Abstract
In the “selective” cholesteryl ester (CE) uptake process, surface-associated lipoproteins [high density lipoprotein (HDL) and low density lipoprotein] are trapped in the space formed between closely apposed surface microvilli (microvillar channels) in hormone-stimulated steroidogenic cells. This is the same location where an HDL receptor (SR-BI) is found. In the current study, we sought to understand the relationship between SR-BI and selective CE uptake in a heterologous insect cell system. Sf9 (Spodoptera frugiperda) cells overexpressing recombinant SR-BI were examined for (i) SR-BI protein by Western blot analysis and light or electron immunomicroscopy, and (ii) selective lipoprotein CE uptake by the use of radiolabeled or fluorescent (BODIPY-CE)-labeled HDL. Noninfected or infected control Sf9 cells do not express SR-BI, show microvillar channels, or internalize CEs. An unexpected finding was the induction of a complex channel system in Sf9 cells expressing SR-BI. SR-BI-expressing cells showed many cell surface double-membraned channels, immunogold SR-BI, apolipoprotein (HDL) labeling of the channels, and high levels of selective HDL-CE uptake. Thus, double-membraned channels can be induced by expression of recombinant SR-BI in a heterologous system, and these specialized structures facilitate both the binding of HDL and selective HDL-CE uptake.
Early studies from this laboratory described an intricate microvillar system on the surface of steroidogenic cells of rodent tissues (1–4) programmed to internalize bulk quantities of lipoprotein-derived cholesteryl esters (CEs) by the “selective” CE uptake process (5). In the selective process, surface-associated cholesterol-rich lipoproteins [high density lipoprotein (HDL) or low density lipoprotein (LDL), regardless of apolipoprotein composition or chemical modification] release CEs directly into the steroidogenic cells without internalization of the lipoprotein particle itself (6–9). It was found that cells of the trophic hormone-stimulated intact rodent adrenal or luteinized ovary relied heavily on selective CE uptake to fulfill their cholesterol needs in steroid hormone production (1, 3, 10, 11), and during this process, exogenously supplied lipoproteins (human or rodent HDL or LDL) became trapped in space formed between closely apposed microvilli on the surface of the cells (2–4). We refer to this general region of the steroidogenic cell surface as the microvillar compartment and the specialized space created between adjacent microvilli as microvillar channels (2–4). Often inverted microvilli form double-membraned channels within the peripheral cytoplasm of the cells as they make close contact with the invaginated portion of the adjacent cytoplasm (1–3).
With the recent understanding that the initial or extracellular phase of the selective uptake process involves lipoprotein binding to a regulatable HDL receptor protein (SR-BI) (8, 9, 12), the relationship of microvillar channel membranes to the localization and expression of SR-BI in different steroidogenic tissues has been of interest. It turns out that in intact tissues of the hormone-primed rat adrenal and ovary, SR-BI is highly expressed in the microvillar compartment of the steroidogenic cells and is shown by electron microscopy to be almost exclusively localized to the membranes lining the microvillar channels. These are the very same sites where circulating lipoproteins are trapped before selective CE uptake (13). SR-BI expression in these sites is hormone regulatable increasing with use of trophic hormones or Bt2cAMP (14), even under conditions where excessive, superphysiological levels of hormone have led to tissue desensitization with shutdown of the steroidogenic pathway and steroid hormone production (13, 15). In hormone-stimulated steroidogenic cells maintained in culture (which have lost the majority of microvilli seen in intact tissues), SR-BI expression at the cell surface appears limited to regions with ample amounts of microvillar remnants (E.R., unpublished observations).
However, in some steroidogenic cells, the majority of double-membraned channels immunolabeled for SR-BI are not found on the cell surface (as in the adrenal and ovary), but are found relatively deep within the peripheral cytoplasm of the cells. Leydig cells of the rat testis demonstrate this kind of channel formation when the animals have been treated for several days with human chorionic gonadotrophin (15). In Leydig cells of the normal mature rat, SR-BI is barely detectable by Western blot analyses or immunocytochemical techniques, and it is believed that such Leydig cells do not use peripheral lipoprotein-CEs for hormone production (15). In contrast, after treatment with human chorionic gonadotropin and desensitization of the testis (i.e., loss of human chorionic gonadotropin receptors and the consequent low levels of testosterone production), Leydig cell-selective HDL-CE uptake is doubled and SR-BI expression is increased 20-fold (15). Under these conditions, occasional Leydig cells show SR-BI localized to surface microvilli (as in cells of the adrenal and ovary), but for the most part, the SR-BI is localized to an elaborate and complex membrane channel system deep within the cytoplasm of the cell (15). We believe that the intracellular channels observed in Leydig cells represent deeply invaginated membranes that may have originated at the cell surface.
