Abstract
A characteristic feature of developing neural circuits is that they are spontaneously active. There are several examples, including the retina, spinal cord and hippocampus, where spontaneous activity is highly correlated amongst neighboring cells, with large depolarizing events occurring with a periodicity on the order of minutes. One likely mechanism by which neurons can “decode” these slow oscillations is through activation of second messengers cascades that either influence transcriptional activity or drive posttranslational modifications. Here we describe recent experiments where imaging has been used to characterize slow oscillations in the cAMP/PKA second messenger cascade in retinal neurons. We review the latest techniques in imaging this specific second messenger cascade, its intimate relationship with changes in intracellular calcium concentration, and several hypotheses regarding its role in neurodevelopment.
Introduction
Spontaneous neuronal activity is a common feature in the developing central nervous system, occurring in many regions including the retina (Wong et al., 1993; Feller et al., 1996), spinal cord (Gorbunova and Spitzer, 2002; Hanson and Landmesser, 2003), hippocampus (Ben-Ari et al., 1989; Garaschuk et al., 1998; Colin-Le Brun et al., 2004; Khalilov et al., 2005), neocortex (Schwartz et al., 1998; Aguilo et al., 1999; Adelsberger et al., 2005), and hindbrain (Gust et al., 2003). Although spontaneous activity is generated by circuits with vastly different synaptic connectivity, each circuit exhibits episodic events that are correlated across large populations of cells with a periodicity on the order of minutes (Feller, 1999; O'Donovan, 1999). These events are for the most part generated by depolarizations of neurons, and cause significant increases in intracellular calcium concentration.
A model system for understanding how periodic activity translates into long-term changes in neural circuits is the developing visual system. Spontaneous activity in the retina is critical for the retinotopic and eye-specific refinement of retinal projections to the thalamus and superior colliculus prior to vision (for review, see Wong, 1999; Torborg and Feller, 2005). Retinal waves are spatially correlated bursts of activity that propagate laterally across the retina prior to the development of light responses. This activity is also periodic, occurring in a given retinal ganglion cell approximately once per minute. Most hypotheses regarding how retinal waves drive the refinement of retinal projections have been based on the assumption that spontaneous activity drives synaptic competition that in turn leads to axonal refinement (Katz and Shatz, 1996; Huberman, 2006; Butts et al., 2007). There is also some indication that cell autonomous processes are dependent on patterned activity (Ruthazer and Cline, 2004). For example, rhythmic activity in retinal ganglion cells significantly enhances the rate of axonal outgrowth (Goldberg et al., 2002; Rodger et al., 2005). In addition, neuronal activity determines the polarity of responsiveness to chemorepulsive or chemoattractive signals (Wang and Zheng, 1998; Ming et al., 2001; Nicol et al., 2007).
Little is known about the specific mechanisms by which patterned activity is read out by the cell to produce changes in protein expression or regulation of existing proteins. Though it is likely that increases in intracellular calcium concentration and downstream calmodulin-dependent kinases are responsible for some of the resulting developmental changes, other second messenger cascades, such as the cAMP pathway, may be involved. Calcium and cAMP pathways are known to be closely interrelated. Calcium activates some adenylyl cyclases (ACs), the enzymes that convert ATP to cAMP to directly increase cAMP concentration (Xia and Storm, 1997). Indeed, mutant mice lacking AC1 show defects in projections to both the superior colliculus and lateral geniculate nucleus, implying that calcium dependent cAMP concentration is important for map refinement (Ravary et al., 2003; Plas et al., 2004). In some cells, increases in calcium concentration lead to decreases in cAMP levels either by inhibiting some classes of ACs (Wayman et al., 1995; Wang and Storm, 2003), or by activating a distinct class of phosphodiesterases that are responsible for the breakdown of cAMP (Sonnenburg et al., 1998).
Cyclic AMP is a ubiquitous second messenger that is involved in myriad cellular processes. Downstream targets of cyclic AMP include cAMP dependent protein kinase (PKA), a guanine nucleotide exchange factor called Exchange protein activated by cAMP (Epac) and cyclic nucleotide gated ion channels. PKA in turn phosphorylates a wide variety of targets, including many receptors and ion channels that govern the excitability of neurons. In addition, cAMP also influences actin dynamics (Meberg et al., 1998) and is critical for growth cone motility (Lohof et al., 1992; Kim and Wu, 1996; Song and Poo, 1999; Munck et al., 2004).
To determine the various roles of second messenger cascades in development, it is critical to characterize the effects of neural activity on second messengers dynamics. However, tools for assaying activity of second messenger pathways other than calcium have been sparse until recently. Particularly, the cAMP/PKA pathway has seen the advent of several indicators suitable for use in both mammalian systems and model organisms. Here, we review advances in the field of fluorescence-based assays of the cAMP/PKA pathway and the application of these techniques to developing neurons.
Indicators for dynamic imaging of cAMP/PKA cascade
Although biochemical techniques to quantify cAMP concentration and PKA activity have existed for many years, these techniques are not appropriate for live imaging since they require the lysing of cells to obtain the measurements. The first assay with the spatiotemporal sensitivity to do dynamic imaging was the protein FlCRhR, a tetrameric holoenzyme consisting of the catalytic and regulatory subunits of PKA fused to fluorescein and rhodamine, respectively (Adams et al., 1991). FlCRhR was used to measure evoked changes of cAMP levels by forskolin or GPCR agonists in invertebrate neurons (Bacskai et al., 1993; Hempel et al., 1996) and mammalian hippocampal neurons, (Vincent and Brusciano, 2001; Goaillard and Vincent, 2002), as well as to image spontaneous cAMP transients in spinal cord neurons (Gorbunova and Spitzer, 2002). However, the use of chemically modified recombinant proteins implies it needs to be microinjected into cells, which has more limited use in mammalian neurons where whole cell recordings dialyze the intracellular contents that may modulate second messenger signaling.
