Abstract
Background
Hepatic ischemia/reperfusion (I/R) injury significantly influences short and long-term outcomes after liver transplantation (LTx). The critical step initiating the injury is known to include sinusoidal endothelial cell (SEC) alteration during the cold preservation period. As carbon monoxide (CO) has potent cytoprotective functions on vascular endothelial cells, this study examines if CO treatment of excised liver grafts during cold storage could protect SEC and ameliorate hepatic I/R injury.
Methods
Rat liver grafts were preserved in UW solution containing 5% CO (CO-UW) for 18–24 hrs and transplanted into syngenic Lewis rats.
Results
After 18 hrs cold preservation, SEC damage was evident with Propidium Iodide (PI) nuclear stain on SEC, and the frequency of PI+ SEC was significantly lower in grafts stored in CO-UW than in control UW. SEC protection with CO associated with decreased ICAM translocation and less MMP release during cold preservation. After LTx with 18 hrs cold preservation, serum ALT levels and hepatic necrosis were significantly less in CO-UW than in control UW group. With 24 hrs cold storage, 35% (7/20) survived with control UW, while the survival with CO-UW improved to 80% (8/10). These beneficial effects of CO-UW associated with significant reduction of neutrophil extravasation, down-regulation of hepatic mRNA for TNF-α and ICAM-1, and less hepatic ERK activation. Liver grafts from Kupffer cell depleted donors or pseudogerm-free donors showed less SEC death during cold preservation, and CO-UW further reduced SEC death.
Conclusion
CO delivery to excised liver grafts during cold preservation efficiently ameliorates SEC damage and hepatic I/R injury.
Keywords: ex vivo treatment, ischemia/reperfusion, liver transplantation
INTRODUCTION
Ischemia/reperfusion (I/R) injury of the liver graft has been considered as one of major problems complicating posttransplant patient care and influencing short- and long-term outcomes after liver transplantation (LTx). Since surgical procedures of transplantation obligate cold preservation and warm reperfusion of grafts, inevitable I/R injury, in some degree, occurs in every case of LTx. I/R injury could be severe and associate with serious morbidity and mortality particularly when expanded criteria donors are utilized. Hence, developing strategies to minimize I/R injury could potentially have an enormous impact on patient care and transplant outcomes. Furthermore, new therapeutic approaches would help to expand donor pools and actively use marginal liver grafts for transplantation to combat current problems of organ shortage.
The process of I/R injury is a sequence of events involving many interconnected factors. Although warm reperfusion of liver grafts displays striking injurious changes of liver grafts, transplant-induced I/R injury is initiated during cold preservation period mainly due to the lack of oxygen, depletion of ATP, and deterioration of the intracellular homeostasis (1). Hepatic sinusoidal endothelial cells (SEC) are particularly vulnerable to stress, and they develop serious morphological alteration during cold storage, such as retraction, cell body detachment, and sinusoidal denudation (2–5). Thus, significant SEC injuries during cold preservation appears to be an early crucial event of hepatic I/R injury, which provokes graft malcirculation, platelet activation, persistent vasoconstriction, and adhesion molecule upregulation, as well as Kupffer cell activation, neutrophil infiltration, and hepatocyte death after reperfusion (6–8).
CO is endogenously generated simultaneously with biliverdin and iron during the degradation of heme by heme oxygenases, and has been shown to function as a signaling molecule in the body (9, 10). Exogenously provided low dose CO also has been shown to have cytoprotective effects in a variety of experimental injury models by mediating vasodilative, anti-inflammatory, anti-platelet aggregation, anti-proliferative and anti-apoptotic activities (11, 12). Furthermore, previous in vitro studies have shown that CO protects vascular endothelial cells from hypoxia-induced injury (13, 14). We therefore hypothesized that exposure of SEC to CO during cold storage period prevented initial stage of SEC injury and ameliorated subsequent hepatic I/R injury. Accordingly, we examined in this study the efficacy of ex vivo CO treatment of excised liver grafts during cold storage in CO containing UW solution in inhibiting SEC damages. Subsequently, liver grafts that were modified with CO during cold storage were transplanted into syngenic rats to determine graft function and severity of the injury after reperfusion.
MATERIALS AND METHODS
Animals
Male Lewis (LEW, RT.1l) rats (8–10 weeks old) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Animals were maintained in laminar flow, specific-pathogen-free atmosphere at the University of Pittsburgh with a standard diet and water supplied ad libitum. All procedures were performed according to the guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh and the National Research Council’s Guide for the Humane Care and Use of Laboratory Animals.
CO supplementation to the UW solution
For each transplant experiment, 100 ml UW solution was vigorously bubbled for 5 min at 4°C with compressed 5% or 100% CO gas mixed in air (PRAXAIR, Danbury, CT) (CO-UW). For the control group, UW solution was either untreated or bubbled with air using the same procedure. Since there was no difference between untreated and air-bubbled UW, the results were put together and used as the control group (control-UW).
Orthotopic liver transplantation (LTx)
Liver harvesting and orthotopic transplantation without hepatic arterial reconstruction were performed according to the method previously described (15). LEW to LEW syngenic LTx in this study was performed by a single person to maintain technical consistency. The recipient unhepatic time was less than 15 min, and there was no difference between groups.
Experimental design
Liver grafts were in situ flushed with 20 ml control or CO-UW via the abdominal aorta with an additional 3 ml infusion of the same solution from the portal vein. Excised grafts then underwent static cold storage for 18–24 hrs in control or CO-UW using tightly secured containers filled with respective solutions, and transplanted into syngenic recipients. As shown in our previous studies (15–17), cold ischemic time (CIT) for 18 hrs of liver grafts in normal UW resulted in hepatic injury with peak serum alanine transaminase (ALT) levels ~4,000 IU/L at 24 hrs after LTx without mortality. Therefore, recipients were sacrificed at 1–48 hrs of reperfusion, and blood and liver graft samples (n=4–6 for each time point) were obtained to evaluate the efficacy of CO-UW. In contrast, cold preservation for 24 hrs in normal UW associated with approximately 70% mortality after LTx (15). In experiments using 24 hr cold preservation, animals were followed for 14 days to evaluate the effects of CO-UW on liver graft survival.
Additionally, two separate sets of experiments were conducted. First, donor animals were treated with intravascular injection of gadolinium chloride (GdCl3, 7.5mg/kg) at 24 hrs before liver graft retrieval to inhibit phagocytotic activity of Kupffer cells in the graft liver (18, 19). Another group of donor animals received twice daily oral antibiotic treatment of a mixture of neomycin (250 mg/kg/day), polymyxin B (9 mg/kg/day), and metronidazole (50 mg/kg/day) for 3 days before liver graft retrieval. The antibiotics regimen has been shown to completely sterilize the intestine of the rat (20, 21). In both groups, liver grafts were preserved for 18 hrs in UW with and without 5% CO to examine SEC injury.
Carboxyhemoglobin (COHb) Measurement
Heparinized blood sample (0.2 ml) was obtained from recipient animals, and blood COHb levels were measured using OSM3 Hemoximeter (Radiometer Copenhagen, Copenhagen, Denmark).