Thus, many questions remain. What is the relationship of extracellular vs. intracellular double-membraned channels to hormone-induced SR-BI in steroidogenic cells? Do double-membraned channels always bear SR-BI? Do such channels exist only in steroidogenic cells? When present, do they always function in selective lipoprotein CE uptake? Is SR-BI a prerequisite for channel production?
The current study seeks to address these issues by using a heterologous cell system which does not normally express SR-BI but is induced to do so by using a baculovirus expression system.
Materials and Methods
Materials.
Na125I (carrier free; specific activity, 644 GBq/mg; 17.4 Ci/mmol) was obtained from New England Nuclear. [1α, 2α, 6,7-N-3H]cholesteryl oleolyl ether (COE) (specific activity, 1.78 TBq/mmol; 48 Ci/mmol) was obtained from Amersham Pharmacia. Enhanced chemiluminescence Western blotting kit was obtained from Kirkegaard & Perry Laboratories. Biotinylated goat anti-rabbit IgG, FITC-avidin, cholesteryl oleate, cholesterol, egg phosphatidylcholine, sphingomyelin, plasma fibronectin, and BSA were supplied by Sigma. Cholesteryl BODIPY FL C12 (BODIPY-CE; cholesteryl 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoate) was purchased from Molecular Probes. All other reagents were analytical grade.
SR-BI Expression Vector.
A full-length rat SR-BI cDNA was cloned by reverse transcription of rat granulosa cell RNA followed by PCR amplification of the cDNA by using Pfu DNA polymerase. Oligonucleotide primers were designed against rat ovarian SR-BI nucleotide sequence (16) in which the forward primer 5′-aagcttCACGCGGACATGGGCGTCAGC-3′, and the reverse primer 5′-tctagaGTCTGACCAAGCTATCAGGTT-3′ contained HindIII or XbaI endonuclease restriction sites, respectively (designated in lowercase). The resulting cDNA was subcloned into pCR2.1 (Invitrogen) and sequenced in entirety on both strands. After sequencing, the full-length cDNA was subjected to restriction digest (HindIII/XbaI), Klenow treatment, and subsequent blunt-end ligation into the Klenow-filled EcoRI site of the baculovirus transfer vector pAcMP3 (PharMingen).
Insect Sf9 (Spodoptera frugiperda) cells were grown and maintained in TNM-FH medium (PharMingen) as monolayer cultures at 27°C. Cells were cotransfected with BaculoGold baculovirus DNA and pAcMP3-SR-BI as per manufacturer's instructions (PharMingen). In addition, one cotransfection contained empty pAcMP3 vector to generate baculovirus that would serve as a control for infection. After 5 days, medium was collected and amplified one time before plaque assay. Viral clones were tested for recombinant SR-BI protein expression by immunoblot analysis. Clones expressing the highest yield of recombinant protein were further amplified (2–3 cycles) until working stock titers exceeded 2 × 108 plaque-forming units/ml as determined by plaque assay.
Morphological Techniques.
Sf9 cells were plated in protein-free insect cell medium (PharMingen) and infected with SR-BI or control baculovirus stocks (multiplicity of infection of 10) in protein-free medium containing 10% lipoprotein-deficient FBS. Cells were harvested and processed for morphological procedures 48 h after infection.
For immunohistochemical localization of SR-BI, cells were grown on fibronectin-coated slides, fixed for 10 min in 4% paraformaldehyde in PBS, treated with ethanolamine/0.2% Triton X-100/2% normal goat serum 1 h at room temperature, and incubated with polyclonal anti-SR-BI (1:1,000–2,000) at 4°C overnight. Antibody-treated cells were subjected to standard immunochemical-labeling techniques (6, 14, 15, 17, 18) and viewed by confocal microscopy (Stanford University Cell Science Imaging Facility), and the resulting images were subsequently colorized by using photoshop technology (Adobe Systems, Mountain View, CA).