A solution to this problem is to use genetically encoded indicators. One approach is to express genetically encoded cAMP-gated cation channels (Rich et al., 2001). Though this works well in kidney cells, the introduction of novel calcium conductances may alter the excitability of neurons. The first genetically encoded indicator of cAMP was based on FlCRhR, replacing fluorescein and rhodamine with GFP and BFP (Zaccolo et al., 2000). This indicator was soon updated to use CFP/YFP fluorophores, which are more suitable for FRET (Zaccolo and Pozzan, 2002) and the linker range was modified to optimize FRET efficiency (Lissandron et al., 2005). The PKA-based cAMP sensor has four binding sites for cAMP – two on each regulatory subunit – each with different affinities for cAMP due to cooperative binding. The PKA-based cAMP sensor relies on the dissociation of the regulatory and catalytic subunits to decrease FRET efficiency. The indicator is expressed throughout the cell, but since it utilizes the RII subunit of PKA, it can also localize to A-Kinase Anchoring Proteins (AKAPs). AKAPs are thought to be involved in organizing microdomains (for review, Beene and Scott, 2007), and therefore this indicator is likely to be useful in studying very localized changes in cAMP concentration. Indeed, the PKA-based cAMP sensor has been used to detect microdomains of cAMP signaling in cardiac myocytes (Zaccolo and Pozzan, 2002; Lissandron and Zaccolo, 2006). A similar indicator has been recently developed that uses a truncated form of the CFP-RII subunit targeted to the membrane. Evanescent wave microscopy is then used to detect the translocation of the YFP-catalytic subunit away from the membrane when cAMP levels increase (Dyachok et al., 2006)
Recently, novel indicators of cAMP have been developed using proteins that have a single cAMP binding site. In particular, three groups independently developed FRET sensors based on the Exchange protein activated by cAMP (Epac) (DiPilato et al., 2004; Nikolaev et al., 2004; Ponsioen et al., 2004). Epac is a guanine nucleotide exchange factor with a single cAMP binding site, whose activity links cAMP to the Ras-ERK pathway (for review Holz et al., 2006). Upon binding of cAMP to the indicators, there is a decrease in FRET efficiency. There has yet to be a rigorous comparison of the various Epac indicators, which are truncated differently but appear to have similar dynamic ranges of FRET ratios. Indicators with a single cAMP binding site provide an advantage over those that rely upon cooperative binding of cAMP in that their response times are more likely to directly reflect changes in changes in cAMP levels. FRET-based indicators have also been developed from the cAMP binding sites of the PKA regulatory subunit (Nikolaev et al., 2004) and the cAMP gated channel HCN2 (Nikolaev et al., 2006).
A third type of indicator was developed to report on PKA activity. This indicator was designed as an archetype for reporting kinase activity via FRET. AKARs consist of a fusion of CFP, a PKA target substrate, a phosphobinding region, and the YFP variant citrine (Zhang et al., 2001). Efforts to further improve AKAR have focused on increasing the off-kinetics (Zhang et al., 2005) and the dynamic range of the indicator by optimizing the orientation of YFP with respect to CFP using circular permutations of the YFP variant, Venus (Nagai et al., 2004; Allen and Zhang, 2006). The AKAR FRET paradigm has also been used to study the activity of other kinases, including PKB/Akt (Aktus, Sasaki et al., 2003), PKC (CKAR, Violin et al., 2003), PKD (DKAR, Kunkel et al., 2007), and tyrosine kinases (Ting et al., 2001).
One advantage of genetically encoded indicators is that they can be localized to specific populations of cells using specific promoters. In addition, they can be mutated to allow targeting to different subcellular compartments. For example, short peptide tags have been used to localize the indicators to nuclear, mitochondrial, cytosolic, and membrane regions in hippocampal neurons (DiPilato et al., 2004; Allen and Zhang, 2006; Gervasi et al., 2007). This ability to obtain subcellular localization is likely to be critical for distinguishing amongst the variety of targets of the cAMP/PKA pathways in neurons.