Measurement of tissue CO content
Tissue CO content was measured using the method as previously described (22). Briefly, one ml of homogenized liver grafts and 3 ml of phthalate solution (pH 4.01) was transferred into the vacuum tube and 0.5 ml of potassium ferricyanide (Sigma, St. Louis, MO) was added. The solution was mixed vigorously to release CO at room temperature. Gas phase (1 ml) was taken into 10 ml vacuum tube, and released CO was measured by TRI lyzer. Results were expressed as CO (pmol) per mg tissue weight.
Liver Enzymes
Hepatic injury was assessed by serum ALT levels using the standard methods in clinical laboratory at the UPMC.
Histopathology
Liver graft tissues were fixed in 10% formalin, embedded in paraffin, sectioned into 6 µm thickness and stained with hematoxylin and eosin (HE). The percentage of necrotic area was determined by morphometric analysis of rondomly selected 10 low-power fields (× 40) per each HE stained section. Neutrophils were stained using a naphthol AS-D chloroacetate esterase staining kit (Sigma). Positively stained cells were counted in 20 high-power fields (× 200) per each section and expressed as the number of cells per 1 mm2. All histopathological quantifications were conducted in a blinded fashion.
Assessment of cell viability using propidium iodide (PI)
Cell viability in liver grafts was determined by PI according to the previously described method (23). Briefly, at the end of preservation, the liver was ex vivo flushed via the portal vein with a 50% mixture of saline solution containing rat plasma and 0.19 mg/ml PI (Sigma) at a flow rate of 2.5 ml/min for 2 min. Liver grafts then were washed for 4 min, fixed in 2% paraformaldehyde, embedded into OCT compound at −80°C. Cryosections were stained with SEC specific antibody SE-1 (Immuno-Biological Laboratories Co., LTD., Gunma, Japan) followed by Alexa 488-conjugated goat anti-mouse IgG antibody (Invitrogen Co., Carlsbad, CA). Sections were nuclear DNA stained with Hoechst dye (bisBenzimide), coverslipped with Crystal mount, and reviewed with a Fluoview 1000 confocal microscope (Olympus, Center Valley, PA). PI+ and SE-1+ dead SEC were counted in randomly selected >10 high power microscopic field (× 600) per section in a blinded fashion.
Ex vivo examination of ICAM-1 expression on cell surface
ICAM-1 expression on SEC at the end of preservation was determined by perfusing the liver grafts from the portal vein with 1.5 ml of monoclonal anti-rat ICAM-1 antibody (1:100, R&D Systems Inc., Minneapolis, MN). After 1 hr incubation, liver grafts were fixed in 2% paraformaldehyde, and frozen into OCT compound. Liver sections were stained with PE-conjugated goat anti-mouse antibody (1:100, Invitrogen Corp.). Nuclear DNA was stained with Hoechst dye, coverslipped with Crystal mount, and visualized with an Olympus BX51 epifluorescence microscope (Malvern, NY).
Gelatinase activity of liver graft effluents
At the end of cold preservation, liver grafts were flushed with 4 ml of UW solution through the portal vein, and effluents were collected from the vena cava and centrifuged at 2,000 rpm for 10 min to remove cellular components. Gelatinase activity of effluents was identified on gels according to the previously described method (24, 25). The gels were stained for 1 to 2 hrs with 0.5% Coomassie Blue R-250, washed and photographed.
Western blot analysis
Western blot analysis was performed using 50 µg of cytosolic protein from graft liver tissue as previously described (17). After the blocking of nonspecific binding with non-fat dry milk, membranes were incubated overnight with primary antibodies including mouse monoclonal for eNOS (BD biosciences Pharmingen, San Diego, CA) and phospho(p)-ERK (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit polyclonal for ERK 1 (Santa Cruz Biotechnology, Inc.), p-p38 MAPK, p38 MAPK, p-JNK, JNK (Cell Signaling Technology, Beverly, MA), and β-actin (Sigma-Aldrich). After incubation with secondary goat anti-rabbit or mouse antibody (1:10,000, Pierce Chemical, Rockford, IL), membranes were developed with the SuperSignal® West Femto Maximum Sensitivity Substrate detection systems (Pierce Chemical) and exposed to film. The band intensities were measured by NIH Image Analysis software.
Real-time RT-PCR
Total RNA was extracted from the liver tissues using the TRIzol reagent (Life Technologies, Inc., Grand Island, NY) according to the manufacturer’s instructions. RNA content was measured using 260/280 UV spectrophotometry. mRNA expression was quantified by SYBR Green two-step, real-time RT-PCR using primers as previously described (17).
Statistical analysis
Data are presented as the mean ± SEM. Comparisons between groups at different time points were performed using Student’s t-test or ANOVA using the Statview program (Abacus Concepts, Inc., Berkeley, CA). Graft survivals were plotted with the Kaplan-Meier method, and the difference among groups was analyzed using the log-rank test. Differences were considered significant at a P value less than .05.
RESULTS
Hepatic CO tissue concentration increases after cold storage in CO-UW
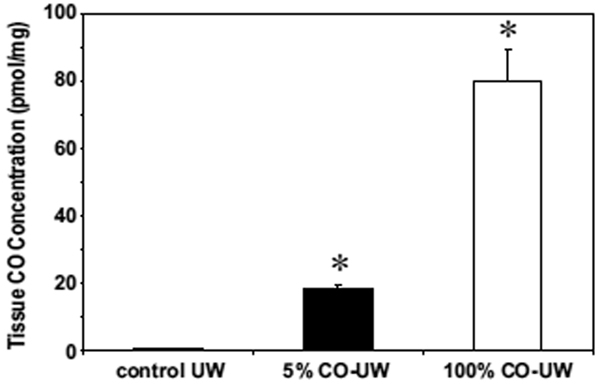
Soluble CO levels in UW solution elevated to 40.6 ± 1.6 and 820 ± 204 µmol/L after bubbling with 5% and 100% gaseous CO, respectively, and were maintained at these levels during cold preservation period when the solution was kept in a tightly sealed container without an air layer (22). At the end of 18 hrs cold preservation, liver tissue CO levels dramatically increased to 18.3 ± 1.3 and 79.9 ± 9.1 pmol/mg with 5% and 100% CO-UW, respectively (Fig. 1). In contrast, CO level of livers in control UW was 0.84 ± 0.08 pmol/mg, and was not different from that of normal naïve liver (0.21 ± 0.01 pmol/mg). These results indicate that CO in UW solution binds to liver tissues (most likely heme proteins) during cold preservation.
Figure 1. CO tissue concentration in liver grafts.
Liver graft samples were obtained at the end of 18 hrs cold preservation in control UW, or 5% or 100% CO-UW. CO tissue concentration was determined by TRI lyzer, as described in the Method section. * p <0.01 vs. control UW (n=4 for each group).
