Abstract
Objective:
The aim of this study was to investigate in heart failure (HF) patients whether acute mental stress induces increases in the HF-severity biomarker B-type natriuretic peptide (BNP) and if alcohol consumption is associated with such stress-induced increases.
Method:
Twenty-one male HF patients and 19 male non-HF controls (M = 56 years, SEM = 2) underwent a 15-minute acute mental stress test combining public speaking and mental arithmetic. Plasma levels of BNP were determined immediately before as well as 2 hours after the stress test. Alcohol consumption was assessed by self-reported number of drinks per month and history of use.
Results:
HF patients had higher BNP levels before and after stress, F(1, 38) = 23.42, p < .001, and showed greater stress-induced increases in BNP levels, F(1, 38) = 4.52, p = .04, compared with controls. HF status (β = .32, p = .015, ΔR2 = .10) and higher alcohol consumption (β = .61, p< .001, ΔR2 = .37) were independently associated with higher BNP stress increases. Moreover, higher alcohol consumption moderated the greater BNP stress increases in HF patients but not in controls (β = .49, p < .001, ΔR2 = .20), although alcohol consumption did not differ between groups.
Conclusions:
For individuals with HF, particularly those who drink moderate to more substantial amounts of alcohol, exposure to acute psychological stress leads to increases in circulating levels of BNP, a biomarker which is associated with increased morbidity and mortality in HF.
Heart failure (hf) is a major public health concern associated with significant mortality and morbidity (Haldeman et al., 1999; Rosamond et al., 2008). The diagnosis of HF and the prediction of mortality and HF events are closely associated with elevated plasma levels of B-type natriuretic peptide (BNP; Moe, 2006; Waldo et al., 2008). The physiological effects of BNP are multiple and include natriuresis/diuresis, peripheral vasodilation, and inhibition of the renin-angiotensin-aldosterone system as well as the sympathetic nervous system (Weber et al., 2006; Woods, 2004). The major source of BNP synthesis and secretion is the ventricular myocardium (de Bold et al., 1996). BNP is synthesized and released into the circulation in response to myocardial wall stress due to volume expansion and pressure overload (de Bold et al., 1996; Weber et al., 2006; Woods, 2004).
Although numerous studies document the diagnostic and prognostic properties of BNP for HF severity and mortality (Moe, 2006; Waldo et al., 2008), fewer studies have examined acute changes in BNP levels. Accumulating evidence suggests that in coronary artery disease (CAD) and HF patients' exercise stress, particularly if strenuous, induces immediate acute increases in BNP plasma levels, probably via induction of myocardial ischemia (Marumoto et al., 1995; Sabatine et al., 2004). Given that acute exercise stress has been identified as a potent trigger of acute coronary syndromes (ACSs) in vulnerable persons (Mittleman et al., 1993; Strike et al., 2006b; Willich et al., 1993), one might speculate that acute increases in BNP levels may precede adverse outcomes in HF patients. Indeed, elevated BNP levels have been associated with the sudden onset of ACSs (de Lemos et al., 2001; Moe, 2006; Waldo et al., 2008). Acute psychological stress occurring within 1-2 hours of onset of symptoms has been identified as a further ACS trigger (Strike et al., 2006a, 2006b; Strike and Steptoe, 2005). However, although animal studies suggest that stress hormones can induce up-regulation of natriuretic peptide genes (Nishi-mori et al., 1997), changes in plasma BNP following acute psychological stress have not yet been investigated, either in HF patients or in healthy/asymptomatic individuals.
Alcohol abuse or dependence can be an etiological factor in the development of HF (Djousse and Gaziano, 2008; Kloner and Rezkalla, 2007), whereas light to moderate alcohol consumption seems beneficial in preventing CAD and CAD-related HF in healthy persons (Djousse and Ga-ziano, 2007; Klatsky et al., 2005; O'Keefe et al., 2007). The literature on associations between alcohol consumption and BNP in HF is scarce. To date, one human study assessed associations between alcohol consumption and BNP, although not in HF patients, and those researchers found that current alcohol consumption (yes/no) was independently associated with higher BNP plasma levels, with a 1.5-fold increased risk of having high plasma BNP (Kanda et al., 2005).
It is unclear whether alcohol consumption is associated with acute BNP changes in HF patients. A study of CAD patients found that acute heavy alcohol intake led to transient ischemia episodes in reaction to 10-minute exercise intervals (Rossinen et al., 1996). Given that myocardial ischemia is known to induce increases in BNP levels (de Lemos et al., 2003; Weber et al., 2006), one might speculate that alcohol intake is associated with BNP increases in response to exercise. Associations between alcohol consumption and BNP following psychological stress have not yet been studied, however.
The objective of the present study was to investigate whether psychological stress induces BNP changes in HF patients and controls and to examine the impact of alcohol consumption on this phenomenon. We measured plasma levels of BNP immediately before as well as 2 hours after stress, because ACSs are known to occur within the 2 hours after an emotional trigger (Strike et al., 2006a, 2006b; Strike and Steptoe, 2005). We hypothesized that HF patients would exhibit greater increases in BNP levels following psychological stress and that alcohol consumption levels would be associated with BNP measures in response to stress.