For standard electron microscopy, cells were fixed in 2% glutaraldehyde overnight and processed as described (6). The extent of intracellular channel formation was quantified in randomly selected experimental and control cells. Large single thin sections of each block were placed on 100-mesh formvar-coated grids and stained by using lead citrate and uranyl acetate (6). All virus-positive cells were photographed at the 12 o'clock and 6 o'clock position (at 14,000×) without regard to the content of the cell cytoplasm. About 100 cells were examined in this strict manner from each preparation, and ≈100–200 more cells from additional preparations were freely searched for evidence of double-membraned structures. Double-membraned channels were marked on the developed prints and channel lengths were measured. Total channel length for the photographed prints (adjusted for the number of cells screened in different preparations) was used to estimate channel frequency in SR-BI vs. infected control cells. Channel diameters were measured and compared with microvillar channels found in mammalian steroidogenic cells.
For immunocytochemistry (14, 15), the cells were fixed with 4% paraformaldehyde plus 0.05–0.5% glutaraldehyde for 3 h, processed, and embedded in LR Gold resin (Ted Pella, Redding, CA). Ultrathin sections on grids were blocked with 3% normal goat serum or 3% BSA, incubated with primary antibody [polyclonal SR-BI (1:100), human or mouse apolipoprotein A1 (apoA-I) (1:2,000), or preimmune serum (1:100–2,000)] plus control homogenates (10% supernatant of centrifuged homogenate of noninfected Sf9 cells) cells for 1–2 h at room temperature. The control homogenates were used to reduce the substantial nonspecific staining encountered. Labeling was carried out with goat anti-rabbit IgG-10 nm gold (BB International, Cardiff, United Kingdom) for 1 h at room temperature. The sections were stained with osmium vapor, Reynolds' lead citrate, and uranyl acetate before viewing with the electron microscope.
Western Blot Analyses of SR-BI.
Cells were gently removed from tissue culture dishes and washed twice with protein-free medium, and cell pellets (600 × g for 5 min) were stored at −20°C. Cell pellets were thawed on ice and incubated in lysis buffer (100 μl) (19, 20) for 30 min at 37°C before sonication (three bursts of 1 s). Equivalent amounts of total protein (10–20 μg) were subjected to SDS/PAGE (6–7%), transferred to Immobilon-P membranes (Millipore), and probed for SR-BI as described (13–15).
Lipoprotein Preparation.
Apolipoprotein E-free high-density human (h) HDL3 was isolated as described (11, 21). For uptake and internalization studies, hHDL3 preparations were conjugated with residualizing labels, i.e., 125I-labeled dilactitol tyramine (125I-DLT) and [3H]COE (6, 7, 21). For fluorescence microscopy, reconstituted BODIPY-CE-HDL particles were prepared as described (9, 14, 15, 17, 18).
Selective CE Uptake Using 125I-DLT-[3H]COE-hHDL3 or BODIPY-Labeled HDL.
For the dual-radiolabeled HDL studies, ≈106 cells were seeded into 60-mm culture dishes and incubated with 125I-DLT-[3H]COE-hHDL3 (33 μg/ml medium) in the presence or absence of excess unlabeled hHDL3 500 μg/ml medium, to determine nonspecific binding (6, 11, 21). After incubation for 5 h, the HDL-containing medium was removed, and the cells were washed three times with protein-free medium. The cells were lysed with 0.1 M NaOH and lysate was then processed to determine trichloroacetic acid soluble and insoluble 125I radioactivity and organic solvent-extractable 3H radioactivity (5, 11, 21). The values for the cell-associated HDL apolipoprotein, the endocytosed and degraded HDL apolipoprotein, and the selective uptake of HDL CE were obtained as described (5, 11, 21).
To assess selective uptake of lipoprotein CEs morphologically, cells were incubated with reconstituted BODIPY-CE-HDL (12.5 μg/ml) at 27°C for 5 h, washed three times in PBS, fixed in 1.5% glutaraldehyde, and washed and stored in PBS until viewed by confocal microscopy (7, 14).