CO-UW protects hepatic SEC during cold storage period
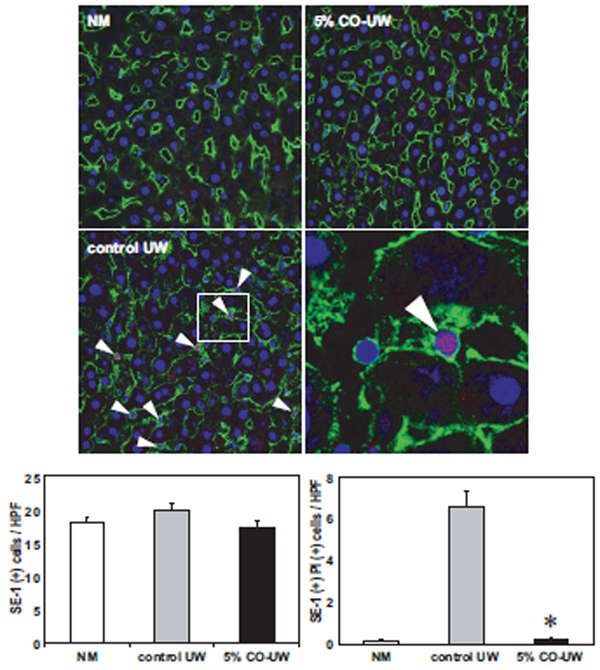
The binding of CO to hepatic heme proteins could alter the behavior of liver grafts during cold preservation. As cold preservation alone has been shown to induce significant SEC injury in liver grafts (2–6), and CO has been shown to protect VEC from hypoxia-induced apoptosis (13, 14), we hypothesized that CO binding to liver grafts during cold preservation prevented hypothermia/hypoxia-induced SEC injury. Accordingly, the viability of SEC was determined by ex vivo perfusion of PI at the end of 18 hrs CIT and subsequent staining with SEC-specific monoclonal antibody, SE-1. SEC in the normal liver showed flat nucleus and smooth SE-1 surface staining, and there was no detectable PI+ SE-1+ damaged SEC. In contrast, in liver grafts preserved for 18 hrs in control UW, many SEC were identified with nuclear PI and irregular SE-1 staining (Fig. 2). In liver grafts stored in 5% CO-UW, there were only few PI+ SEC. The numbers of PI+ SE-1+ SEC were significantly lower with 5% CO-UW compared to those with control UW.
Figure 2. SEC viability at the end of 18 hrs cold storage in UW.
Liver grafts preserved for 18 hrs in control UW or 5% CO-UW were perfused with PI solution, fixed, cut into 6 µ sections, and stained with SE-1 mAb (green) and Hoechst nuclear dye (blue). Upper left: Normal liver showed the networks of healthy SEC with smooth SE-1 surface staining and flat nuclear staining. Lower left: In liver grafts in control UW, many SEC showed co-localization (shown as pink, white arrow heads) of nuclear Hoechst (blue) and PI (red). Boxed area is enlarged and shown in lower right panel. Upper right: There were only few PI+ SE-1+ injured SEC with 5% CO-UW. Hepatcytes did not show any nuclear PI staining. Lower graphs show the number of SE-1+ (left) and SE-1+ PI+ (right) cells. * p <0.01 vs control UW (n=3 for each group).
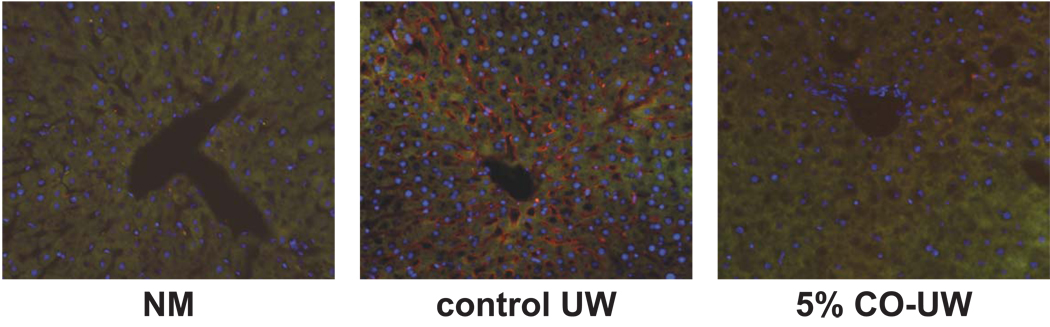
As cold preservation increases ICAM expression on SEC due to the translocation of cytosolic ICAM to the plasma membrane surface (26), we next detected extracellular ICAM protein in liver grafts by perfusing grafts at the end of 18 hrs cold storage with anti-rat ICAM-1 antibody. Significant amount of extracellular ICAM protein was detected in liver grafts preserved in control UW; on the other hand, grafts stored in 5% CO-UW showed negligible levels of ICAM, indicating that CO binding to liver graft inhibited ICAM translocation on the surface of SEC (Fig. 3).
Figure 3. ICAM-1 expression on SEC at the end of preservation.
Translocation of ICAM protein on cellular surface was detected by portal perfusion of liver grafts with anti-ICAM mAb at the end of 18 hrs cold preservation (see Methods). ICAM protein (red) was detected on the sinusoidal surface at the end 18 hrs preservation in control UW. Liver grafts in 5% CO-UW showed nearly no detectable ICAM protein on SEC surface. Representative immunofluorescent images of n=3 in each group. NM: normal liver perfused with anti-ICAM mAb.
Effluents from preserved liver grafts
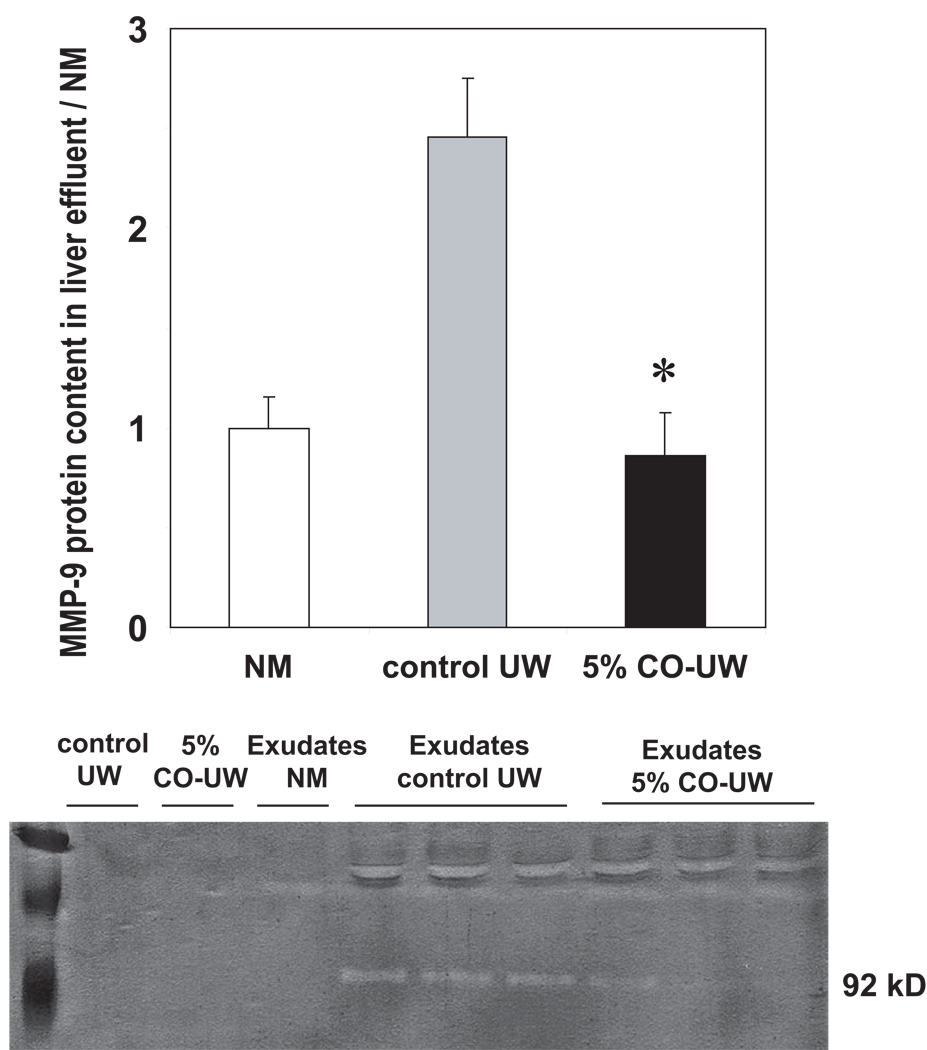
Matrix metalloproteinase (MMP), a specialized group of metalloproteinases, play roles in graft injury by digesting connective tissue matrix in the extracellular milieu and are shown to be released from SEC during cold preservation (24). MMP activity in the effluent from liver grafts was analyzed at the end of cold preservation. UW solution showed minimum gelatinelysis in gelatin zymography. Effluent obtained from liver grafts in control UW showed MMP activity at 92 kD band, indicating that the main MMP released from liver grafts during cold preservation was MMP9. MMP release from liver grafts was significantly suppressed with 5% CO-UW (Fig. 4).