Method
Study participants
The study sample consisted of 21 male patients diagnosed with HF and, as a control group, 19 male individuals with no cardiovascular pathology except for elevated blood pressure. Patients were recruited from the San Diego Veterans Affairs Medical Center and the University of California, San Diego, Medical Center Heart Failure Program as part of a larger study on the effects of depression on cellular adhesion and inflammation. We recruited the control non-HF individuals from the local community via various advertisements (e.g., newspaper, flyers, brochures, and Web sites) and word-of-mouth referrals.
Inclusion criteria for all study participants included being age 30-85 years and having hypertension (with a systolic blood pressure of less than 180 mm Hg or a diastolic blood pressure of less than 110 mm Hg); men of all ethnicities and races were eligible. Inclusion criteria for HF patients included New York Heart Association (NYHA) classes II through IV, symptoms of HF for at least 3 months that have been optimally treated with beta-blockers, diuretics, and angiotensin-converting enzyme (ACE) inhibitors, and systolic dysfunction (defined by a left ventricular ejection fraction [LVEF] ≤ 45%) or diastolic dysfunction (defined by echocardiography using mitral inflow patterns and tissue Doppler velocities to detect abnormalities according to recommended criteria) (Nagueh et al., 2009). We assessed LVEF by echocardiography as part of the patient's routine medical evaluation. To assess physical function capacity in all subjects, we used the 6-minute walk test (O'Keeffe et al., 1998). Exclusion criteria included recent myocardial infarction (1 month), recent stroke or significant cerebral neurological impairment, severe chronic obstructive pulmonary disease, major depression, and other psychiatric illnesses.
The protocol was approved by the University of California, San Diego, Institutional Review Board, and participants gave written informed consent. The study was carried out in accordance with the Declaration of Helsinki principles.
Acute mental stress testing
Subjects were admitted to the General Clinical Research Center of the University of California, San Diego, Medical Center the night before testing. Testing was performed at 9:00 a.m. the next morning, following a light standardized non-caffeine-containing breakfast. Participants abstained from food and drink (other than water) for 2 hours before the experiment and from physical exercise, alcohol, and caf-feinated beverages starting the evening before the test day.
Following a 30-minute quiet rest, resting blood pressure was taken using an automated blood-pressure monitor (Dinamap Compact BP monitor; Critikon, Tampa, FL) and venous blood was taken via an intravenous catheter that was placed in the antecubital vein 1 hour prior. Subjects were then given instructions for performing a moderate mental challenge test that combined a 5-minute serial subtraction mental arithmetic task and a public speech task. The speaking task involved preparing (5 minutes) and presenting (5 minutes) a speech in response to an automobile dealer not honoring a warranty (Mills et al., 2003). Subjects were told that the performance would be videotaped and rated by experts on poise and articulation. A video camera was displayed prominently during the procedure. If subjects stopped talking before the end of the speech, they were reminded to continue the talk by reiterating or summarizing the main points. After the stress test, all subjects relaxed in a quiet room in the General Clinical Research Center to recover. Blood samples were obtained before the stressor and 2 hours after cessation of the stressor. Testing was terminated if a subject expressed discomfort, chest pain, or shortness of breath or if blood pressure exceeded a systolic blood pressure of 220 mm Hg or a diastolic blood pressure of 100 mm Hg or dropped below resting levels.
Assessment of alcohol consumption
A brief questionnaire, consisting of questions regarding past and current alcohol consumption, was administered by the study personnel. We asked whether subjects were current or former drinkers, and we assessed current alcohol consumption by a self-report of the estimated number of consumed alcoholic beverages per month.
Biochemical analyses
Blood was drawn into ethylenediaminetetraacetic-acid (EDTA)-coated vacutainer tubes (BD Biosciences; San Jose, CA). Blood samples were centrifuged for 10 minutes at 3,000 g and 4 °C and plasma was stored at -80 °C until analysis. Plasma BNP levels were determined by the Bayer/Centaur BNP Assay (Bayer Diagnostics, New York, NY). The Bayer BNP assay is an enzyme-linked immunosorbent assay (ELISA) measuring BNP concentrations up to 2,500 pg/ml with minimum detectable concentrations of < 1.0 pg/ml. The interassay coefficient of variation was 1.8 % and the intra-assay coefficient of variance was 2.2 %. To minimize intra-assay error variance, both BNP samples from each subject were analyzed in the same run.
Statistical analyses
All calculations were performed using SPSS Inc. (Version 11.0.1; SPSS, Chicago, IL) software packages. Data are presented as mean ± SEM. Results were considered statistically significant at the p ≤ .05 level, and all tests were two-tailed. In case of missing data, cases were excluded listwise. BNP levels were normally distributed in both groups as verified by the Kolmogorow-Smirnow test. We calculated mean arterial pressure (MAP) from resting-blood-pressure readings (1/3 systolic blood pressure + 2/3 diastolic blood pressure) and body mass index was calculated by the formula: weight in kg/(height in m)2.
Power analyses (Faul, 2007) revealed that with a sample size of 40, the study would have adequate power (.95) to predict BNP stress increases of a large effect size of f 2 = .35 in regression analyses using the maximum of five predictors. It should be noted that this investigation was based on a post hoc data analysis addressing the question of BNP responses to stress in HF patients and their relation to alcohol consumption.
To test for group differences in sociodemographic and medical characteristics, we computed univariate analyses of variance (Table 1).
Table 1.
