Abstract
Mammalian oocytes are arrested at prophase I until puberty when luteinizing hormone (LH) induces resumption of meiosis of follicle-enclosed oocytes. Resumption of meiosis is tightly coupled with regulating cyclin-dependent kinase 1 (CDK1) activity. Prophase I arrest depends on inhibitory phosphorylation of CDK1 and anaphase-promoting complex—(APC–CDH1)-mediated regulation of cyclin B levels. Prophase I arrest is maintained by endogenously produced cyclic adenosine monophosphate (cAMP), which activates protein kinase A (PKA) that in turn phosphorylates (and activates) the nuclear kinase WEE2. In addition, PKA-mediated phosphorylation of the phosphatase CDC25B results in its cytoplasmic retention. The combined effect maintains low levels of CDK1 activity that are not sufficient to initiate resumption of meiosis. LH triggers synthesis of epidermal growth factor-like factors in mural granulosa cells and leads to reduced cGMP transfer from cumulus cells to oocytes via gap junctions that couple the two cell types. cGMP inhibits oocyte phosphodiesterase 3A (PDE3A) and a decline in oocyte cGMP results in increased PDE3A activity. The ensuing decrease in oocyte cAMP triggers maturation by alleviating the aforementioned phosphorylations of WEE2 and CDC25B. As a direct consequence CDC25B translocates into the nucleus. The resulting activation of CDK1 also promotes extrusion of WEE2 from the nucleus thereby providing a positive amplification mechanism for CDK1 activation. Other kinases, e.g. protein kinase B, Aurora kinase A and polo-like kinase 1, also participate in resumption of meiosis. Mechanisms governing meiotic prophase I arrest and resumption of meiosis share common features with DNA damage-induced mitotic G2-checkpoint arrest and checkpoint recovery, respectively. These common features include CDC14B-dependent activation of APC–CDH1 in prophase I arrested oocytes or G2-arrested somatic cells, and CDC25B-dependent cell cycle resumption in both oocytes and somatic cells.
Keywords: resumption of meiosis, prophase I arrest, oocyte, G2-checkpoint, checkpoint recovery
Introduction
The female germ cells, oocytes, arise from the primordial germ cells during development of the fetus. Following a round of DNA replication the cells enter prophase of the first meiotic division (prophase I), and following chromosome condensation and recombination reach the dictyate stage, at which point the chromosomes become dispersed and the oocytes are surrounded by a single layer of flattened epithelial-like somatic cells (granulosa cells; Rodrigues et al., 2008). Such prophase I arrested oocytes are referred to as primordial oocytes and reside in primordial follicles. Prophase I arrest remains in effect until puberty when oocytes enter the growth phase, and fully grown oocytes present in pre-ovulatory antral follicles resume meiosis in response to luteinizing hormone (LH) in mammals (Neal and Baker, 1975; Lei et al., 2001). Fully grown oocytes removed from their follicle also resume spontaneous resumption of meiosis in vitro when placed in a suitable culture medium (Sato and Koide, 1984).
Oocytes arrested at prophase I have intact nuclear envelope or germinal vesicle (GV) and germinal vesicle break down (GVBD) is the first clear visible marker of resumption of meiosis. Following GVBD, a metaphase I spindle forms and when all chromosome bivalents have established stable microtubule-kinetochore interactions, anaphase I occurs. Following completion of meiosis I, oocytes enter directly into meiosis II without an intervening S-phase, at which point they arrest for the second time at the metaphase II. Fertilization triggers resumption and completion of meiosis II. The road from GV-stage oocytes to metaphase II arrested eggs is principally governed by meiosis promoting factor that consists of cyclin-dependent kinase 1 (CDK1) and cyclin B1 (CCNB1) (Brunet and Maro, 2005).
In this minireview, we focus on the signalling pathways responsible for prophase I arrest and resumption of meiosis in mouse oocytes. Resumption of meiosis in oocytes and recovery from G2-arrest of somatic cells have many similarities, and accordingly we highlight some common features.
CDK1 regulation
Although oocytes are arrested in the first meiotic prophase, resumption of meiosis has historically been viewed as a model system to study the G2-M transition, because oocytes have a 4C DNA content and the chromosomes remain relatively decondensed. The G2-M transition is largely governed by activating CDK1. CDK1 is positively regulated by CCNB1 binding but also negatively regulated by WEE1/MYT kinase family-mediated phosphorylation on Thr14 and Tyr15 (Fig. 1A). Dephosphorylation of these residues is mediated by CDC25 phosphatases. The mammalian genome contains three CDC25 genes: A, B and C. CDC25C-deficient mice are viable and fertile, and mouse embryo fibroblasts (MEFs) from these mice have normal timing of entry into mitosis and a normal response to DNA damage (Chen et al., 2001). Although CDC25B-deficient mice are also viable and their MEFs proliferate normally and mount a strong DNA damage response, oocytes do not resume meiosis and remain arrested at prophase I (Lincoln et al., 2002).