Figure 4. MMP activity in effuluents from preserved liver grafts.
Exudates were obtained from liver grafts preserved in control and CO-UW at the end of 18 hrs cold preservation, and gelatin zymography was conducted. Control UW and CO-UW solutions, as well as exudates from normal liver without cold storage (NM) were used as controls. Increased MMP activity was seen when liver grafts were preserved in control UW. 5% CO-UW significantly inhibited MMP activity in hepatic effluents. * p <0.05 vs control UW (n=3 for each group).
The data indicates that significant SEC injury during cold preservation of liver grafts could be inhibited with ex vivo exposure and binding to CO. Accordingly, we evaluated the efficacy of graft-targeted CO application in the rat liver transplantation model.
CO-UW attenuates hepatic cold I/R injury and improves graft survival
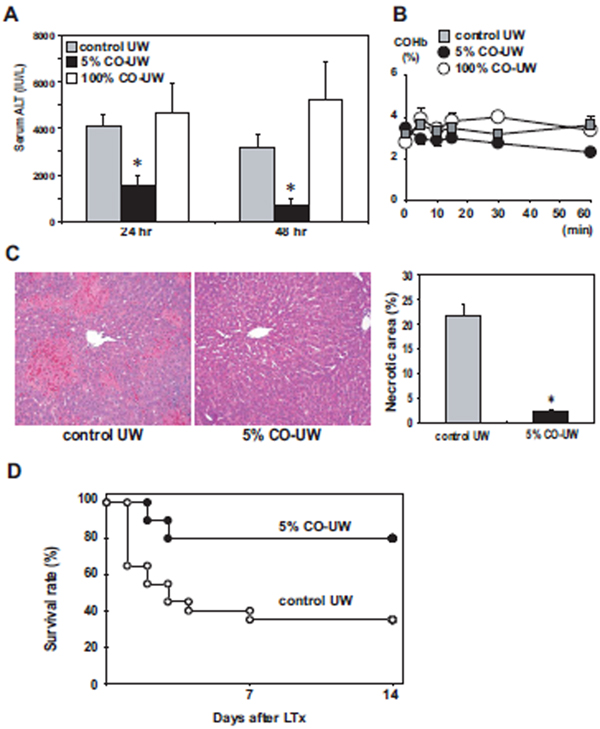
With 18 hrs cold preservation, all animals survived for 14 days; however, liver graft injury was evident with elevated ALT levels to 4068 ± 546 IU/L at 24 hrs after LTx in control UW group. When liver grafts were preserved in 5% CO-UW, ALT levels at 24 hrs significantly reduced to 1576 ± 446 IU/L (Fig. 5A). Blood carboxyhemoglobin levels were not elevated in any recipient sample during the first hour after LTx (Fig. 5B). Routine histopathology of liver samples at 48 hrs after reperfusion revealed significant reduction of hepatic necrotic area in 5% CO-UW preserved liver grafts (2.3 ± 0.4% vs. 21.7 ± 2.5% in control UW) (Fig. 5C). In contrast, storage in 100% CO-UW did not show beneficial effects and ALT levels were compatible to those in control UW group.
Figure 5. Liver graft injury and animal survival.
(A) Serum ALT levels with 18 hrs cold ischemic time (CIT): Liver grafts were preserved in control UW, 5% CO-UW, or 100% CO-UW for 18 hrs, and serum samples were obtained at 24 and 48 hrs after LTx. *p <0.01 vs. control UW at same time point (n=5 for each group).
(B) COHb levels were measured in recipient blood samples after LTx (n=3 for each group).
(C) Hepatic graft necrosis at 48 hrs after 18 hrs CIT and reperfusion: Necrotic area in H&E sections was significantly reduced with 5% CO-UW compared to that in control UW. * p <0.01 vs. control UW (n=5 for each group). Original magnification: ×100.
(D) Animal survival with 24 hrs CIT: Liver grafts were preserved for 24 hrs in control UW (n=20) or 5% CO-UW (n=10). Recipient animals were followed for 14 days after LTx. Animal survival was significantly (p <0.05) improved with 5% CO-UW.
To further establish the beneficial effects of 5% CO-UW in protecting liver grafts from hepatic I/R injury, cold preservation time was extended to 24 hrs. When liver grafts were stored for 24 hrs in control UW, 13 of 20 recipients died within 7 days, resulting in 14-day animal survival of 35%. In contrast, graft survival was significantly improved to 80.0% (n=10), when stored in 5% CO-UW (Fig. 5D).