Spontaneous cAMP/PKA transients in retinal ganglion cells
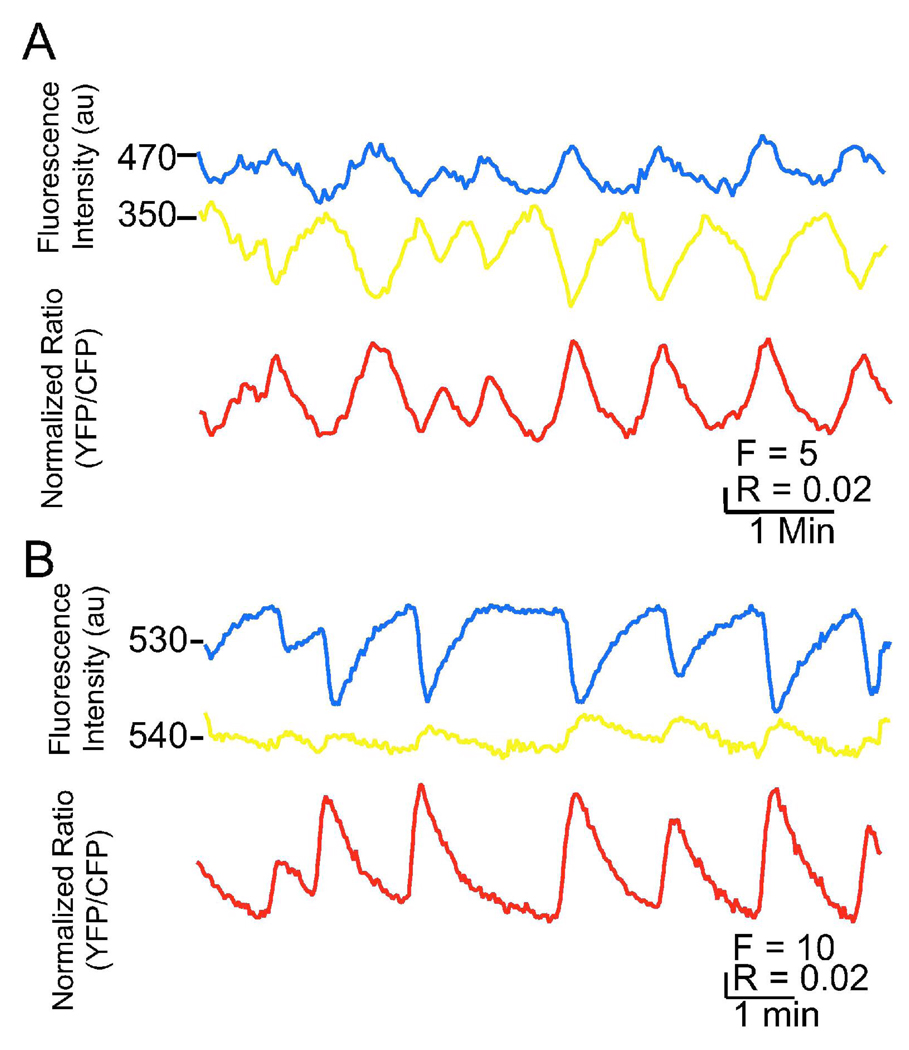
We recently used two FRET indicators, ICUE2 and AKAR2.2 to assay the spontaneous activation of the cAMP/PKA pathway in retinal ganglion cells during retinal waves (Dunn, Wang, et al. 2006). Calcium influx due to retinal waves occurs approximately once per minute, and calcium dependent adenylyl cyclases are present in retinal ganglion cells suggesting cAMP/PKA might be activated by calcium influx. However, it was unknown whether this pattern of calcium transients would cause transient or sustained activation of the cAMP/PKA pathway. Briefly, retinal explants were isolated from P0-P4 rats, electroporated with either ICUE2 or AKAR2.2, and cultured overnight to allow expression of the indicator. We found that some retinal ganglion cells exhibit spontaneous transient increases in PKA activity and cAMP concentration across the cell soma (Figure 2). Blockade of retinal waves eliminated the PKA transients. We also found that PKA activity increased within 5 seconds following a retinal wave. This is consistent with the hypothesis that retinal waves drive activation of the cAMP/PKA pathway through a calcium mediated mechanism. The implications for a role of these second messenger transients in neurodevelopment are explored below. Here was use these data as an example for illustrating various properties of cAMP and PKA indicators.
Fig. 2. Spontaneous changes in cAMP levels and PKA activity in retinal ganglion cells during retinal waves.
A. Time course of FCFP (blue), FYFP (yellow) recorded simultaneously and averaged over the cell body of a RGC expressing ICUE2. The ICUE2 FRET ratio is computed as FYFP/FCFP, inverted to show cAMP increases as upward deflections, corrected for CFP bleedthrough into YFP channel, and corrected for differential bleaching of CFP and YFP.
B. Time course of FCFP (blue), FYFP (yellow) recorded simultaneously and averaged over the cell body of a RGC expressing AKAR2.2. The ratio is computed as FYFP/FCFP, corrected for CFP bleedthrough into YFP channel and corrected for differential bleaching. From Dunn, Wang et al., 2006.
While these indicators provide significant opportunities to advance our understanding about the dynamics of the cAMP/PKA pathway, the results must be interpreted with caution. First, there may be a limitation to the sensitivity of the reporters. For example, during waves cAMP and PKA activity transients were observed in only a small subset of RGCs although all RGCs have spontaneous depolarizations. We found that depolarizations lasting less than one second did not reliably induce a detectable change in the FRET ratio of AKAR2.2 while depolarizations greater than two seconds did. We cannot determine from these experiments whether depolarizations less than one second induced changes in PKA activity that were below the sensitivity of our imaging system or whether activation of the cAMP/PKA pathway requires a threshold influx of calcium.
Second, the timecourse of the response is a convolution of the kinetics of the indicators and the second messenger cascade. For example, we found that the PKA-based cAMP sensor had a slower rise time to bath application of forskolin and IBMX than ICUE2. This may be due to the fact that a change in FRET ratio for the PKA-based cAMP sensor requires the binding of four cAMP molecules while ICUE2 requires the binding of a single cAMP molecule. Other indicators featuring a single cAMP binding site had comparable kinetics to ICUE2 (Nikolaev et al., 2004; Nikolaev et al., 2006). The timecourse of AKAR response is determined by the rate at which AKAR is determined by three components: cAMP accumulation. PKA phosphorylation rate, and the intrinsic kinetics of the indicator. Uncaging cAMP in cardiac myocytes led to an immediate increase in FRET ratio change of AKAR2, suggesting that the delay due to phosphorylation was less than five seconds (Saucerman et al., 2006). Therefore the predominant component of AKAR’s time course is cAMP accumulation, However, since AKAR reports PKA activity, and a single PKA catalytic subunit can phosphorylate many target substrates, AKAR may have faster response kinetics than the indicators relying upon cAMP binding sites. Since AKAR rapidly responds to changes in cAMP concentration, the time to peak has been used as measure of the timecourse of the propagation of cAMP/PKA signals across the cytosol. In response to bath application of forskolin, the AKAR2.2 FRET ratio in the soma of hippocampal neurons increases immediately upon application of the drug, and reaches to 90% of its maximum in 2.5- min (Gervasi et al., 2007). In response to short depolarizations and short applications of forskolin the AKAR2.2 FRET ratio in the soma of retinal ganglion cells reaches to its maximum in 20 s. This difference with hippocampal neurons is likely due to the shorter stimulus time, as cAMP will continue to accumulate during constant exposure to forskolin. It is important to note that the temporal response of AKAR to phosphorylation by PKA, whether it is the onset of the response, the rate at which the ratio change increases and decreases, or the maximum FRET ratio change, may be different than the endogenous targets of PKA.