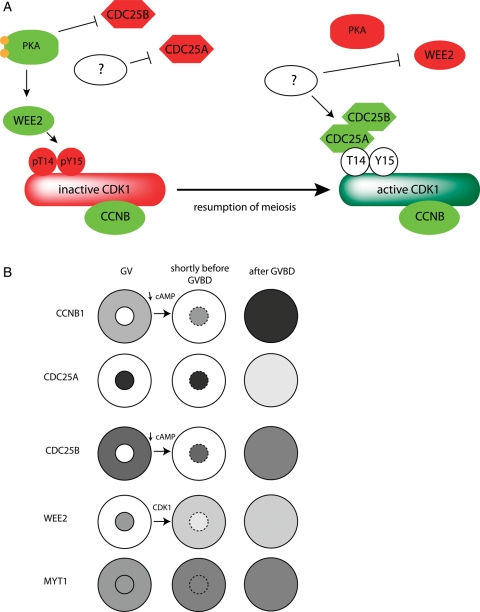
Figure 1.
CDK1 and its regulators during resumption of meiosis. (A) Inhibition of CDK1 during prophase I arrest and CDK1 activation during resumption of meiosis. (B) Localization of cyclin B (CCNB1), phosphatases CDC25A and CDC25B, kinases WEE2 and MYT1 during prophase I arrest and CDC25B and CCNB1 nuclear translocation and partial WEE2 nuclear extrusion shortly before GVBD. The dependency of the changes in localization of cAMP and CDK1 is marked.
Recently, we (Solc et al., 2008) and others (Li et al., 2008) demonstrated that CDC25A participates in resumption of meiosis, e.g. over-expressing CDC25A in mouse oocytes overcomes cyclic adenosine monophosphate (cAMP)-mediated maintenance of meiotic arrest. CDC25A is exclusively localized within the nucleus prior to GVBD (Fig. 1B). In contrast, CDC25B localizes to cytoplasm in GV-intact oocytes and translocates to the nucleus shortly before GVBD (Solc et al., 2008). Interestingly, CCNB1 also translocates from cytoplasm into the nucleus shortly before GVBD (Marangos and Carroll, 2004; Reis et al., 2006). After GVBD, CDC25B remains stable but CDC25A is degraded (Solc et al., 2008). WEE2 (formerly WEE1B) is an oocyte-specific member of WEE1/MYT kinase family and transgenic RNAi-mediated down-regulation causes leaky prophase I arrest of follicle-enclosed oocytes (Han et al., 2005). WEE2 and MYT1 kinases synergistically maintain meiotic arrest in mouse oocytes (Oh et al., 2010). Moreover, WEE2 is an exclusively nuclear protein whereas MYT1 exhibits both cytoplasmic and nuclear localization. Before GVBD, CDC25B translocation, which depends on a decline in cAMP concentration (see below), precedes the partial WEE2 nuclear extrusion promoted by CDK1 (Oh et al., 2010). Although CCNB1 (together with CDK1) is mainly cytoplasmic in GV-intact oocytes (Marangos and Carroll, 2004; Reis, et al., 2006), based on published data from both mouse oocytes (Marangos and Carroll, 2004) and somatic cells (Hagting et al., 1998; Toyoshima et al., 1998; Yang et al., 1998), we believe that the cytoplasmic localization of CCNB1 is a result of the rapid nuclear export and slow nuclear import (CCNB1 cytoplasmic-nuclear shuttle). Therefore, nuclear WEE2 should prevent nuclear CDK1 activation at GV-stage.
In summary, the decision to resume meiosis is regulated at the level of CDK1 kinase activity that in turn is regulated by a balance between CDC25A and CDC25B phosphatase activities and WEE2 and MYT1 kinases activities (Fig. 1A). Localization of these important molecules plays a critical regulatory role for prophase I arrest. We propose that cytoplasmic CDC25B activity is likely counteracted by MYT1, whereas nuclear CDC25A action is blocked by nuclear WEE2. Before GVBD translocation of both CCNB1 and CDC25B to the nucleus permits an increase in the activity of the CDC25 that ultimately results in an increase in CDK1 activity that is further amplified by CDK1-mediated loss of nuclear WEE2.