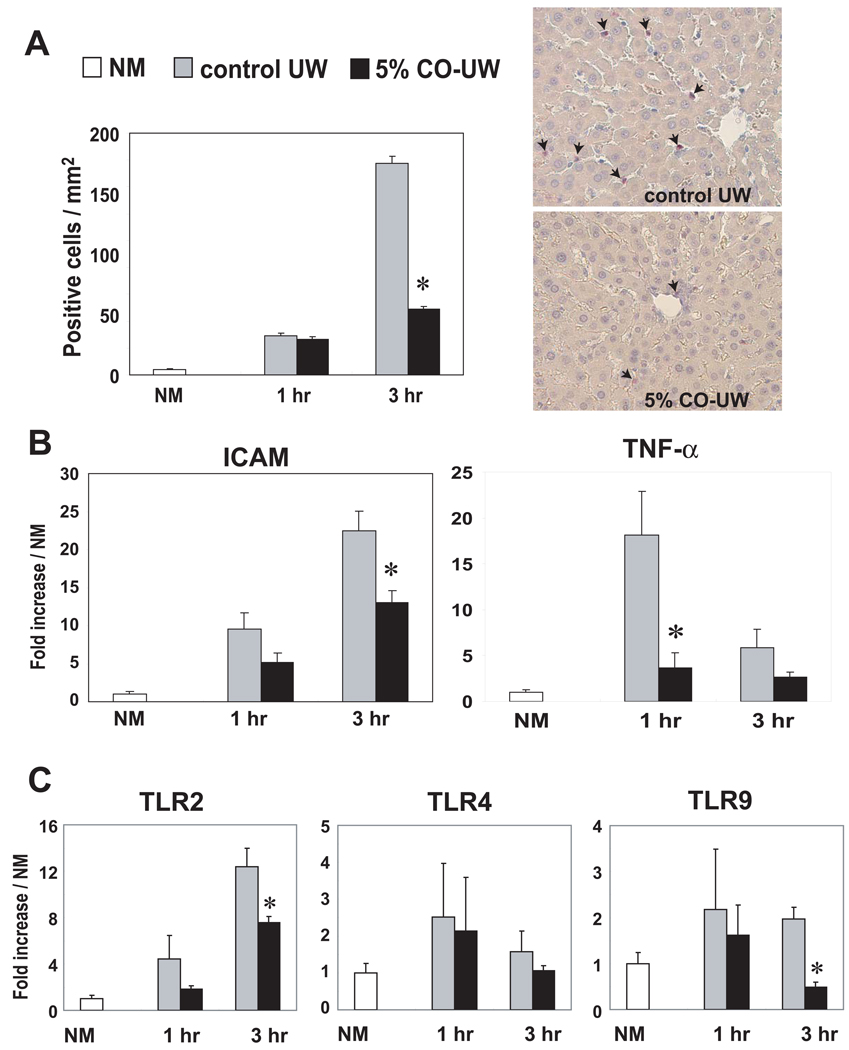
CO-UW suppresses early neutrophil accumulation and proinflammatory responses after LTx
At 1–3 hrs after LTx, numbers of infiltrating neutrophils rapidly increased in liver grafts preserved in control UW for 18 hrs (Fig. 6A). Significantly reduced numbers of neutrophils were seen in grafts preserved in 5% CO-UW at 3 hrs.
Figure 6. Hepatic proinflammatory responses early after LTx.
(A) Neutrophil infiltration: Liver grafts preserved in control or 5% CO-UW for 18 hrs were transplanted into syngenic recipients, and graft samples obtained at 1 and 3 hrs were stained for naphthol AS-D chloroacetate esterase. Positively stained cells were counted and shown as the number per 1 mm2. Representative images at 3 hrs showed less frequent neutrophils (red, arrows) in liver grafts preserved in 5% CO-UW. * p <0.01 vs. control UW (n=3 for each group). NM: normal liver.
(B) ICAM-1 and TNF-α mRNA levels: Liver grafts were preserved for 18 hrs in control UW or 5% CO-UW. Liver samples obtained at 1 and 3 hrs were analyzed by real-time qPCR for ICAM and TNF-α mRNA. I/R injury-induced mRNA upregulation was significantly reduced in liver grafts preserved in 5% CO-UW * p <0.05 vs. control UW (n=3 for each group).
(C) TLR mRNA levels: TLR2 and TLR9, but not TLR4, upregulation was inhibited in liver grafts stored in 5% CO-UW. * p <0.05 vs. control UW (n=3 for each group).
Upregulation of adhesion molecules on hepatic SEC is known to promote leukocyte sequestration, and hepatic ICAM-1 expression has been shown to be significantly upregulated after reperfusion (27–29). To examine the effects of CO-UW on ICAM upregulation, RT-PCR analysis was conducted. There was a prompt increase of mRNA levels for ICAM after reperfusion in liver grafts of control UW group after reperfusion. In contrast, liver grafts preserved in 5% CO-UW showed significantly lower ICAM mRNA at 3 hr after LTx (Fig. 6B).
Hepatic TNFα mRNA expression peaked at 1 hr after reperfusion of liver grafts in control UW; however, significantly reduced TNFα levels were seen in liver grafts preserved in 5% CO-UW (Fig. 6B). Likewise, upregulation of mRNA for TLR2, 4, and 9 was seen at 1–3 hrs after reperfusion of control liver grafts. Liver grafts preserved in 5% CO-UW showed significantly reduced mRNA levels for TLR2 and 9 at 3 hrs after LTx (Fig. 6C).
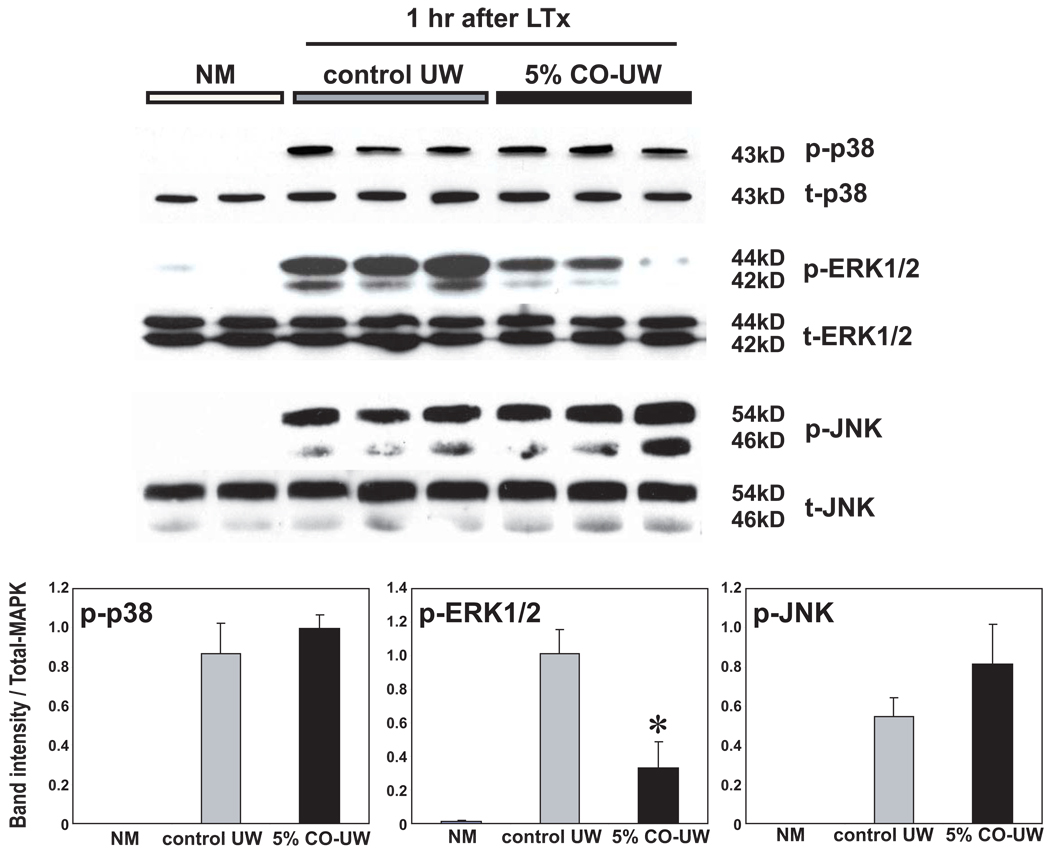
CO-UW inhibits MAPK pathway activation
MAPK intracellular signaling pathways were promptly activated in hepatic I/R injury, and phosphorylation of p38, ERK, and JNK was seen at 1 hr after reperfusion. CO-UW significantly suppressed p-ERK at 1 hr after reperfusion compared with control UW group (Fig. 7).