Miscellaneous Techniques.
The procedure of Markwell et al. (22) was used to quantify protein content of hHDL3 and reconstituted HDL preparations. Protein in the cellular lysates was determined as described by Peterson (23). Cholesterol content of the hHDL3 and reconstituted HDL was determined according to the procedure of Tercyak (24). Polyclonal antibodies against human and rat apoA-I proteins were raised in rabbits. For immunization, rat apoA-I was purified from rat HDL (rHDL) (1.063–1.21 g/ml) by gel filtration followed by heparin-Sepharose affinity chromatography (19). Human apoA-I was purified from hHDL3 essentially as described by Brewer et al. (20).
Results
Expression of SR-BI.
Western blot.
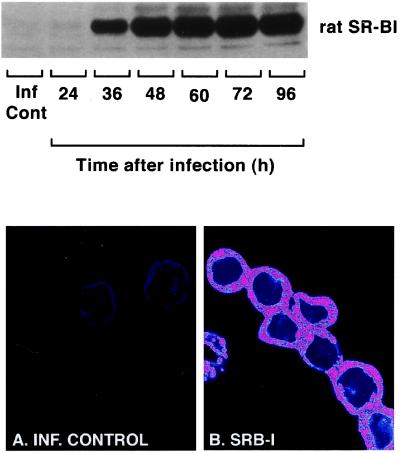
A time course of expression of recombinant rat SR-BI in Sf9 cells is shown in Fig. 1 Upper. SR-BI is detectable at 24 h and reaches maximal expression by 48 h postinfection. Based on these data, cells were infected with recombinant baculovirus for 48 h in all subsequent SR-BI experiments.
Figure 1.
Expression of recombinant rat SR-BI in Sf9 insect cells infected with recombinant baculoviruses encoded for this protein. (Upper) Cell lysates (20 μg) prepared from cells 24, 36, 48, 60, 72, or 96 h postinfection subjected to 7% SDS/PAGE and immunoblotted with anti-SR-BI. The immunoreactive bands were detected by chemiluminescence. (Lower) Infected control cells (A) or SR-BI-expressing cells (B) immunostained for SR-BI, examined by confocal fluorescence microscopy, and subsequently color adjusted by using photoshop techniques. SR-BI-expressing cells showed patchy expression of the SR-BI protein (pink).
Fluorescence microscopy.
Confocal fluorescence microscopy was used to examine the expression of SR-BI in intact Sf9 cells (Fig. 1 Lower). As expected, confocal slices through infected control Sf9 cells (Fig. 1A) exhibit no fluorescence. In contrast, confocal sections through Sf9 cells expressing recombinant SR-BI (Fig. 1B) show substantial background fluorescence of the cell cytoplasm (purple) with patches of high fluorescence (pink). The royal blue color of the enlarged cell nuclei indicates negligible fluorescence.
Electron microscopy.
Morphometric measurements indicate that a large number (≈30%) of SR-BI-expressing Sf9 cells in any given thin section display double-membraned channels both at the cell surface and throughout the cytoplasm (Fig. 2A arrowheads). In contrast, double-membraned channels are never seen in uninfected cells and are found on only extremely rare occasions (0.3%) in infected control cells. The shape of the double-membraned structures are often circular as if cross sections were made through a series of complicated infoldings of the cell surface. The structural detail of these double membranes (e.g., channel width and intermembrane bridges) resemble the microvillar channels described for a variety of rodent steroidogenic tissues (1–4). Viral particles (v) are found on the cell surface and throughout the cell, but rarely with invaginated double membranes.
Figure 2.
Electron micrographs of surface regions of SR-BI-expressing Sf9 cells. (A) A complicated network of double-membraned channels formed at a junction of two Sf9 cells with the cell surface. Standard electron microscopic techniques permit visualization of the convoluted channel membranes with intermembrane striations (➤) plus viral particles (v) near the surface of the cell (★). (B) Similar channel membranes are immunostained by using antiserum to SR-BI and show immunogold particles specifically tracing the outlines of the double membranes (➤). (C) A similar region of the same cell preparation immunostained with preimmune serum is shown, which demonstrates essentially no gold labeling of the channel membranes.