Sociodemographic and medical characteristics of the study subjects
| Variable | HF patients | Non-HF controls | p |
| Alcohol consumption | |||
| No. drinks per month, M ± SEM (range) | 5.8 ± 3.0 (0-56) | 5.4 ± 2.7 (0-45) | .93 |
| 0 | 55.0% | 41.2% | |
| 1-5 | 25.0% | 41.2% | |
| 6-15 | 10.0% | 5.9% | |
| 15-56 | 10.0% | 11.8% | |
| Positive history of drinking | 33% | 16% | |
| Age, years, M ± SEM (range) | 61.8 ±3.4 (34-81) | 49.4 ± 1.6(37-62) | .003 |
| Body mass index, kg/m2, M± SEM (range) | 31.1 ± 1.6(23.7-41.5) | 29.6 ± 1.0(23.8-51.2) | .43 |
| Mean arterial blood pressure, mm Hg, M ± SEM (range) | 77.9 ±2.1 (73.5-82.3) | 93.9 ± 2.8 (88.0-99.8) | <.001 |
| Cigarettes per day, M ± SEM (range) | 3.2 ± 2.9 (0-60) | 1.9 ± 1.3(0-20) | .70 |
| Current smokers | 5% | 16% | |
| Coronary artery disease | 31.6% | 0% | |
| Systolic dysfunction | 90.5% | 0% | |
| Diastolic dysfunction | 57.1% | 0% | |
| HF severity | |||
| 6-minute walk test, meters M ± SEM (range) | 364.7 ±20.7 (190-511) | 518.8 ± 19.4(375-724) | <.001 |
| LVEF, M± SEM (range) | 29.1% ± 1.75% (16%-45%) | — | |
| NYHA classifications II/III | 81%/19% | 0%/0% | |
| Medications | |||
| ACE inhibitors | 71% | 0% | |
| Beta blockers | 95% | 0% | |
| Calcium channel blockers | 5% | 0% | |
| Statin | 57% | 0% | |
| Aspirin | 52% | 5% | |
| Diuretics | 91% | 0% | |
| Anti-arrhythmics | 9.5% | 0% | |
| Digoxin | 62% | 0% | |
Notes: Data are presented as mean ± standard error of means (range) or percentage value. HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; ACE = angiotensin-converting enzyme.
Because BNP levels of control subjects were in the very low detection range of the assay, we decided to use absolute BNP levels in all analyses and abstained from additionally calculating percentage changes to guarantee a satisfying reliability.
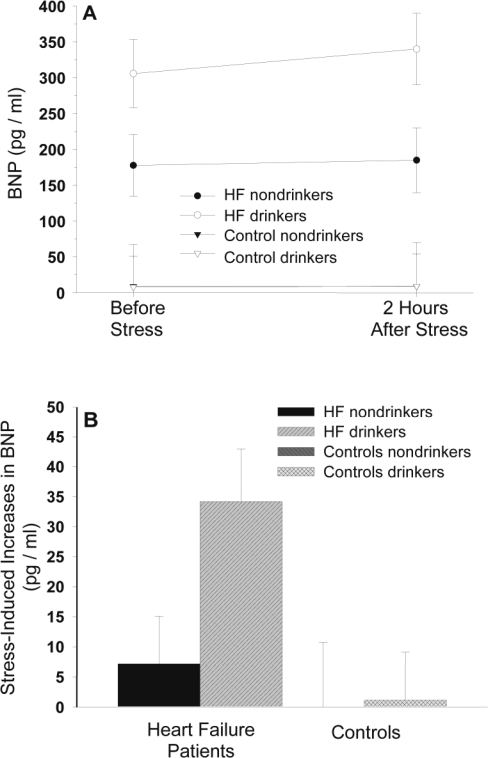
Repeated measures analysis of variance was applied to test for stress-induced changes in BNP plasma levels in HF patients and controls, with subject group as independent and BNP measures as repeated dependent variable (Figure 1). We applied the Huynh-Feldt correction for repeated measures.
Figure 1.
Values are means ± SEM. The figure depicts biomarker B-type natriuretic peptide (BNP) levels before and 2 hours after acute stress (Panel A) and stress-induced BNP increases (Panel B) in heart failure (HF) patients and non-HF controls who are alcohol drinkers and nondrinkers. Regression analyses revealed that belonging to the HF patient group (main effect group: β = .32, p = .015, ΔR2 = .10) and having higher alcohol consumption as continuously measured by number of drinks per month (main effect drinks per month: β = .61, p < .001, ΔR2 = .37) were associated with higher BNP stress increases. Moreover, higher alcohol consumption was associated with higher BNP stress increases in the HF group as compared with the non-HF control group (Group x Stress interaction: β = .49, p < .001, ΔR2 = .20).