Figure 7. MAPK signaling pathways.
Western blot analysis of liver grafts at 1 hr after LTx for MAPK signaling pathways. 5% CO in UW significantly suppressed phosphorylation of ERK, but not p38 or JNK, compared with those in control UW group. * p <0.05 vs control UW (n=3 for each group).
These results indicate that ex vivo treatment of excised liver grafts with CO before transplantation has significant in vivo effects in reducing hepatic injury and regulating inflammatory reactions after transplantation.
Kupffer cells are involved in SEC injury during cold storage
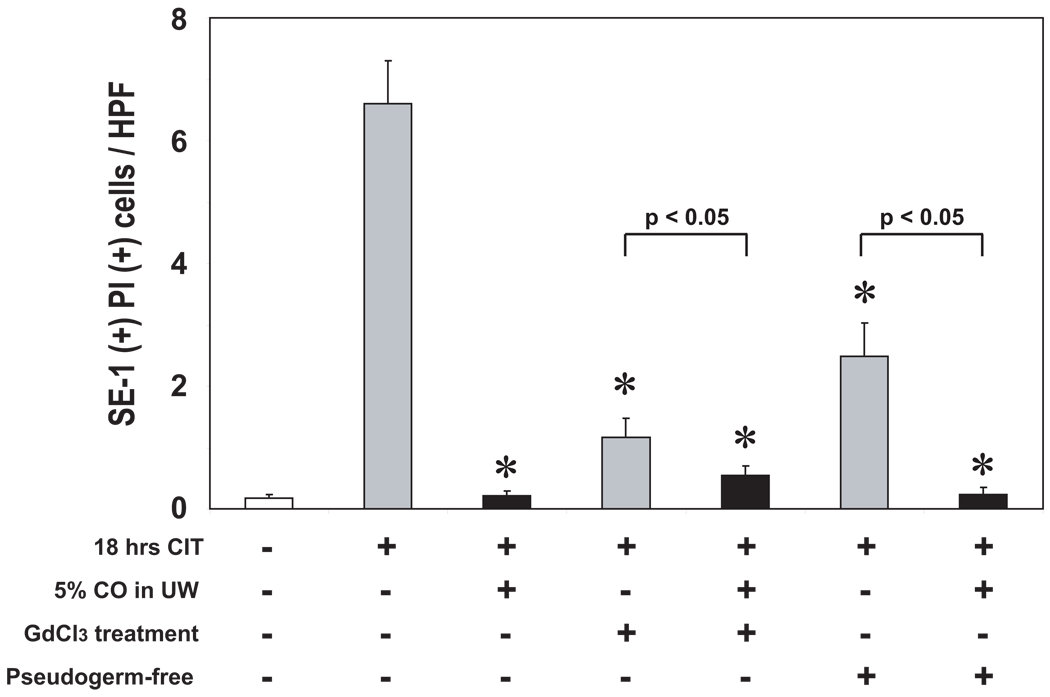
SEC protection with CO-UW during cold storage could be mediated via the direct binding of CO to SEC, as previous in vitro studies demonstrate CO’s protective actions on cultured VEC (13, 30, 31). However, CO also is able to downregulate hepatic Kupffer cell activation during hepatic I/R injury (32), and SEC injury during cold preservation might be prevented with CO through the modulation of Kupffer cells. To explore this possibility, we inhibited Kupffer cells in liver grafts by donor pretreatment with GdCl3 and examined SEC viability at the end of cold storage in either control or 5% CO-UW. PI+ SE-1+ SEC were significantly reduced in GdCl3-pretreated liver grafts compared to untreated grafts when preserved in control UW (Fig. 8). The number of PI+ SE-1+ SEC was further significantly reduced, when GdCl3-pretreated liver grafts were preserved in 5% CO-UW. Although GdCl3 does not inhibit all Kupffer cell activity, these results indicate that Kupffer cells play important roles in SEC injury during cold preservation. Additionally, as CO-UW provides additional protection by further reducing SEC death in GdCl3-treated liver grafts, CO-UW might in some degree function directly on SEC and inhibits SEC injury.
Figure 8. SEC viability in liver grafts obtained from GdCl3 treated or pseudogerm-free donors.
Liver grafts were obtained from normal, GdCl3-pretreated, or pseudogerm-free donor animals. After 18 hrs cold preservation in control UW or 5% CO-UW, SEC viability was analyzed with PI and SE-1 stain (see Methods). Numbers of PI+ SE-1+ SEC were blindly counted in >10 randomly selected high power field (×600). *p< 0.01 vs control UW (n=4–6 per group).
To investigate the possible signals that activate Kupffer cells during cold storage, we preserved liver grafts from pseudogerm-free donors in control or 5% CO-UW, and SEC injury was determined at the end of 18 hrs cold storage. When preserved in control UW, the number of PI+ SE-1+ SEC was significantly less in germ-free donor livers than in untreated controls. Preservation of liver grafts from germ-free donors in 5% CO-UW further significantly reduced SEC death.
These data strongly suggest the roles of Kupffer cells and intestinal bacterial flora on initial SEC damage during cold storage. The protection of SEC with CO might be mediated via the direct actions on SEC as well as indirect actions through Kupffer cell regulation.
DISCUSSION
The current study demonstrates significant protection of SEC during cold storage with ex vivo delivered CO in UW solution. Severe SEC damage during cold storage is evident in this study as increases of PI+ SEC, MMP release, and ICAM translocation in control liver grafts. In contrast, liver grafts exposed to CO during cold storage show a significant reduction of these SEC-related changes during cold preservation period. Further, after transplantation, ALT levels, hepatic graft necrosis, inflammatory responses, and graft survival are significantly improved in liver grafts exposed to CO. These beneficial effects associate with elevated tissue CO levels in liver grafts by the end of cold preservation, suggesting that ex vivo delivered CO in UW binds to and persists in liver graft tissues to exert protection. Although specific binding sites and target proteins, as well as target cell types need to be identified, ex vivo treatment of excised grafts would be an attractive strategy, as the procedure modifies liver grafts before the actual transplantation.
CO has been shown to exert potent cytoprotective actions in numerous studies. In the majority of these experimental studies as well as in our previous study (17), inhaled CO has been utilized to deliver gaseous CO to tissues; however, in vivo use of CO associates with CO binding to hemoglobin and raises concerns for serious adverse effects. We have previously shown that gaseous CO can be bubbled into UW preservation solution, and intestine and kidney grafts preserved in CO-UW were protected from I/R injury associating with transplantation (22, 33). The current study is an extension of ex vivo CO application to the liver graft which is an active heme-producing tissue in the body and considered to be a major source of endogenous CO due to its role in removing damaged red blood cells and free hemoglobin from circulation. The study shows that ex vivo CO treatment can protect SEC from hypothermic and hypoxic injury and inhibits following reperfusion injury in heme-rich liver grafts.