With immunocytochemical staining, it is clear that the double-membraned channels specifically label for SR-BI. One sees this in Fig. 2B where the immunogold labeling faithfully trace the outlines of the double membranes as they appear in the cytoplasm. Similar channel membranes remain unlabeled when treated with preimmune serum (Fig. 2C). In addition, cells overexpressing SR-BI show labeling specific to a few other structures including viral particles and the peripheral membranes of large cytoplasmic vacuoles that may contain vesicles (data not shown).
SR-BI-Expressing Sf9 Insect Cells Bind HDL and Function in Selective CE Uptake.
Use of double-radiolabeled HDL.
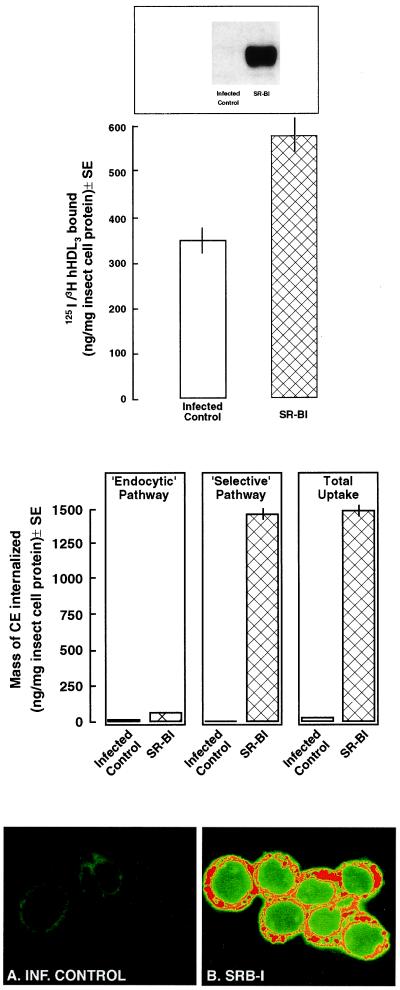
Insect Sf9 cells overexpressing recombinant SR-BI bind substantial amounts of 125I-DLT-[3H]COE-hHDL3 during the 5-h incubation period (Fig. 3 Top). Surface binding of hHDL3 is 40% greater in cells overexpressing SR-BI when compared with infected control cells. Also, noninfected Sf9 cells (data not shown) and infected control cells are found to bind considerable levels of hHDL3; presumably this occurs through an endogenous receptor that is capable of binding lipoprotein but does not crossreact with antiserum to rat SR-BI.
Figure 3.
Binding of 125I-/[3H]hHDL3 and selective CE uptake in Sf9 cells expressing SR-BI. (Top) Sf9 cells infected with recombinant baculovirus coding for SR-BI or infected control incubated for 5 h with 125I-DLT-[3H]COE-hHDL3 plus unlabeled hHDL3. Specific binding was defined as the difference between total and nonspecific binding (in the presence of 500 μg/ml unlabeled hHDL3). The expression of SR-BI protein was confirmed by immunoblotting (see blot at the top of the figure in which 20 μg of cellular lysate was loaded). (Middle) The mass of CEs internalized via the endocytic and selective pathways is shown for infected control and SR-BI-expressing cells. Determination of the mass of CEs internalized via the endocytic and selective pathways is described in Materials and Methods. (Bottom) Infected control and SR-BI-expressing cells were incubated with reconstituted BODIPY-CE-HDL to microscopically identify CE uptake. By using confocal microscopy, the infected control cells (A) show no fluorescence, whereas SR-BI-expressing cells (B) show patchy areas of high (red) or medium (orange) uptake of CEs.
Despite the noted HDL surface binding, essentially no HDL-CE is internalized by infected control cells (Fig. 3 Middle). Also, little HDL-CE is internalized via the endocytic (LDL receptor/LDL receptor-related protein) pathways in either infected control or cells overexpressing SR-BI. Therefore, during the 5-h period in which the cells are incubated with lipoproteins, CE is internalized almost exclusively by the selective pathway in cells expressing SR-BI.
Uptake of BODIPY-labeled HDL-CE.