For assessment of associations between alcohol consumption and BNP measures, we applied the following procedure. To address associations at rest, we calculated linear regression analyses with pre-stress measures of BNP as a dependent variable and number of drinks per month as a continuous independent variable while controlling for the group variable (HF or control) in a first step. In a second step, we additionally controlled for those group characteristics that significantly differed between patients and controls (i.e., age, MAP, and 6-minute walk test). It is of particular importance to control for age, because BNP levels increase with aging (McKie and Burnett, 2005). We did not control for all group characteristics, to avoid statistical overcontrol-ling given our sample size (Babyak, 2004). To address associations between alcohol consumption and BNP changes in response to stress, we again calculated linear regression analyses with BNP stress change as dependent variable. BNP stress change was calculated as BNP post-stress level minus pre-stress level. We entered the number of drinks per month as an independent continuous variable while controlling for subject group in a first step. In a second step, we again additionally controlled for group characteristics significantly differing between the groups. To address the potential influence of alcohol consumption on group differences in BNP, we calculated moderator analyses as described by Baron and Kenny (1986), both for resting BNP and for BNP stress changes. We performed regression analyses with BNP stress changes as the dependent variable, and we entered the number of drinks per month, the group variable, and their interaction as independent variables. We centered the group variable to the mean to allow calculation of interaction terms. To graphically illustrate our continuously calculated regression results, we divided each subject group into drinkers and nondrinkers based on their current drinking report, rendering four subgroups of subjects (i.e., HF patients who are nondrinkers [n = 11] and drinkers [n = 9] and controls who are nondrinkers [n = 6] and drinkers [n = 11]; Figure 1).
Results
Sociodemographic and medical characteristics of the study group
Table 1 presents the biological and medical characteristics of the 21 HF patients and 19 controls studied. Most of the HF patients were NYHA class II and were taking ACE inhibitors, beta-blockers, and diuretics. In addition, a high percentage of patients were taking digoxin (Lanoxicaps and Lanoxin) and statins. Most patients had systolic dysfunction (n = 19) and more than half of the patients had diastolic dysfunction (n = 12). HF patients walked fewer meters in the 6-minute walk test compared with controls, F(1, 37) = 29.4, p < .001. Moreover, HF patients were older, F(1, 38) = 10.3, p = .003, and had a lower MAP, F(1, 38) = 21.3, p < .001, probably as a result of medication intake and/or their underlying LVEF dysfunction. Although a higher percentage of HF patients did not currently consume alcohol compared with healthy controls (55% vs. 41%), the average number of drinks per month was similar between the two groups.
BNP reactivity to mental stress
Table 2 depicts BNP baseline and post-stress levels as well as stress-induced increases in BNP levels. Mental stress induced significant increases in absolute BNP levels among all participants, main effects of stress: F(1, 38) = 5.5, p = .024, ηp 2 = .13, f = .39. Post hoc testing by separate recalculation in each subject group revealed that this effect was carried by HF patients, as stress significantly increased BNP levels in HF patients (p = .028) but not in controls (p = .10). As expected, compared with controls, HF patients had higher BNP levels at rest as well as after exposure to the acute stressor, main effect group: F(1, 38) = 23.42, p <.001, ηp 2 = .38, f = .78. Moreover, HF patients showed greater stress-induced increases in BNP levels, interaction group-by-stress: F(1, 38) = 4.52, p = .04, ηp 2 = .11, f = .35.
TABLE 2.
BNP levels and increases in HF patients and controls before and after acute mental stress
| BNP levels | HF patients | Non-HF controls | p |
| Baseline, pg/ml | 227.0 ± 43.3 | 8.8 ± 1.4 | <..001 |
| Post-stress, pg/ml | 245.8 ± 46.2 | 9.7 ± 1.7 | <..001 |
| Stress changes, pg/ml | 18.8 ± 7.9 | 0.9 ± 0.6 | <..001 |
Notes: Values are means ± SEM; baseline: immediately before stress; post-stress: 2 hours after stress cessation; stress changes: BNP post-stress levels minus baseline levels. BNP = biomarker B-type natriuretic peptide; HF = heart failure.
Alcohol consumption, BNP, and control variables
At rest.
Regression analyses revealed that HF group (main effect group: β = .62, p <.001, ΔR2 = .38) but not alcohol consumption (p = .10) was associated with higher absolute BNP baseline levels. The group effect remained significant when controlling for age and MAP (p = .002). When additionally controlling for meters walked in the 6-minute walk test, the main effect of group (β = .37, p = .087, ΔR2 = .05) as well as the main effect of alcohol consumption (β = .27, p = .059, ΔR2 = .07) became borderline significant. The moderation effect of alcohol consumption on group differences in baseline BNP levels did not reach statistical significance, either with (p = .08) or without (p = .23) controlling for age, MAP, and the 6-minute walk test.
Stress reactivity
Both group and alcohol consumption were independently associated with higher BNP stress changes. Being a HF patient (main effect group: (β = .32, p = .015, ΔR2 = .10) and higher alcohol consumption (main effect drinks per month: β = .61, p < .001, ΔR2 = .37) were associated with higher stress-induced increases in absolute BNP levels. Additional controlling for age, MAP, and meters walked in the 6-minute walk test did not significantly change the main effect of drinks per month (p < .001), but the group effect lost significance (p = .11). Regression analyses testing moderation revealed that the interaction between group and drinks per month was significantly associated with higher BNP increases following stress (β = .45, p < .001, ΔR2 = .19), even when additionally controlling for age, MAP, and the 6-minute walk test (β = .45, p < .001, ΔR2 = .18). This suggests a statistical moderation effect of alcohol on group differences in BNP stress increases, with higher alcohol consumption being associated with higher BNP stress increases in the HF group as compared with the control group.
Figure 1 depicts BNP levels before and after stress (Panel A) as well as stress-induced BNP increases (Panel B) in HF patients and controls with greater and fewer numbers of drinks per month. Table 3 summarizes all regression results.