Third, overexpression of the various FRET indicators may disrupt the cAMP/PKA pathway in neurons through a variety of ways. 1) Indicators that contain high affinity binding sites for cAMP, may buffer cAMP and therefore interfere with the endogenous signaling and feedback on the pathway. 2) Indicators, particularly the PKA-based and full length Epac-based indicators, may provide diffusion barriers that spatially sequester the cAMP more than usual. It has been suggested that the limited diffusibility of PKA allows it to serve as a barrier to cAMP diffusion in the same way that some calcium binding proteins limit the diffusion of calcium, thereby overemphasizing the presence of microdomains (Saucerman et al., 2006). 3) Catalytically active enzymes may alter PKA signaling in basal conditions, as was reported for FlCRhR (Goaillard et al., 2001). However, in hippocampal neurrons, AKAR overexpression did not block PKA-dependent phosphorylation of an endogenous ion channel indicating endogenous PKA substrates were not affected (Gervasi et al., 2007).
Fourth, it has been reported that FRET ratios decrease in response to nonspecific biochemical interactions with ATP (Willemse et al., 2007). Hence, it is critical to determine whether changes in FRET ratio are a result of activation of cAMP as opposed a result of changes in ATP. For this reason, it is advantageous to use pairs of indicators that have opposite FRET ratio changes in response to increases in the cAMP/PKA pathway, for example AKARs increase FRET ratio with increased PKA activity, while PKA- and Epac-based FRET sensors decrease FRET ratio with increased cAMP concentration.
Imaging the interplay of intracellular cAMP and calcium concentrations
The close interplay of cAMP and calcium pathways and the importance for both in guiding cellular responses to various inputs make combined imaging of the two an enticing prospect (DeBernardi and Brooker, 1996). For example in developing spinal cord neurons, certain patterns of calcium influx lead to transient increases in cAMP, and, reciprocally, cAMP levels regulated the frequency of calcium transients (Gorbunova and Spitzer, 2002) (Figure 3). This has led to the idea that the frequency of calcium transients is tuned to drive cAMP transients (Zaccolo and Pozzan, 2003; Borodinsky and Spitzer, 2006; Willoughby and Cooper, 2006). Simultaneous imaging of Epac based FRET reporters and the calcium indicator, fura-2, have led to detailed description of the interactions between calcium and cAMP oscillations in various non-neuronal cell types (Landa et al., 2005; Harbeck et al., 2006; Willoughby and Cooper, 2006). Applying these quantitative methods to spontaneously active neurons will yield tremendous insight into the function of periodic calcium and cAMP transients.
Figure 3. Spontaneous oscillations in cAMP are driven by certain patterns of calcium spikes in spinal neurons.
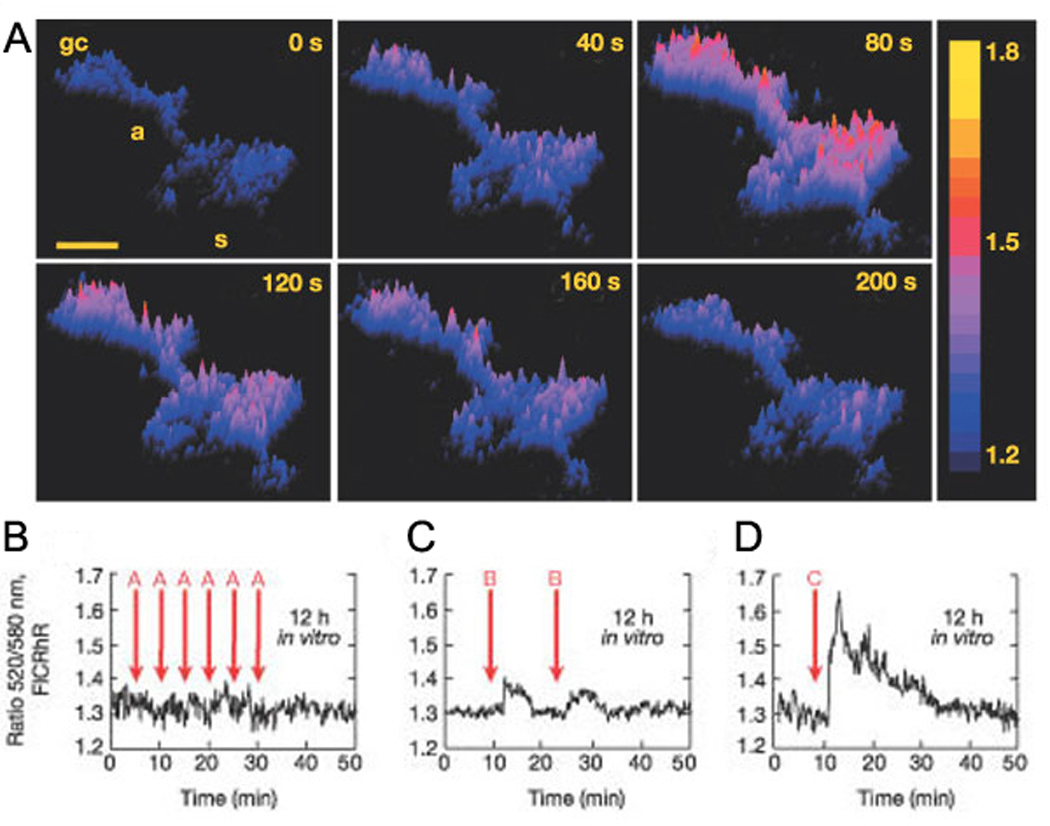
A. Surface plot images show a spontaneous, transient increase in cAMP concentration in an immature spinal neuron loaded with the ratiometric dye, FlCRhR. Color scale represents the 520 nm/580 nm fluorescence ratio. (s) soma, (a) axon, (gc) growth cone. Scale bar, 10 µm. B-D. Fluorescence ratio traces of F520/F580 in a spinal neuron loaded with FlCRhR show cAMP response to induced calcium influx. Arrows indicate stimulated Ca2+ spikes. (A) single spikes, (B) three spikes, (C) five spikes. From Gorbunova and Spitzer, 2002.