Cyclic adenosine monophosphate
After acquisition of meiotic competence (early antral follicle stage), the cell cycle block at prophase I (GV arrest) is maintained by a high level of cAMP via the cAMP-dependent protein kinase, protein kinase A (PKA) (Bornslaeger et al., 1986, 1988; Horner et al., 2003). Meiotically incompetent oocytes do not resume meiosis presumably because they do not contain sufficient concentrations of regulatory proteins such as CDK1 and CCNB1 (Mitra and Schultz, 1996; Kanatsu-Shinohara et al., 2000) that are essential for meiosis resumption (Mehlmann et al., 2002, 2004).
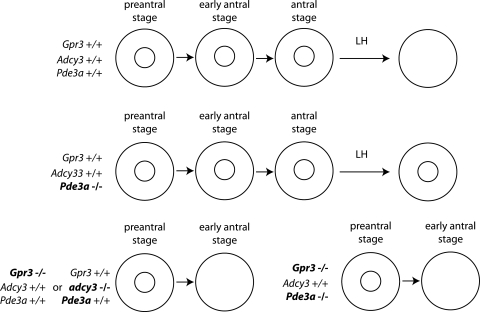
In mouse oocytes, cAMP is produced mainly by adenylate cyclase 3 (ADCY3) because ADCY3-deficient oocytes resume meiosis within the follicle when oocytes become competent to resume meiosis (Horner et al., 2003). Production of cAMP and meiotic arrest depends on oocytes expressing stimulatory subunits of the trimeric G-protein (Gs) (Mehlmann et al., 2002), whose activity is stimulated by the G-protein coupled receptor 3 (GPR3) (Mehlmann et al., 2004; Hinckley et al., 2005; Norris et al., 2007). The central function of cAMP produced in oocytes to maintain meiotic arrest (Fig. 2) was clearly documented by generating mouse oocytes defective in both cAMP synthesis and degradation (GPR3−/−; PDE3A−/−) (Vaccari et al., 2008). Oocytes derived from PDE3A-deficient mice do not resume meiosis either after an LH surge or spontaneously when placed in culture (Masciarelli et al., 2004), i.e. PDE3A is the essential PDE isoform required to promote the cAMP decrease that initiates resumption of meiosis. Depletion of GPR3, a major oocyte G-protein coupled receptor, results in spontaneous resumption of meiosis of oocytes enclosed in antral follicles (Mehlmann et al., 2004). Depleting both of GPR3 and PDE3A genes allows the same level of spontaneous meiosis resumption in vivo as depletion of GPR3 alone (Vaccari et al., 2008), and microinjection of inhibitory Gs antibody into follicle-enclosed oocytes induces resumption of meiosis (Mehlmann et al., 2002). These results strongly implicate that cAMP produced directly in oocytes is essential and sufficient for meiotic arrest at prophase I and that cAMP supplied from surrounding cumulus cells and entering the oocyte via the heterologous gap junctions between cumulus cells and oocytes (Dekel et al., 1981) is not required for maintenance of meiotic arrest as previously proposed (Dekel et al., 1981).
Figure 2.
Effect of genetic disruption of molecules involved in oocytes cAMP production (GPR3 or ADCY3) or cAMP destruction (PDE3A) on the maintenance of prophase I arrest at oocytes in early antral follicles and during LH-induced resumption of meiosis, respectively.
Protein kinase B (PKB or AKT) is also involved in resumption of meiosis (Kalous et al., 2006), possibly by phosphorylating PDE3A and thereby potentiating PDE3A activity (Han et al., 2006). These findings raise the question as to what activates PKB during resumption of meiosis. The mechanism of PKB activation in mouse oocytes, however, is not understood but may be linked to the ability of high levels of cAMP to inhibit PKB activity (Kim et al., 2001). The maturation-associated decrease in cAMP could be a mechanism that reinforces PKB activation, but is unlikely to account for its initial activation.
Consistent with a central role for cAMP to maintain meiotic arrest through the action of PKA is that (i) microinjection of the heat-stable PKA inhibitor into oocytes induces resumption of meiosis when oocytes are cultured under conditions that normally maintain concentrations of cAMP that inhibit spontaneous maturation in vitro and (ii) microinjection of the catalytic subunit of PKA inhibits spontaneous maturation in vitro (Bornslaeger et al., 1986). The action of PKA appears spatially restricted because microinjection of oocytes cultured in the presence of competitive PDE inhibitor 3-isobutyl-1-methylxanthine-containing medium with peptide H31, which disrupts association of A-kinase anchor proteins (AKAPs) with regulatory subunits of PKA, induces resumption of meiosis (Newhall et al., 2006). However, oocytes from AKAP1-deficient mice have a significantly lower ability to resume meiosis in vivo (Newhall et al., 2006) suggesting that other AKAPs are involved in regulating PKA during GV arrest (Newhall et al., 2006; Webb et al., 2008).