Discussion
This study examined BNP reactivity to acute psychological stress in HF patients and its relation to light to moderate alcohol consumption. Our findings of stress-induced BNP increases in HF patients extend previous studies of comparable sample sizes reporting increased cardiovascular reactivity in terms of heart rate and/or sympathetic nerve activity to mental stress in HF (Holmes et al., 2007; Middlekauff et al., 1997). Cardiovascular hyperreactivity has been shown to predict worsening of HF symptoms (e.g., increased left ventricular mass) and clinical events such as ACSs (Alderman et al., 1990; Treiber et al., 2003). Similarly, heightened BNP reactivity in HF might be associated with disease progression. Previous HF studies investigating reactivity to exercise challenges report rapid increases in BNP plasma levels (Ciampi et al., 2009; Foote et al., 2004; Marumoto et al., 1995; Win et al., 2005). As BNP levels are associated with myocardial wall stress due to volume expansion and pressure overload (de Bold et al., 1996; Weber et al., 2006; Woods, 2004), it has been suggested that BNP responses to exercise in HF patients reflect increases in cardiac strain (Ciampi et al., 2009). Accordingly, our findings of BNP level changes following mental stress in HF patients may indicate strain on the heart with mental stress. It is interesting to note that exercise-induced increases in BNP levels seem to return to baseline levels within 10-15 minutes after exercise (Win et al., 2005), whereas we observed significant BNP increases 2 hours after cessation of our stressor. Given that ACSs are known to occur within 2 hours after emotional triggering (Strike et al., 2006a, 2006b; Strike and Steptoe, 2005), our observed BNP stress-increases in HF patients might be associated with clinical endpoints.
The clinical relevance of short-term changes in BNP plasma levels is, however, unclear. Prior research on prognostic properties of BNP shows that baseline BNP levels and increases in natriuretic peptides over several weeks are indicators of HF progression and elevated mortality risk (de Lemos et al., 2001; Yan et al., 2005). Given that a baseline BNP level of more than 80 pg/ml has been shown to be associated with an increased 10-month mortality rate in ACS patients (de Lemos et al., 2001), our results might be of clinical relevance. We observed mean increases of 19 pg/ml, with a standard deviation of 36 pg/ml, in our HF patients 2 hours after a relatively moderate mental stress task. One could speculate that a more strenuous life stressor might induce even higher BNP increases in HF patients and potentially reach a critical threshold, especially if prolonged. Moreover, the kinetics of mental-stress-induced BNP changes have not yet been studied. Therefore, peak BNP levels might occur after 2 hours or might have been higher before 2 hours, as observed after exercise in some HF patients (Ciampi et al., 2009). In addition, it is possible that repeated mental stressors that induce increases in BNP might accumulate strain on the heart and have adverse clinical effects.
Few studies have examined biomarkers of HF severity— such as BNP—to determine alcohol-associated influences on cardiac activity. In one animal study of chronic moderate ethanol consumption, there were significant elevations in ventricular BNP concentration found in spontaneously hypertensive but not Wistar-Kyoto rats (Guillaume et al., 1996). This suggests that alcohol consumption in a more vulnerable population may be particularly deleterious to cardiac function. In another study, alcohol consumption as assessed by current drinking status (yes/no) was independently associated with higher BNP plasma levels, with a 1.5-fold increased risk of having high plasma BNP (Kanda et al., 2005). Results of the present study also suggest that alcohol consumption levels may be dose-dependently associated with increased BNP levels. The clinical relevance of this finding is unclear, however. Although excessive alcohol consumption is recognized as a cause of HF (Haddad et al., 2008), light to moderate alcohol consumption (up to one drink daily for women and one or two drinks daily for men) seems beneficial in preventing CAD and CAD-related HF in healthy persons (Djousse and Gaziano, 2007; Klatsky et al., 2005; O'Keefe et al., 2007). However, because alcohol consumption increases blood pressure and thereby hypertension risk in a dose-dependent fashion (Beilin and Puddey, 2006), it may be speculated that benefits of moderate alcohol consumption on CAD might be counterbalanced in HF patients who are more vulnerable to blood pressure effects (Klatsky, 2004; Klatsky and Gunderson, 2006; Walsh et al., 2002).
Whereas acute alcohol consumption seems to exert dampening effects on cardiovascular reactivity to stress (Hoaken et al., 2003; Sayette, 1993; Sher et al., 2007), less is known about levels of chronic alcohol consumption (i.e., light, moderate, or heavy) interactions with psychological stress on cardiac function, and no prior studies have examined this question in HF patients. Current and abstinent alcoholics have been reported to have blunted cortisol reactivity to mental stress (Lovallo et al., 2000). In contrast, some heavy social drinkers showed greater cortisol stress reactivity (Nesic and Duka, 2008), and research in primates shows that chronic moderate alcohol consumption, along with psychological stress, elicited significant increases in resting heart rate (Shively et al., 2007). As glucocorticoids can induce up-regulation of natriuretic peptide genes (Nishimori et al., 1997) and as heart rate increases may add to pressure overload, mental stress may generate deleterious effects to cardiac function in vulnerable persons, such as HF patients. Indeed, the present study found higher alcohol consumption moderated greater stress-induced BNP increases in HF patients compared with controls. More precisely, HF patients who were "drinkers" had mean BNP increases of approximately 35 pg/ml, whereas HF nondrinkers had mean increases of only 7 pg/ml. Given the observed linear relationship, patients with higher alcohol consumption had even higher increases, with an observed maximum increase of 130 pg/ml. This suggests increased cardiac strain in response to emotional stress in HF patients that consume larger quantities of alcohol.