Hypotheses regarding the role of periodic activation of second messengers in neurodevelopment
There are several examples throughout the nervous system that indicate that infrequent activation of neurons is critical for driving specific developmental programs. These examples can be broken into two categories – regulation of transcription and post-translational modification. Using transcriptional assays, several groups have found that certain transcription factors are preferentially activated by rhythmic stimuli, not continuous stimuli (Itoh et al., 1995; Fields et al., 1997; Itoh et al., 1997; Dolmetsch et al., 1998; Li et al., 1998). In addition, altering the pattern of rhythmic activity in immature spinal cord neurons alters neurotransmitter expression, likely through transcriptional regulation (Borodinsky et al., 2004). Similarly, Hanson and Landmesser found that the frequency of rhythmic bursting in spinal motoneurons was necessary for expression of ephrinA4 (Hanson and Landmesser, 2004).
There is also evidence that periodic activation of networks alters post-translational modifications of existing proteins. Post-translation modifications such as phosphorylation are typically thought of as rapid onset, transient alterations of proteins. The repetitive nature of spontaneous activity offers a mechanism by which post-translational modifications might be maintained over long periods (Wu et al., 2001). For example, decreasing the frequency of periodic bursting in spinal cord neurons leads to a lack of polysialic acid on NCAM in spinal motoneurons which leads to axon pathfinding errors, probably by way of failed defasiculatuion (Hanson and Landmesser, 2004). Whether this represents a general function of spontaneous activity in developing neurons remains to be determined.
Implications for activity-dependent developmental processes in visual system
Do slow oscillations in cAMP levels play a role in refinement of retinal ganglion cell projections? The cAMP/PKA pathway has been implicated by findings that show mice that lack adenylyl cyclase 1 (AC1) have both reduced eye-specific segregation of retinogeniculate axons within the dLGN (Ravary et al., 2003) and reduced retinotopic refinement in the SC (Ravary et al., 2003; Plas et al., 2004). AC1 is found in the retina, dLGN, and SC (Ravary et al., 2003; Plas et al., 2004; Nicol et al., 2005). Recently, Nicol et al. (Nicol et al., 2006) used a co-culture system to demonstrate that retinal explants from WT mice refine normally in the presence of explants from an AC1−/− superior colliculus, while retinal explants from AC1−/− mice failed to refine in the presence of SC explants from WT mice. Based on these studies, it was proposed that the failure in segregation is due to a defect of AC1 in the retina.
How does AC1 in the retina regulate activity-dependent refinement? First, it may be functioning cell autonomously in retinal ganglion cells. Recently, it has been reported that AC1 is necessary in retinal ganglion cells for repulsive responses to exogenous ephrin (Nicol et al., 2006). In addition, it was demonstrated spontaneous retinal activity is necessary for repulsive responses to ephrin (Nicol et al., 2007). This lack of responsiveness is rescued by uncaging cAMP in a periodic manner. These results lead to the intriguing hypothesis that retinal waves drive periodic activation of the cAMP/PKA pathway that is required for ephrin mediated repulsion. Second, AC1 modulates synaptic transmission at retinocollicular synapses (Shah et al., 2007), and therefore may be that the level of reading out the activity-dependent competition that leads to refinement (Katz and Shatz, 1996; Crair, 1999; Butts, 2002; Butts et al., 2007). Third, mice lacking normal level of phosphorylated CREB, a target of PKA activity have reduced map refinement, indicating a role of postsynaptic cAMP/PKA signaling in LGN neurons (Pham et al., 2001). By using imaging to determine the spatial and temporal properties of cAMP/PKA signaling, we will be able to gain insights in the specific developmental mechanisms by which activity influences several aspects of neural development.