The major molecular targets of CO most likely are transition metals, and iron is the most abundant element of the body's transition metals. The body iron is found in hemoglobin (66%), myoglobin (3%), heme enzymes (0.1%), intracellular storage as ferritin (30%) or labile iron (chelatable iron, 1%), and extracellular transferrin (0.1%). Although hemoglobin is the largest pool of the body iron, excised bloodless liver grafts contain minimum amounts of erythrocytes, and the binding of CO to hemoglobin is negligible. Thus, CO in this study might induce beneficial effects by efficiently binding to graft heme enzymes that play crucial roles in regulating cellular function (e.g. catalase, cyclooxygenase, cytochrome c, cytochrome P450, cytochrome c oxidase, guanylyl cyclase, NADPH oxidase, NO synthases) (34). Indeed, CO has been shown to activate bovine guanylyl cyclase (35) and inhibit cytochrome c oxidase in RAW 264.7 cells (36). These observations suggest that ex vivo exposure of bloodless liver grafts to CO not only minimizes adverse effects, but also increases efficacy by efficiently binding to target heme enzymes.
However, at the same time, detrimental effects with 100% CO could be induced by the impaired function of hepatic heme proteins. We have observed relative decreases of hepatic CYP450 functions with CO (data not shown). COHb levels in recipient blood samples did not increase after transplanting liver grafts stored in 100% CO-UW, suggesting that CO was not released from the liver to the recipient circulation and that oxygen delivery was not affected in this study. However, available oxygen binding sites on heme proteins and intracellular uptake of oxygen might be affected by high concentrations of CO. Further studies certainly are required to determine the mechanisms of beneficial and adverse effects induced by CO.
In a relevant approach, CO releasing molecules (CORM) have been developed using a heavy metal and surrounding carbonyl (CO) groups as coordinated ligands. In particular, water-soluble complexes with tricarbonyldichlororuthenium (II) dimer liberate CO in the physiological condition and have been shown to elicit similar biological activities to those seen with inhaled gaseous CO (37). In isolated perfusion circuit, Celsior preservation solutions supplemented with CORM showed improved renal function and mitochondrial respiration in 24 hrs cold preserved rabbit kidneys (38). CORM added into St Thomas cardioplegic solution also showed less cardiac injury and improved cardiac function in isolated rat heart perfusion circuit after cold ischemic storage for 4 or 6 hrs (39), supporting that CO can be delivered to organ grafts during cold preservation and ameliorate I/R injury.
The fundamental mechanism responsible for I/R injury associating with LTx is hepatic microcirculatory disturbance mainly due to SEC injury initiated during cold preservation. Ultrastructure studies reveal progressive SEC damage during cold preservation of animal as well as human liver grafts, while parenchymal cells maintain a relatively normal appearance (2, 40). As exposure of isolated SEC to hypothermia results in elevated intracellular calcium concentration, increased calpain activity, and actin disassembly (24, 41), SEC injury could be a direct consequence of cold storage. However, Niwano et al demonstrate that liver grafts from GdCl3-treated and Kupffer cell inactivated donors have reduced SEC injury evidenced by less sinusoidal denudation in SEM and reduced sinusoidal discontinuity and extravasation in microvascular casting (42). Kupffer cells in cold-stored liver tissues have been shown to develop signs of activation, such as progressive rounding, cell surface ruffling, polarization, appearance of wormlike densities, vacuolization and degranulation (43, 44). It is conceivable that activated Kupffer cells during hypoxic and hypothermic storage secrete various inflammatory mediators (e.g. cytokines), arachidonate metabolites (e.g., thromboxanes, leukotrines), and ROS, and induce SEC damage. The mechanism by which CO protected SEC during cold storage remains to be fully elucidated; however, CO in this study might decrease SEC damage and reduce subsequent I/R injury by inhibiting Kupffer cell activation, in addition to directly protecting SEC during cold preservation.
Kupffer cell activation during cold preservation might be a direct result of hypothermic/hypoxic condition; however, substances in the portal blood stream that are taken up by Kupffer cells during donor surgery could also have some effects. TLRs specifically recognize pathogen-associated molecular patterns that are expressed on infectious agents (e.g. LPS, bacterial DNA, viral double-stranded RNA), as well as molecules released from damaged or stressed tissues (e.g. heat-shock proteins, hyaluronan, HMGB1). TLR-ligation on Kupffer cells activates intracellular signal transduction pathways for inflammatory responses, and liver grafts from pseudogerm-free donors had less SEC injury during cold storage, suggesting the involvement of intestinal microbes in the early phase of hepatic I/R injury.
In conclusion, this study demonstrates gaseous cytoprotective molecule CO in UW cold preservation solution can protect SEC during cold storage and improve liver graft function and survival after LTx. Kupffer cell inactivation with donor pretreatment with GdCl3 as well as pseudogerm-free donor experiments suggests that Kupffer cells play roles in SEC injury. CO-UW appears to inhibit SEC injury by directly protecting SEC and indirectly regulating Kupffer cells. Excised liver graft treatment with CO before transplantation might be an attractive strategy to reduce hepatic I/R injury.
ACKNOWLEDGEMENT
We thank Lifang Shao, Sue L. Gleixner, and Lee Sonis for their excellent technical support and Carla Forsythe for preparation and organization of the manuscript.
This work was supported by the National Institutes of Health grants DK54232, CA76541 and DK062313.
