberg laboratory, Alex Keene, and Jens Rister for comments and discussion. We thank Hans Kaderschabek and Konrad Öchsner for help with the behavioral setup. This research was supported by Grant 1R01DC006252 from the National Institute on Deafness and Other Communication Disorders and by the John Merck Scholars Program (K.S.). K.S. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009318107/-/DCSupplemental.
References
- 1.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: From mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Marella S, et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 6.Chyb S, Dahanukar A, Wickens A, Carlson JR. Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14526–14530. doi: 10.1073/pnas.2135339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meunier N, Ferveur JF, Marion-Poll F. Sex-specific non-pheromonal taste receptors in Drosophila. Curr Biol. 2000;10:1583–1586. doi: 10.1016/s0960-9822(00)00860-5. [DOI] [PubMed] [Google Scholar]
- 10.Hiroi M, Marion-Poll F, Tanimura T. Differentiated response to sugars among labellar chemosensilla in Drosophila. Zoolog Sci. 2002;19:1009–1018. doi: 10.2108/zsj.19.1009. [DOI] [PubMed] [Google Scholar]
- 11.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- 12.Dethier VG. The Hungry Fly. Cambridge, MA: Harvard Univ Press; 1976. [Google Scholar]
- 13.Médioni J, Vaysse G. Conditional suppression of a reflex in Drosophila melanogaster: Acquisition and extinction. C R Seances Soc Biol Fil. 1975;169:1386–1391. [PubMed] [Google Scholar]
- 14.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ja WW, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 20.Gerber B, Tanimoto H, Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol. 2004;14:737–744. doi: 10.1016/j.conb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Fiala A. Olfaction and olfactory learning in Drosophila: Recent progress. Curr Opin Neurobiol. 2007;17:720–726. doi: 10.1016/j.conb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila. Prog Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 24.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 25.Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 26.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 27.Masek P, Heisenberg M. Distinct memories of odor intensity and quality in Drosophila. Proc Natl Acad Sci USA. 2008;105:15985–15990. doi: 10.1073/pnas.0804086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia S, Tully T. Segregation of odor identity and intensity during odor discrimination in Drosophila mushroom body. PLoS Biol. 2007;5:e264. doi: 10.1371/journal.pbio.0050264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herness MS, Pfaffmann C. Generalization of conditioned taste aversions in hamsters: Evidence for multiple bitter receptor sites. Chem Senses. 1986;11:347–360. [Google Scholar]
- 30.Spector AC, Grill HJ. Differences in the taste quality of maltose and sucrose in rats: Issues involving the generalization of conditioned taste aversions. Chem Senses. 1988;13:95–113. [Google Scholar]
- 31.Breslin PA, Kemp S, Beauchamp GK. Single sweetness signal. Nature. 1994;369:447–448. doi: 10.1038/369447a0. [DOI] [PubMed] [Google Scholar]
- 32.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: Implications for the neural coding of bitter-tasting compounds. J Neurosci. 2002;22:1937–1941. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dotson CD, Spector AC. Behavioral discrimination between sucrose and other natural sweeteners in mice: Implications for the neural coding of T1R ligands. J Neurosci. 2007;27:11242–11253. doi: 10.1523/JNEUROSCI.1227-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strausfeld NJ, Li Y. Organization of olfactory and multimodal afferent neurons supplying the calyx and pedunculus of the cockroach mushroom bodies. J Comp Neurol. 1999;409:603–625. [PubMed] [Google Scholar]
- 35.Ehmer B, Gronenberg W. Segregation of visual input to the mushroom bodies in the honeybee (Apis mellifera) J Comp Neurol. 2002;451:362–373. doi: 10.1002/cne.10355. [DOI] [PubMed] [Google Scholar]
- 36.Schulz RA, Chromey C, Lu MF, Zhao B, Olson EN. Expression of the D-MEF2 transcription in the Drosophila brain suggests a role in neuronal cell differentiation. Oncogene. 1996;12:1827–1831. [PubMed] [Google Scholar]
- 37.Zars T, Wolf R, Davis R, Heisenberg M. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: In search of the engram. Learn Mem. 2000;7:18–31. doi: 10.1101/lm.7.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brembs B. Operant learning of Drosophila at the torque meter. J Vis Exp 731. 2008 doi: 10.3791/731. 10.3791/731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu SY, et al. Thermal nociception in adult Drosophila: Behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 40.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.