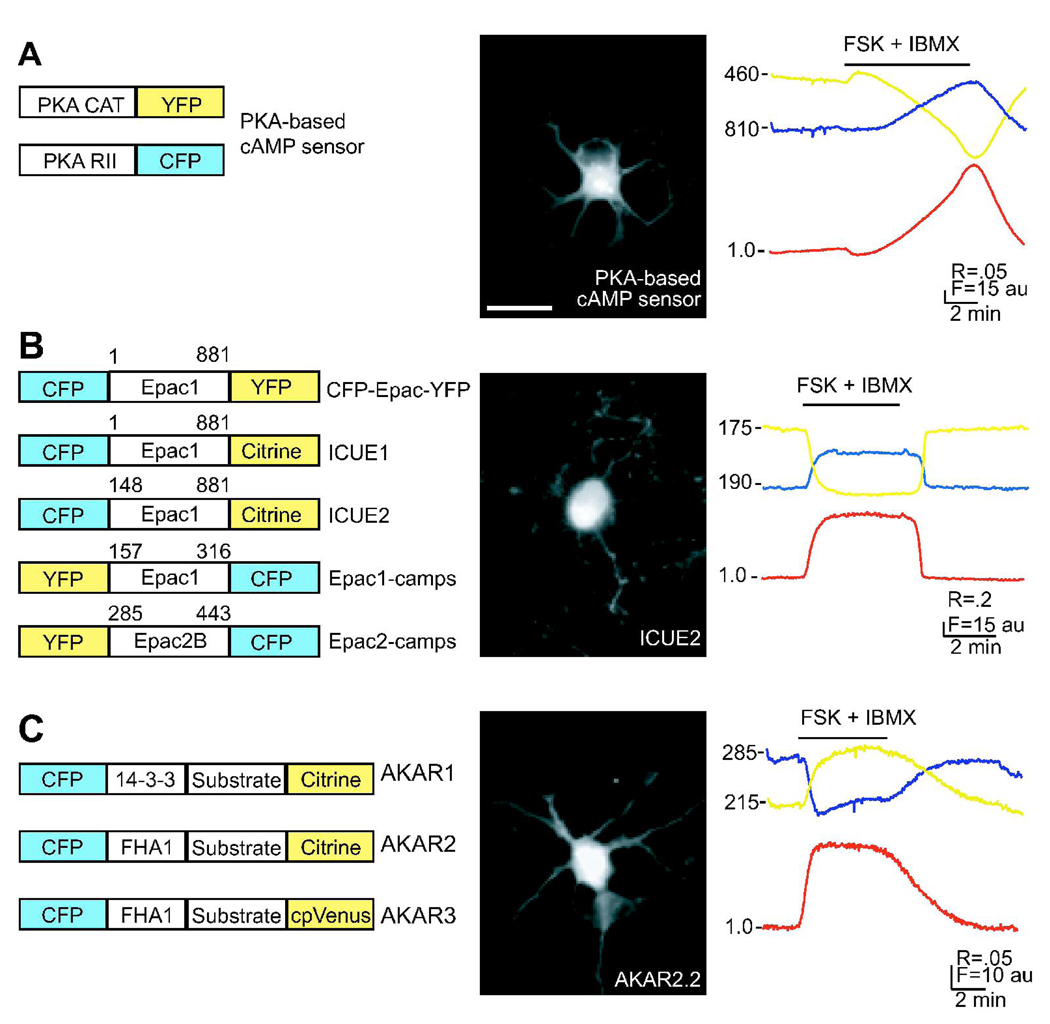
Figure 1. Schematics of genetically encoded, FRET based cAMP level and PKA activity sensors.
A. LEFT: Schematic of the PKA-based cAMP sensors. CFP and YFP are C-terminal fusions to the regulatory and catalytic subunits of PKA (Lissandron, 2005). MIDDLE: Fluorescence image of a dissociated retinal neuron expressing PKA based cAMP sensor. RIGHT: Time course of FCFP (blue), FYFP (yellow), and the FRET ratio (red) of FYFP/FCFP for the PKA-based cAMP sensor during application of both the adenylyl cyclase activator forskolin (10µM) and phosphodiesterase inhibitor IBMX (100µM). Bar represents time of drug applications. All ratios are corrected for CFP bleedthrough into YFP channel and differential bleaching of the two fluorophores. Elevation of cAMP leads to decreases in FRET ratio. Ratio trace is inverted to show increases in cAMP as upward deflections.
B. LEFT: Schematics of EPAC-based sensors: Different truncations of the exchange protein activated by CAMP (Epac) are sandwiched between a CFP/YFP FRET pair. The different variations have different binding affinities for cAMP (Ponsioen, 2004). MIDDLE: Fluorescence image of a dissociated retinal neuron expressing ICUE2. RIGHT: Time course of FCFP (blue), FYFP (yellow), and the FRET ratio (red) of FYFP/FCFP for ICUE2 where elevation of cAMP leads to decreases in FRET ratio. Ratio trace is inverted to show increases in cAMP as upward deflections.
C. LEFT: Schematic of the PKA activity reporter, AKAR3. Modified from Zhang, 2001. A-kinase-activity reporter (AKAR) is a fusion of CFP and YFP to either a FHA1 or 14-3-3 phosphobinding region and PKA target substrate. Different variations have varying dynamic range and dephosphorylation rates. MIDDLE: Fluorescence image of a dissociated retinal neuron expressing AKAR2.2. RIGHT: Time course of FCFP (blue), FYFP (yellow), and the FRET ratio (red) of FYFP/FCFP for the AKAR2.2 where elevation of PKA activity leads to increases in FRET ratio.