Fluorescent (BODIPY)-labeled hHDL3 was used to monitor the selective uptake of HDL-CEs in Sf9 cells overexpressing SR-BI. In Fig. 3 Bottom, infected control Sf9 cells incubated for 5 h with the reconstituted HDL (Fig. 3A) showed little uptake (green) of BODIPY-CE. In contrast, similarly treated cells overexpressing SR-BI (Fig. 3B) selectively internalized large amounts of HDL-CEs (high fluorescence, red; and moderate fluorescence, yellow), confirming the results of the dual-radiolabeled hHDL experiments described above.
Relationship of Double-Membraned Channels to HDL Binding and Selective HDL-CE Uptake.
Localization of bound HDL.
To determine whether the newly formed double-membraned channels of SR-BI-infected cells are responsible for the binding (or trapping) of such lipoproteins, Sf9 cells expressing SR-BI were incubated for 5 h with a saturating quantity (500 μg/ml) of hHDL3 or rHDL. The cells were examined at the electron microscope level for the expression of apoA-I, the major apolipoprotein of both hHDL and rHDL, by using immunocytochemical techniques.
In the examined cells, rHDL was easily visualized in surface-associated microvilli of the incubated cells (data not shown), but because hHDL3 particles are substantially smaller than rHDL, it was more difficult to definitively identify the channel particles as newly acquired HDL lipoproteins (rather than the filamentous striations often seen in the channel space). However, confirming results for both incubated particles were observed by using immunocytochemical techniques, i.e., cells expressing SR-BI and incubated with hHDL3 demonstrated surface-associated double-membraned channels prominently labeled with antibodies to hHDL apoA-1 (Fig. 4A), and such cells incubated with rHDL demonstrated surface-associated double-membraned channels labeled with antibodies to rHDL apoA-I (Fig. 4B). No labeling was found in cells similarly incubated with preimmune rabbit serum (data not shown).
Figure 4.
Localization of h- and rHDL after incubation with SR-BI-expressing Sf9 cells. Incubated cells were processed for immunogold staining at the electron microscope level by using the techniques described in Materials and Methods. Cells incubated with hHDL3 (A) were immunolabeled by using antisera against human apoA1; cells incubated with rHDL (B) were immunolabeled by using antisera against rat apoA1. (➤) The specific immuno-(gold)- labeling of both classes of lipoproteins associated with surface microvillar channels in SR-BI-expressing Sf9 cells. ★, Cell surface.
Discussion
The surprising findings of this study have led us to re-examine our thinking regarding the formation, characteristics, and function of double-membraned (microvillar) channels in cells. In physiologically competent adrenals or ovaries of rats and mice, the double-membraned channels are formed by the juxtaposition of adjacent surface microvilli and/or the occasional invagination of a single microvillus of one cell into its own cytoplasm or into the cytoplasm of a neighboring cell (1, 3, 25). In the latter case, one membrane of the channel wall is formed by the inverted microvillus plasma membrane pushing into the cell cytoplasm, whereas the plasma membrane of the probed cell provides the outer channel wall; thus, the channel space itself is lined with only exofacial surfaces of plasma membranes (2). During in situ perfusions of adrenal and luteinized ovarian tissue, the perfused lipoproteins are trapped in such channels and selective uptake of lipoprotein CEs is believed to begin at these sites (1–3, 6, 26). Enriched membrane preparations isolated from the luteinized ovary retain many such microvillar channels in vitro, which are morphologically intact, functionally able to trap lipoproteins, and able to carry out selective HDL-CE uptake (4, 27). Recent studies have shown that in all these situations, the double-membraned channels strongly express the HDL receptor protein, SR-BI. The microvillar channel space itself contains fine (100 Å) cross striations variously referred to as putative HDL remnant particles (15) or unknown structural proteins (2, 3, 13).
Results from the current study have added insights regarding microvillar channels. For example, it is now clear that the existence of double-membraned channels is not restricted to steroidogenic cells. Indeed, channel structures can form in very primitive cells such as Sf9 ovary cells in direct response to the overexpression of recombinant SR-BI. Also, double-membraned channels are not always strictly related to microvilli. The SR-BI-infected Sf9 cells have occasional large patches of microvilli, yet show an abundance of intracellular double membranes strongly and specifically expressing SR-BI. The existence of such intracellularly located double-membraned channels in cells expressing SR-BI is not unknown because they are present also in rat testicular Leydig cells primed with the trophic hormone human chorionic gonadotropin (15).