There are a few noteworthy positive aspects to the study. We decided on a long sampling interval and measured BNP levels 2 hours after stress cessation. This time interval may have implications for the clinical relevance of our findings, because ACSs are also known to occur within the 1-2 hours after emotional triggering in vulnerable persons. We used a statistical approach allowing assessment of linear associations between alcohol consumption entered as continuous variable and changes in BNP plasma levels in the study groups.
The study also has its limitations, however. First, medication differences between subjects are inevitable. Therefore, we cannot rule out that our observed findings, particularly our observed group difference in BNP stress reactivity and its association with the amount of reported alcohol consumption may be, in part, the result of medication effects. Second, our study sample size did not allow us to control for more variables than those group characteristics significantly differing between the study groups. Moreover, it is possible that baseline associations between BNP and alcohol did not become significant because our study was underpowered to reveal medium or small effects. Future studies using larger sample sizes need to address this question. Third, we measured BNP levels only once after stress (i.e., 2 hours afterward). Future studies should extend the measurement of BNP to further sampling intervals in order to shed light on the kinetics of BNP stress reactivity. Fourth, our assessment of alcohol consumption is based on self-reports of the number of drinks per month. We cannot exclude underreporting or false reporting of alcohol consumption in our study subjects. Moreover, we did not assess the pattern of alcohol consumption (i.e., whether the alcohol was consumed continuously [every day] or intermittently [e.g., only during weekends]) or the type of consumed beverage. Fifth, our HF patients were older than our controls, which we statistically controlled by covarying for age. Thus, conclusions on associations between BNP and alcohol consumption in our study need to be interpreted with care. Moreover, we did not recruit for heterogeneous levels of alcohol consumption among patients and controls but restricted ourselves to assess the natural range of reported alcohol consumption. Indeed, given a continuous consumption pattern, the self-reported range of consumed drinks per month did not exceed a moderate amount of alcohol consumption, as the reported maximum was 56 drinks per month (i.e., 2 drinks per day). This implies that our findings cannot be generalized to heavy drinking or alcoholism. Taken together, these limitations suggest that our findings should be considered as preliminary. The clinical implications are limited because of a lack of disease-outcome assessment. Prospective designs are needed to study whether the BNP increases following stress in the range observed in our HF patients might be associated with higher mortality (e.g., resulting from ACS onset following emotional triggering) and whether alcohol consumption plays a role in this association.
In conclusion, our findings suggest that exposure to mental stress in HF patients is associated with increases in BNP and that such increases are positively associated with alcohol consumption. Future investigations are needed to verify our findings in larger populations, to determine mechanisms associated with BNP responses to stress, and to study potential clinical implications—for example, whether increased BNP reactivity to stress and alcohol consumption interact to produce deleterious health consequences.
Table 3.
Hierarchical regression analyses for associations between alcohol consumption and BNP levels in HF patients and non-HF controls
| Variables entered | Standardized β coefficient | t | P | R2 change |
| BNP baseline levels | ||||
| Group (HF vs. controls) | .62 (.37)a | 4.8(1.8)a | <.001 (.09)a | .38 (.05)a |
| Drinks per month | .22 (.27)a | 1.7(2.0)a | .10 (.06)a | .05 (.09)a |
| Group by drinks per monthb | .18 (.24)a | 1.2(1.8)a | .23 (.08)a | .03 (.05)a |
| BNP stress change | ||||
| Group (HF vs. controls) | .32 (.32)a | 2.6(1.7)a | .015 (.11)a | .10 (.04)a |
| Drinks per month | .61 (.56)a | 4.9 (4.4)a | <.001 (<.001)a | .37 (.28)a |
| Group × Drinks per Monthb | .49 (.45)a | 4.3 (4.6)a | <.001 (<.001)a | .19 (.18)a |
Notes: BNP stress change = difference between post-stress level and baseline. BNP = biomarker B-type natriuretic peptide; HF = heart failure.
After controlling for age, mean arterial pressure, and meters walked in the 6-minute walk test;
regression results for the interaction of Group × Drinks per Month after controlling for group and drinks per month.
Footnotes
This research was supported by National Institutes of Health (NIH) grants HL-073355 and HL-57265 awarded to Paul J. Mills; NIH grant RR-00827 awarded to the University of California, San Diego (UCSD), General Clinical Research Center; and Swiss National Foundation grant IZKOBO-122843/1 awarded to Petra H. Wirtz.