MIDDLE and RIGHT, Modified from Dunn, Wang et al. 2006
References
- Adams SR, Harootunian AT, Buechler YJ, Taylor SS, Tsien RY. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 1991;349:694–697. doi: 10.1038/349694a0. [DOI] [PubMed] [Google Scholar]
- Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nat Neurosci. 2005;8:988–990. doi: 10.1038/nn1502. Epub 2005 Jul 2010. [DOI] [PubMed] [Google Scholar]
- Aguilo A, Schwartz TH, Kumar VS, Peterlin ZA, Tsiola A, Soriano E, Yuste R. Involvement of cajal-retzius neurons in spontaneous correlated activity of embryonic and postnatal layer 1 from wild-type and reeler mice. J Neurosci. 1999;19:10856–10868. doi: 10.1523/JNEUROSCI.19-24-10856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochemical And Biophysical Research Communications. 2006;348:716–721. doi: 10.1016/j.bbrc.2006.07.136. [DOI] [PubMed] [Google Scholar]
- Bacskai BJ, Hochner B, Mahaut-Smith M, Adams SR, Kaang BK, Kandel ER, Tsien RY. Spatially resolved dynamics of cAMP and protein kinase A subunits in Aplysia sensory neurons. Science. 1993;260:222–226. doi: 10.1126/science.7682336. [DOI] [PubMed] [Google Scholar]
- Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr Opin Cell Biol. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. Epub 2007 Feb 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Spitzer NC. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE. 2006;2006:pe22. doi: 10.1126/stke.3362006pe22. [DOI] [PubMed] [Google Scholar]
- Butts DA. Retinal waves: implications for synaptic learning rules during development. Neuroscientist. 2002;8:243–253. doi: 10.1177/1073858402008003010. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A Burst-Based "Hebbian" Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS Biol. 2007;5:e61. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin-Le Brun I, Ferrand N, Caillard O, Tosetti P, Ben-Ari Y, Gaiarsa JL. Spontaneous synaptic activity is required for the formation of functional GABAergic synapses in the developing rat hippocampus. J Physiol. 2004;559:129–139. doi: 10.1113/jphysiol.2004.065060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC. Neuronal activity during development: permissive or instructive? Curr Opin Neurobiol. 1999;9:88–93. doi: 10.1016/s0959-4388(99)80011-7. [DOI] [PubMed] [Google Scholar]
- DeBernardi MA, Brooker G. Single cell Ca2+/cAMP cross-talk monitored by simultaneous Ca2+/cAMP fluorescence ratio imaging. Proc Natl Acad Sci U S A. 1996;93:4577–4582. doi: 10.1073/pnas.93.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci U S A. 2004;101:16513–16518. doi: 10.1073/pnas.0405973101. Epub 12004 Nov 16515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression [see comments] Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Dunn TA, Wang CT, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, Tsien RY, Feller MB. Imaging of cAMP levels and protein kinase a activity reveals that retinal waves drive oscillations in second-messenger cascades. Journal Of Neuroscience. 2006;26:12807–12815. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature. 2006;439:349–352. doi: 10.1038/nature04410. [DOI] [PubMed] [Google Scholar]
- Feller MB. Spontaneous correlated activity in developing neural circuits. Neuron. 1999;22:653–656. doi: 10.1016/s0896-6273(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science. 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;507(Pt 1):219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi N, Hepp R, Tricoire L, Zhang J, Lambolez B, Paupardin-Tritsch D, Vincent P. Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J Neurosci. 2007;27:2744–2750. doi: 10.1523/JNEUROSCI.5352-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Vincent P. Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline thalamic neurones by activating 5-HT(7) receptors. J Physiol. 2002;541:453–465. doi: 10.1113/jphysiol.2001.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM, Vincent PV, Fischmeister R. Simultaneous measurements of intracellular cAMP and L-type Ca2+ current in single frog ventricular myocytes. J Physiol. 2001;530:79–91. doi: 10.1111/j.1469-7793.2001.0079m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca(2+) spikes. Nature. 2002;418:93–96. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- Gust J, Wright JJ, Pratt EB, Bosma MM. Development of synchronized activity of cranial motor neurons in the segmented embryonic mouse hindbrain. J Physiol. 2003;550:123–133. doi: 10.1113/jphysiol.2002.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. Journal Of Neuroscience. 2003;23:587–600. doi: 10.1523/JNEUROSCI.23-02-00587.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Harbeck MC, Chepurny O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Simultaneous optical measurements of cytosolic Ca2+ and cAMP in single cells. Sci STKE. 2006;2006:16. doi: 10.1126/stke.3532006pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel CM, Vincent P, Adams SR, Tsien RY, Selverston AI. Spatio-temporal dynamics of cyclic AMP signals in an intact neural circuitm. Nature. 1996;384:166–169. doi: 10.1038/384166a0. [DOI] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD. Nob mice wave goodbye to eye-specific segregation. Neuron. 2006;50:175–177. doi: 10.1016/j.neuron.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Itoh K, Ozaki M, Stevens B, Fields RD. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Khalilov I, Le Van Quyen M, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron. 2005;48:787–796. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Kim YT, Wu CF. Reduced growth cone motility in cultured neurons from Drosophila memory mutants with a defective cAMP cascade. J Neurosci. 1996;16:5593–5602. doi: 10.1523/JNEUROSCI.16-18-05593.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. J Biol Chem. 2007;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa LR, Jr., Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem. 2005;280:31294–31302. doi: 10.1074/jbc.M505657200. Epub 32005 Jun 31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Lissandron V, Terrin A, Collini M, D'Alfonso L, Chirico G, Pantano S, Zaccolo M. Improvement of a FRET-based indicator for cAMP by linker design and stabilization of donor-acceptor interaction. Journal Of Molecular Biology. 2005;354:546–555. doi: 10.1016/j.jmb.2005.09.089. [DOI] [PubMed] [Google Scholar]