Most importantly, it now appears that expression of SR-BI, by itself, can provide the stimulus or signal for the formation of double-membraned channels in cells. This is an unexpected finding because it was previously assumed that preformed microvillar channel structures in cells secondarily acquire SR-BI protein after a stimulus (in most cases, a hormonal stimulus) activating steroidogenesis and the selective uptake of lipoprotein CEs. In the current study, noninfected Sf9 insect cells, or control-infected Sf9 cells (i.e., cells infected with baculovirus that does not encode for recombinant proteins), do not have double-membraned channels. However, overexpression of recombinant SR-BI appears to induce the formation of these channels. Indeed, the findings of this study are consistent with the idea that SR-BI not only induces the formation of double-membraned channels, but remains constitutively associated with these channels, which can trap cholesterol-rich lipoprotein particles and initiate massive selective uptake of CEs as measured by both fluorescent and isotope techniques.
It seems likely, however, that the role of SR-BI in the double-membraned channels goes beyond simply tethering lipoproteins to the cell surface. As shown in this report and others (9, 11, 21), the degree of lipoprotein binding is often unrelated to the level of SR-BI-associated selective CE flux in cells. Recent biochemical evidence suggests that SR-BI may also regulate changes in the mass, or organization of cholesterol in cell plasma membranes (e.g., changes in membrane rafts/caveolae) which then leads to alterations in plasma membrane function (28–30). The double-membraned channels described in the present report may represent a structural variant of such SR-BI-induced events.
Overall, we have found Sf9 insect cells to be a remarkably clean and efficient system in which to study all of the parameters associated with the selective uptake of lipoprotein-CEs. Cells overexpressing SR-BI present a fully functional reconstituted selective CE uptake system with the added advantage that noninfected and infected control cells do not exhibit any of the characteristics associated with the selective pathway. Control cells do not express SR-BI receptor proteins and the channel membranes believed to be important to the selective pathway, and are unable to internalize CEs by the selective process. This system is also particularly useful in that it isolates the selective pathway from other cellular cholesterol uptake pathways, i.e., there is no endocytic LDL (or LDL receptor-related protein) receptor-related cholesterol uptake from HDL in Sf9 insect cells. Thus, insect cells overexpressing SR-BI represent a high-capacity, high-performance, and highly specific model of the selective pathway, and may prove to be an ideal system in which to probe new ideas regarding the still puzzling details of selective cholesterol uptake.
Acknowledgments
This work is supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and National Institutes of Health Grant HL33881.
Abbreviations
- CE
cholesteryl ester
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- SR-BI
scavenger receptor class B type I
- apoA-I
apolipoprotein A–I
- hHDL3
human HDL
- DLT
dilactitol tyramine
- COE
cholesteryl oleolyl ether
- rHDL
rat HDL
- BODIPY-CE
cholesteryl BODIPY FL C12 or cholesteryl 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoate
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Reaven E, Chen Y-D I, Spicher M, Azhar S. J Clin Invest. 1984;74:1384–1397. doi: 10.1172/JCI111549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven E, Boyles J, Spicher M, Azhar S. Arteriosclerosis. 1988;8:298–309. doi: 10.1161/01.atv.8.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Reaven E, Spicher M, Azhar S. J Lipid Res. 1989;30:1551–1560. [PubMed] [Google Scholar]