References
- Alderman MH, Ooi WL, Madhavan S, Cohen H. Blood pressure reactivity predicts myocardial infarction among treated hypertensive patients. Journal of Clinical Epidemiology. 1990;43:859–866. doi: 10.1016/0895-4356(90)90069-2. [DOI] [PubMed] [Google Scholar]
- Babyak MA. What you see may not be what you get: A brief, nontechnical introduction to overfitting in regression-type models. Psychosomatic Medicine. 2004;66:411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beilin LJ, Puddey IB. Alcohol and hypertension: An update. Hypertension. 2006;47:1035–1038. doi: 10.1161/01.HYP.0000218586.21932.3c. [DOI] [PubMed] [Google Scholar]
- Ciampi Q, Borzillo G, Barbato E, Petruzziello B, Betocchi S, Villari B. Diastolic function and BNP changes during exercise predict oxygen consumption in chronic heart failure patients. Scandinavian Cardiovascular Journal. 2009;43:17–23. doi: 10.1080/14017430802175720. [DOI] [PubMed] [Google Scholar]
- de Bold AJ, Bruneau BG, Kuroski de Bold ML. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovascular Research. 1996;31:7–18. [PubMed] [Google Scholar]
- de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. New England Journal of Medicine. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- Djousse L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians' Health Study I. Circulation. 2007;115:34–39. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- Djousse L, Gaziano JM. Alcohol consumption and heart failure: A systematic review. Current Atherosclerosis Reports. 2008;10:117–20. doi: 10.1007/s11883-008-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Foote RS, Pearlman JD, Siegel AH, Yeo KT. Detection of exercise-induced ischemia by changes in B-type natriuretic peptides. Journal of the American College of Cardiology. 2004;44:1980–1987. doi: 10.1016/j.jacc.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Guillaume P, Jankowski M, Gutkowska J, Gianoulakis C. Effect of chronic moderate ethanol consumption on heart brain natriuretic peptide. European Journal of Pharmacology. 1996;316:49–58. doi: 10.1016/s0014-2999(96)00644-9. [DOI] [PubMed] [Google Scholar]
- Haddad GE, Saunders L, Carles M, Crosby SD, del Monte F, Macgillivray TE, Gwathmey JK. Fingerprint profile of alcohol-associated heart failure in human hearts. Alcoholism: Clinical and Experimental Research. 2008;32:814–821. doi: 10.1111/j.1530-0277.2008.00628.x. [DOI] [PubMed] [Google Scholar]
- Haldeman GA, Croft JB, Giles WH, Rashidee A. Hos-pitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. American Heart Journal. 1999;137:352–360. doi: 10.1053/hj.1999.v137.95495. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Campbell T, Stewart SH, Pihl RO. Effects of alcohol on cardiovascular reactivity and the mediation of aggressive behaviour in adult men and women. Alcohol and Alcoholism. 2003;38:84–92. doi: 10.1093/alcalc/agg022. [DOI] [PubMed] [Google Scholar]
- Holmes SD, Krantz DS, Kop WJ, Del Negro A, Karasik P, Gottdiener JS. Mental stress hemodynamic responses and myocardial ischemia: Does left ventricular dysfunction alter these relationships? Psychosomatic Medicine. 2007;69:495–500. doi: 10.1097/PSY.0b013e3180cabc73. [DOI] [PubMed] [Google Scholar]
- Kanda H, Kita Y, Okamura T, Kadowaki T, Yoshida Y, Nakamura Y, Ueshima H. What factors are associated with high plasma B-type natriuretic peptide levels in a general Japanese population? Journal of Human Hypertension. 2005;19:165–172. doi: 10.1038/sj.jhh.1001792. [DOI] [PubMed] [Google Scholar]
- Klatsky AL. Alcohol-associated hypertension: When one drinks makes a difference. Hypertension. 2004;44:805–806. doi: 10.1161/01.HYP.0000146538.26193.60. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Chartier D, Udaltsova N, Gronningen S, Brar S, Friedman GD, Lundstrom RJ. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. American Journal of Cardiology. 2005;96:346–351. doi: 10.1016/j.amjcard.2005.03.073. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Gunderson E. Alcohol and hypertension. In: Mohler ER, Townsend RR, editors. Advanced therapy in hypertension and vascular disease. Hamilton, Ontario, Canada: B.C. Decker; 2006. pp. 108–117. [Google Scholar]
- Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1317. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcoholism: Clinical and Experimental Research. 2000;24:651–658. [PubMed] [Google Scholar]
- Marumoto K, Hamada M, Hiwada K. Increased secretion of atrial and brain natriuretic peptides during acute myocardial ischaemia induced by dynamic exercise in patients with angina pectoris. Clinical Science. 1995;88:551–556. doi: 10.1042/cs0880551. [DOI] [PubMed] [Google Scholar]
- McKie PM, Burnett JC., Jr B-type natriuretic peptide as a biomarker beyond heart failure: Speculations and opportunities. Mayo Clinic Proceedings. 2005;80:1029–1036. doi: 10.4065/80.8.1029. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nguyen AH, Negrao CE, Nitzsche EU, Hoh CK, Natterson BA, Moriguchi JD. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: Implications for ‘triggering’ adverse cardiac events. Circulation. 1997;96:1835–1842. doi: 10.1161/01.cir.96.6.1835. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Farag NH, Hong S, Kennedy BP, Berry CC, Ziegler MG. Immune cell CD62L and CD11a expression in response to a psychological stressor in human hypertension. Brain Behavior and Immunity. 2003;17:260–267. doi: 10.1016/s0889-1591(03)00055-2. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE for The Determinants of Myocardial Infarction Onset Study Investigators. Triggering of acute myocardial infarction by heavy physical exertion—Protection against triggering by regular exertion. New England Journal of Medicine. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- Moe GW. B-type natriuretic peptide in heart failure. Current Opinion in Cardiology. 2006;21:208–214. doi: 10.1097/01.hco.0000221582.71619.84. [DOI] [PubMed] [Google Scholar]
- Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Journal of the American Society of Echocardiography. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Nesic J, Duka T. Effects of stress on emotional reactivity in hostile heavy social drinkers following dietary tryptophan enhancement. Alcohol and Alcoholism. 2008;43:151–162. doi: 10.1093/alcalc/agm179. [DOI] [PubMed] [Google Scholar]
- Nishimori T, Tsujino M, Sato K, Imai T, Marumo F, Hirata Y. Dexamethasone-induced up-regulation of adrenomedullin and atrial natriuretic peptide genes in cultured rat ventricular myocytes. Journal of Molecular and Cellular Cardiology. 1997;29:2125–2130. doi: 10.1006/jmcc.1997.0460. [DOI] [PubMed] [Google Scholar]
- O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: The razor-sharp double-edged sword. Journal of the American College of Cardiology. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- O'Keeffe ST, Lye M, Donnellan C, Carmichael DN. Re-producibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart. 1998;80:377–382. doi: 10.1136/hrt.80.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hong Y. Heart disease and stroke statistics—2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rossinen J, Partanen J, Koskinen P, Toivonen L, Kupari M, Niemin-en MS. Acute heavy alcohol intake increases silent myocardial ischaemia in patients with stable angina pectoris. Heart. 1996;75:563–567. doi: 10.1136/hrt.75.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, de Lemos JA, Omland T, Desai MY, Tanasijevic M, Braunwald E. Acute changes in circulating natriuretic peptide levels in relation to myocardial ischemia. Journal of the American College of Cardiology. 2004;44:1988–1995. doi: 10.1016/j.jacc.2004.07.057. [DOI] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol's effects on stress responses in social drinkers. Psychological Bulletin. 1993;114:459–476. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Bartholow BD, Peuser K, Erickson DJ, Wood MD. Stress-response-dampening effects of alcohol: Attention as a mediator and moderator. Journal of Abnormal Psychology. 2007;116:362–377. doi: 10.1037/0021-843X.116.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Mietus JE, Grant KA, Goldberger AL, Bennett AJ, Willard SL. Effects of chronic moderate alcohol consumption and novel environment on heart rate variability in primates (Macaca fascicularis) Psychopharmacology. 2007;192:183–191. doi: 10.1007/s00213-007-0709-z. [DOI] [PubMed] [Google Scholar]
- Strike PC, Magid K, Whitehead DL, Brydon L, Bhattacharyya MR, Steptoe A. Pathophysiological processes underlying emotional triggering of acute cardiac events. Proceedings of the National Academy of Sciences. 2006a;103:4322–4327. doi: 10.1073/pnas.0507097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. Triggering of acute coronary syndromes by physical exertion and anger: Clinical and sociodemographic characteristics. Heart. 2006b;92:1035–1940. doi: 10.1136/hrt.2005.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike PC, Steptoe A. Behavioral and emotional triggers of acute coronary syndromes: A systematic review and critique. Psychosomatic Medicine. 2005;67:179–186. doi: 10.1097/01.psy.0000155663.93160.d2. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of pre-clinical and clinical disease states. Psychosomatic Medicine. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Waldo SW, Beede J, Isakson S, Villard-Saussine S, Fareh J, Clopton P, Maisel AS. Pro-B-type natriuretic peptide levels in acute decompensated heart failure. Journal of the American College of Cardiology. 2008;51:1874–1882. doi: 10.1016/j.jacc.2007.12.051. [DOI] [PubMed] [Google Scholar]
- Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, Levy D. Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Annals of Internal Medicine. 2002;136:181–191. doi: 10.7326/0003-4819-136-3-200202050-00005. [DOI] [PubMed] [Google Scholar]
- Weber M, Mitrovic V, Hamm C. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide - Diagnostic role in stable coronary artery disease. Experimental and Clinical Cardiology. 2006;11:99–101. [PMC free article] [PubMed] [Google Scholar]
- Willich SN, Lewis M, Lowel H, Arntz HR, Schubert F, Schroder R for The Triggers and Mechanisms of Myocardial Infarction Study Group. Physical exertion as a trigger of acute myocardial infarction. New England Journal of Medicine. 1993;329:1684–1690. doi: 10.1056/NEJM199312023292302. [DOI] [PubMed] [Google Scholar]
- Win HK, Chang SM, Raizner M, Shah G, Al Basky F, Desai U, Zoghbi WA. Percent change in B-type natriuretic peptide levels during treadmill exercise as a screening test for exercise-induced myocardial ischemia. American Heart Journal. 2005;150:695–700. doi: 10.1016/j.ahj.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Woods RL. Cardioprotective functions of atrial natriuretic peptide and B-type natriuretic peptide: A brief review. Clinical and Experimental Pharmacology and Physiology. 2004;31:791–794. doi: 10.1111/j.0305-1870.2004.04073.x. [DOI] [PubMed] [Google Scholar]
- Yan RT, White M, Yan AT, Yusuf S, Rouleau JL, Maggioni AP, McKelvie RS. Usefulness of temporal changes in neurohormones as markers of ventricular remodeling and prognosis in patients with left ventricular systolic dysfunction and heart failure receiving either candesartan or enalapril or both. American Journal of Cardiology. 2005;96:698–704. doi: 10.1016/j.amjcard.2005.04.048. [DOI] [PubMed] [Google Scholar]