- Lissandron V, Zaccolo M. Compartmentalized cAMP/PKA signalling regulates cardiac excitation-contraction coupling. Journal Of Muscle Research And Cell Motility. 2006;27:399–403. doi: 10.1007/s10974-006-9077-2. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg PJ, Ono S, Minamide LS, Takahashi M, Bamburg JR. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Munck S, Bedner P, Bottaro T, Harz H. Spatiotemporal properties of cytoplasmic cyclic AMP gradients can alter the turning behaviour of neuronal growth cones. Eur J Neurosci. 2004;19:791–797. doi: 10.1111/j.0953-816x.2004.03118.x. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Muzerelle A, Bachy I, Ravary A, Gaspar P. Spatiotemporal localization of the calcium-stimulated adenylate cyclases, AC1 and AC8, during mouse brain development. J Comp Neurol. 2005;486:281–294. doi: 10.1002/cne.20528. [DOI] [PubMed] [Google Scholar]
- Nicol X, Muzerelle A, Rio JP, Metin C, Gaspar P. Requirement of adenylate cyclase 1 for the ephrin-A5-dependent retraction of exuberant retinal axons. J Neurosci. 2006;26:862–872. doi: 10.1523/JNEUROSCI.3385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol X, Voyatzis S, Muzerelle A, Narboux-Neme N, Sudhof TC, Miles R, Gaspar P. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. Epub 32004 Jul 37211. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. Epub 2006 Oct 1012. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr Opin Neurobiol. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Pham TA, Rubenstein JL, Silva AJ, Storm DR, Stryker MP. The cre/creb pathway is transiently expressed in thalamic circuit development and contributes to refinement of retinogeniculate axons. Neuron. 2001;31:409–420. doi: 10.1016/s0896-6273(01)00381-6. [DOI] [PubMed] [Google Scholar]
- Plas DT, Visel A, Gonzalez E, She WC, Crair MC. Adenylate Cyclase 1 dependent refinement of retinotopic maps in the mouse. Vision Res. 2004;44:3357–3364. doi: 10.1016/j.visres.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Ponsioen B, Zhao J, Riedl J, Zwartkruis F, van der Krogt G, Zaccolo M, Moolenaar WH, Bos JL, Jalink K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. Embo Reports. 2004;5:1176–1180. doi: 10.1038/sj.embor.7400290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravary A, Muzerelle A, Herve D, Pascoli V, Ba-Charvet KN, Girault JA, Welker E, Gaspar P. Adenylate cyclase 1 as a key actor in the refinement of retinal projection maps. J Neurosci. 2003;23:2228–2238. doi: 10.1523/JNEUROSCI.23-06-02228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. Epub 12001 Oct 13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J, Goto H, Cui Q, Chen PB, Harvey AR. cAMP regulates axon outgrowth and guidance during optic nerve regeneration in goldfish. Mol Cell Neurosci. 2005;30:452–464. doi: 10.1016/j.mcn.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Cline HT. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J Neurobiol. 2004;59:134–146. doi: 10.1002/neu.10344. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Sato M, Umezawa Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J Biol Chem. 2003;278:30945–30951. doi: 10.1074/jbc.M212167200. [DOI] [PubMed] [Google Scholar]
- Saucerman JJ, Zhang J, Martin JC, Peng LX, Stenbit AE, Tsien RY, McCulloch AD. Systems analysis of PKA-mediated phosphorylation gradients in live cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103:12923–12928. doi: 10.1073/pnas.0600137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TH, Rabinowitz D, Unni V, Kumar VS, Smetters DK, Tsiola A, Yuste R. Networks of coactive neurons in developing layer 1. Neuron. 1998;20:541–552. doi: 10.1016/s0896-6273(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Shah RD, Chandrasekaran AR, Anishchenko A, Dunn TA, Elstrott J, Wang C-T, Feller MB, Crair MC. Personal coummunication. 2007. In.
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Sonnenburg WK, Rybalkin SD, Bornfeldt KE, Kwak KS, Rybalkina IG, Beavo JA. Identification, quantitation, and cellular localization of PDE1 calmodulin-stimulated cyclic nucleotide phosphodiesterases. Methods. 1998;14:3–19. doi: 10.1006/meth.1997.0561. [DOI] [PubMed] [Google Scholar]
- Ting AY, Kain KH, Klemke RL, Tsien RY. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc Natl Acad Sci U S A. 2001;98:15003–15008. doi: 10.1073/pnas.211564598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Progress In Neurobiology. 2005;76:213–235. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Vincent P, Brusciano D. Cyclic AMP imaging in neurones in brain slice preparations. J Neurosci Methods. 2001;108:189–198. doi: 10.1016/s0165-0270(01)00393-4. [DOI] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zheng JQ. cAMP-mediated regulation of neurotrophin-induced collapse of nerve growth cones. J Neurosci. 1998;18:4973–4984. doi: 10.1523/JNEUROSCI.18-13-04973.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Storm DR. Ca2+ inhibition of type III adenylyl cyclase in vivo. J Biol Chem. 1995;270:21480–21486. doi: 10.1074/jbc.270.37.21480. [DOI] [PubMed] [Google Scholar]
- Willemse M, Janssen E, de Lange F, Wieringa B, Fransen J. ATP and FRET--a cautionary note. Nat Biotechnol. 2007;25:170–172. doi: 10.1038/nbt0207-170. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP. J Cell Sci. 2006;119:828–836. doi: 10.1242/jcs.02812. [DOI] [PubMed] [Google Scholar]
- Wong RO, Meister M, Shatz CJ. Transient period of correlated bursting activity during development of the mammalian retina. Neuron. 1993;11:923–938. doi: 10.1016/0896-6273(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Wong ROL. Role of retinal waves in visual system development. Annual Review of Neuroscience. 1999:22. doi: 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. Calmodulin-regulated adenylyl cyclases and neuromodulation. Curr Opin Neurobiol. 1997;7:391–396. doi: 10.1016/s0959-4388(97)80068-2. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, De Giorgi F, Cho CY, Feng LX, Knapp T, Negulescu PA, Taylor SS, Tsien RY, Pozzan T. A genetically encoded, fluorescent indicator for cyclic AMP in living cells. Nature Cell Biology. 2000;2:25–29. doi: 10.1038/71345. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. CAMP and Ca2+ interplay: a matter of oscillation patterns. Trends Neurosci. 2003;26:53–55. doi: 10.1016/s0166-2236(02)00017-6. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts betaadrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–573. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]