ABBREVIATIONS
- I/R
ischemia/reperfusion
- LTx
Liver transplantation
- SEC
sinusoidal endothelial cell
- CO
carbon monoxide
- PI
Propidium iodide
- CIT
cold ischemic time
- ALT
alanine transaminase
- GdCl3
gadolinium chloride
- COHb
carboxyhemoglobin
- MMP
matrix metalloproteinase
REFERENCES
- 1.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134(6):1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKeown CM, Edwards V, Phillips MJ, Harvey PR, Petrunka CN, Strasberg SM. Sinusoidal lining cell damage: the critical injury in cold preservation of liver allografts in the rat. Transplantation. 1988;46(2):178–191. [PubMed] [Google Scholar]
- 3.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology. 1989;10(3):292–299. doi: 10.1002/hep.1840100307. [DOI] [PubMed] [Google Scholar]
- 4.Lemasters JJ, Bunzendahl H, Thurman RG. Reperfusion injury to donor livers stored for transplantation. Liver Transpl Surg. 1995;1(2):124–138. doi: 10.1002/lt.500010211. [DOI] [PubMed] [Google Scholar]
- 5.Stolz DB, Ross MA, Ikeda A, Tomiyama K, Kaizu T, Geller DA, et al. Sinusoidal endothelial cell repopulation following ischemia/reperfusion injury in rat liver transplantation. Hepatology. 2007;46(5):1464–1475. doi: 10.1002/hep.21887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda T, Yanaga K, Kishikawa K, Kakizoe S, Shimada M, Sugimachi K. Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats. Hepatology. 1992;16(2):454–461. doi: 10.1002/hep.1840160226. [DOI] [PubMed] [Google Scholar]
- 7.Selzner N, Rudiger H, Graf R, Clavien PA. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125(3):917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 9.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 10.Suematsu M, Kashiwagi S, Sano T, Goda N, Shinoda Y, Ishimura Y. Carbon monoxide as an endogenous modulator of hepatic vascular perfusion. Biochem Biophys Res Commun. 1994;205(2):1333–1337. doi: 10.1006/bbrc.1994.2811. [DOI] [PubMed] [Google Scholar]
- 11.Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36(2):175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290(3):F563–F571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- 13.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192(7):1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282(3):1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 15.Kaizu T, Ikeda A, Nakao A, Takahashi Y, Tsung A, Kohmoto J, et al. Donor graft adenoviral iNOS gene transfer ameliorates rat liver transplant preservation injury and improves survival. Hepatology. 2006;43(3):464–473. doi: 10.1002/hep.21067. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y, Ganster RW, Gambotto A, Shao L, Kaizu T, Wu T, et al. Role of NF-kappaB on liver cold ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283(5):G1175–G1184. doi: 10.1152/ajpgi.00515.2001. [DOI] [PubMed] [Google Scholar]
- 17.Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, et al. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G236–G244. doi: 10.1152/ajpgi.00144.2007. [DOI] [PubMed] [Google Scholar]
- 18.Hardonk MJ, Dijkhuis FW, Hulstaert CE, Koudstaal J. Heterogeneity of rat liver and spleen macrophages in gadolinium chloride-induced elimination and repopulation. J Leukoc Biol. 1992;52(3):296–302. doi: 10.1002/jlb.52.3.296. [DOI] [PubMed] [Google Scholar]
- 19.Contreras MA, Khan M, Smith BT, Cimini AM, Gilg AG, Orak J, et al. Endotoxin induces structure-function alterations of rat liver peroxisomes: Kupffer cells released factors as possible modulators. Hepatology. 2000;31(2):446–455. doi: 10.1002/hep.510310226. [DOI] [PubMed] [Google Scholar]
- 20.Rozenfeld RA, Liu X, DePlaen I, Hsueh W. Role of gut flora on intestinal group II phospholipase A2 activity and intestinal injury in shock. Am J Physiol Gastrointest Liver Physiol. 2001;281(4):G957–G963. doi: 10.1152/ajpgi.2001.281.4.G957. [DOI] [PubMed] [Google Scholar]
- 21.Sun XM, MacKendrick W, Tien J, Huang W, Caplan MS, Hsueh W. Endogenous bacterial toxins are required for the injurious action of platelet-activating factor in rats. Gastroenterology. 1995;109(1):83–88. doi: 10.1016/0016-5085(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 22.Nakao A, Toyokawa H, Tsung A, Nalesnik MA, Stolz DB, Kohmoto J, et al. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am J Transplant. 2006;6(10):2243–2255. doi: 10.1111/j.1600-6143.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- 23.Katori M, Tamaki T, Takahashi T, Tanaka M, Kawamura A, Kakita A. Prior induction of heat shock proteins by a nitric oxide donor attenuates cardiac ischemia/reperfusion injury in the rat. Transplantation. 2000;69(12):2530–2537. doi: 10.1097/00007890-200006270-00011. [DOI] [PubMed] [Google Scholar]
- 24.Upadhya GA, Strasberg SM. Evidence that actin disassembly is a requirement for matrix metalloproteinase secretion by sinusoidal endothelial cells during cold preservation in the rat. Hepatology. 1999;30(1):169–176. doi: 10.1002/hep.510300130. [DOI] [PubMed] [Google Scholar]
- 25.Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986;261(6):2814–2818. [PubMed] [Google Scholar]
- 26.Topp SA, Upadhya GA, Strasberg SM. Leukocyte adhesion to cold-preserved rat endothelial cells: role of actin disassembly and ICAM-1. Liver Transpl. 2003;9(12):1286–1294. doi: 10.1016/j.lts.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57(3):368–374. [PubMed] [Google Scholar]
- 28.Nishimura Y, Takei Y, Kawano S, Goto M, Nagano K, Tsuji S, et al. The F(ab')2 fragment of an anti-ICAM-1 monoclonal antibody attenuates liver injury after orthotopic liver transplantation. Transplantation. 1996;61(1):99–104. doi: 10.1097/00007890-199601150-00020. [DOI] [PubMed] [Google Scholar]
- 29.El-Wahsh M, Fuller B, Davidson B, Rolles K. Hepatic cold hypoxia and oxidative stress: implications for ICAM-1 expression and modulation by glutathione during experimental isolated liver preservation. Cryobiology. 2003;47(2):165–173. doi: 10.1016/j.cryobiol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Soares MP, Usheva A, Brouard S, Berberat PO, Gunther L, Tobiasch E, et al. Modulation of endothelial cell apoptosis by heme oxygenase-1-derived carbon monoxide. Antioxid Redox Signal. 2002;4(2):321–329. doi: 10.1089/152308602753666370. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280(10):8714–8721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 32.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, et al. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic I/R injury in rats. Hepatology. doi: 10.1002/hep.22482. in press. [DOI] [PubMed] [Google Scholar]
- 33.Nakao A, Faleo G, Shimizu H, Nakahira K, Kohmoto J, Sugimoto R, et al. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney International. 2008;74:989–991. doi: 10.1038/ki.2008.342. [DOI] [PubMed] [Google Scholar]
- 34.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57(4):585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 35.Stone JR, Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33(18):5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, Billiar TR, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. Faseb J. 2007;21(4):1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 37.Motterlini R. Carbon monoxide-releasing molecules (CO-RMs): vasodilatory, anti-ischaemic and anti-inflammatory activities. Biochem Soc Trans. 2007;35(Pt 5):1142–1146. doi: 10.1042/BST0351142. [DOI] [PubMed] [Google Scholar]
- 38.Sandouka A, Fuller BJ, Mann BE, Green CJ, Foresti R, Motterlini R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006;69(2):239–247. doi: 10.1038/sj.ki.5000016. [DOI] [PubMed] [Google Scholar]
- 39.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007;26(11):1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Carles J, Fawaz R, Hamoudi NE, Neaud V, Balabaud C, Bioulac-Sage P. Preservation of human liver grafts in UW solution. Ultrastructural evidence for endothelial and Kupffer cell activation during cold ischemia and after ischemia-reperfusion. Liver. 1994;14(1):50–56. doi: 10.1111/j.1600-0676.1994.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 41.Upadhya GA, Topp SA, Hotchkiss RS, Anagli J, Strasberg SM. Effect of cold preservation on intracellular calcium concentration and calpain activity in rat sinusoidal endothelial cells. Hepatology. 2003;37(2):313–323. doi: 10.1053/jhep.2003.50069. [DOI] [PubMed] [Google Scholar]
- 42.Niwano M, Arii S, Monden K, Ishiguro S, Nakamura T, Mizumoto M, et al. Amelioration of sinusoidal endothelial cell damage by Kupffer cell blockade during cold preservation of rat liver. J Surg Res. 1997;72(1):36–48. doi: 10.1006/jsre.1997.5162. [DOI] [PubMed] [Google Scholar]
- 43.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13(1):83–95. [PubMed] [Google Scholar]
- 44.Arii S, Monden K, Adachi Y, Zhang W, Higashitsuji H, Furutani M, et al. Pathogenic role of Kupffer cell activation in the reperfusion injury of cold-preserved liver. Transplantation. 1994;58(10):1072–1077. [PubMed] [Google Scholar]