- 4.Reaven E, Shi X-Y, Azhar S. J Biol Chem. 1990;265:19100–19111. [PubMed] [Google Scholar]
- 5.Glass C, Pittman R C, Weinstein D B, Steinberg D. Proc Natl Acad Sci USA. 1983;80:5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reaven E, Tsai L, Azhar S. J Lipid Res. 1995;36:1602–1617. [PubMed] [Google Scholar]
- 7.Reaven E, Tsai L, Azhar S. J Biol Chem. 1996;271:16208–16217. doi: 10.1074/jbc.271.27.16208. [DOI] [PubMed] [Google Scholar]
- 8.Krieger M. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 9.Williams D L, Connelly M A, Temel R E, Swarnakar S, Phillips M C, de la Llera-Moya M, Rothblat G H. Curr Opin Lipidol. 1999;10:329–339. doi: 10.1097/00041433-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Azhar S, Cooper A, Tsai L, Maffe W, Reaven E. J Lipid Res. 1988;29:869–882. [PubMed] [Google Scholar]
- 11.Azhar S, Stewart D, Reaven E. J Lipid Res. 1989;30:1799–1810. [PubMed] [Google Scholar]
- 12.Acton S, Rigotti A, Landschulz K T, Xu S, Hobbs H H, Krieger M. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 13.Reaven E, Nomoto A, Leers-Sucheta S, Temel R, Williams D L, Azhar S. Endocrinology. 1998;139:2847–2856. doi: 10.1210/endo.139.6.6056. [DOI] [PubMed] [Google Scholar]
- 14.Azhar S, Nomoto A, Leers-Sucheta S, Reaven E. J Lipid Res. 1998;39:1616–1628. [PubMed] [Google Scholar]
- 15.Reaven E, Zhan L, Nomoto A, Leers-Sucheta S, Azhar S. J Lipid Res. 2000;41:343–356. [PubMed] [Google Scholar]
- 16.Mizutani T, Sonoda Y, Minegishi T, Wakabayashi K, Miyamoto K. Biochem Biophys Res Commun. 1997;234:499–505. doi: 10.1006/bbrc.1997.6646. [DOI] [PubMed] [Google Scholar]
- 17.Azhar S, Luo Y, Medicherla S, Reaven E. J Cell Physiol. 1999;180:190–202. doi: 10.1002/(SICI)1097-4652(199908)180:2<190::AID-JCP7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 18.Reaven E, Lua Y, Nomoto A, Temel R, Williams D L, van der Westhuyzen D R, Azhar S. Biochim Biophys Acta. 1999;1436:565–576. doi: 10.1016/s0005-2760(98)00169-6. [DOI] [PubMed] [Google Scholar]
- 19.Rifici V A, Eder H A, Swaney J B. Biochim Biophys Acta. 1985;834:205–214. doi: 10.1016/0005-2760(85)90157-2. [DOI] [PubMed] [Google Scholar]
- 20.Brewer B, Ronan R, Meng M, Bishop C. Methods Enzymol. 1986;128:223–226. doi: 10.1016/0076-6879(86)28070-2. [DOI] [PubMed] [Google Scholar]
- 21.Azhar S, Tsai L, Reaven E. Biochim Biophys Acta. 1990;1047:148–160. doi: 10.1016/0005-2760(90)90041-u. [DOI] [PubMed] [Google Scholar]
- 22.Markwell M A K, Haas S M, Bieber L L, Tolbert N E. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 23.Peterson G L. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 24.Tercyak A M. J Nutr Biochem. 1991;2:281–292. [Google Scholar]
- 25.Plump A S, Erickson S K, Weng W, Partin J S, Breslow J L, Williams D L. J Clin Invest. 1996;97:2660–2671. doi: 10.1172/JCI118716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reaven E, Chen Y-D I, Spicher M, Hwang S-F, Mondon C E, Azhar S. J Clin Invest. 1986;77:1971–1984. doi: 10.1172/JCI112526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X-Y, Azhar S, Reaven E. Biochemistry. 1992;31:3230–3236. doi: 10.1021/bi00127a026. [DOI] [PubMed] [Google Scholar]
- 28.De la Llera-Moya M, Rothblat G H, Connelly M A, Kellner-Weibel G, Sakr S W, Phillips M C, Williams D L. J Lipid Res. 1999;40:575–580. [PubMed] [Google Scholar]
- 29.Kellner-Weibel G, de la Llera-Moya M, Connelly M A, Stoudt G, Christian A E, Haynes M P, Williams D L, Rothblat G H. Biochemistry. 2000;39:221–229. doi: 10.1021/bi991666c. [DOI] [PubMed] [Google Scholar]
- 30.Uittenbogaard A, Shaul P W, Yuhanna I S, Blair A, Smart E J. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]