Two PKA substrates, CDC25B and WEE2, provide a link between cAMP and inhibition of CDK1 activity that is required to maintain meiotic arrest (Fig. 1A). PKA-mediated phosphorylation of CDC25B on Ser-321 in GV-stage oocytes generates a binding site for a 14-3-3 protein (Pirino et al., 2009) that retains CDC25B in the cytoplasm and thereby inhibits its function (Zhang et al., 2008; Pirino et al., 2009). What remains unresolved is how CDC25A activity is inhibited. Although inhibition of prophase I CDC25A activity may somehow be linked to PKA activity, CDC25A may already be fully active in prophase I oocytes but by being sequestered within the GV CDC25A cannot interact with the cytoplasmically localized CDK1–CCNB1 complex (Fig. 1B). Just prior to GVBD, all of these central components required for maturation—CDK1, CCNB1, CDC25A and CDC25B—become localized in the nucleus.
WEE2 is the other PKA substrate. Phosphorylation of WEE2 by PKA potentiates the activity of WEE2, thereby enhancing its ability to inhibit CDK1 activity (Han et al., 2005). The combined action of these two PKA substrates, inhibiting CDC25B and activating WEE2, likely insures that CDK1 activity remains inhibited in prophase 1-arrested oocytes until that inhibition is overcome in response to hormonal signals in vivo or the inability of oocytes to maintain inhibitory concentrations of cAMP following release from their follicle.
Protein tyrosine phosphatase non-receptor type 13 (PTPN13) is another potential PKA substrate that could function as a positive regulator in resumption of meiosis; PTPN13 is inhibited by PKA phosphorylation. Studies using Xenopus oocytes provide evidence for a role of PTPN13 in resumption of meiosis (Nedachi and Conti, 2004), e.g. siRNA-mediated targeting of PTPN13 mRNA inhibits progesterone-induced maturation. Although PTPN13 is expressed in mouse oocytes, it is unlikely involved in meiosis because mice carrying a mutation of PTPN13 that leads to loss of phosphatase activity are fertile (Wansink et al., 2004).
PDE3A-dependent hydrolysis of cAMP produces AMP, which in turn stimulates the activity of the AMP-activated protein kinase (AMPK). Both pharmacological activation and microinjection of a constitutively active AMPK induces resumption of meiosis in oocytes cultured in dbcAMP-containing medium that normally inhibits spontaneous maturation in vitro (Chen et al., 2006). Moreover, pharmacological inhibition of AMPK significantly delays both amphiregulin (AREG)-induced (Chen and Downs, 2008, and see below) and spontaneous oocyte maturation (Chen et al., 2006). The AMPK substrates that mediate these effects, however, have not been identified. Whether the hydrolysis of cAMP (whose intracellular concentration is ∼1 µM) that leads to resumption of meiosis actually results in an increase in intracellular AMP concentration sufficient to activate AMPK has not been established.
Taken together, these data indicate that endogenously produced cAMP is essential to maintain prophase I arrest. Such endogenously generated cAMP activates PKA that in turn inhibits the action of CDC25B and potentiates the activity of WEE2. Prior to resumption of meiosis as evidenced by GVBD, the decrease in cAMP that occurs during the commitment period results in translocation of CDC25B and CCNB1 to the nucleus and a decrease in WEE2 kinase activity. Furthermore, the initial increase in CDK1 activity fosters a partial loss of WEE2 from the nucleus that permits further activation of CDK1 such that the threshold for CDK1 activity necessary to promote GVBD is surpassed. Critical to this mode of regulation is the localization of CDK1–CCNB1, CDC25A and CDC25B in the nucleus and partial loss of nuclear WEE2 shortly before GVBD.
APC–CDH1-dependent regulation of CCNB1 level
In mitosis entry into anaphase is triggered by the rapid destruction of CCNB1 and securin (pituitary tumour-transforming 1, PTTG1). This decline is initiated by E3 polyubiquitin ligase APC that it is activated in early anaphase by CDC20 and in late anaphase and mainly in G1-phase by CDH1. APC–CDH1 drives destruction of other mitotic molecules including CDC20, Aurora kinases A and B (AURKA and AURKB) and Polo-like kinase 1 (PLK1). The APC-mediated degradation of CCNB1 leads to a decline in CDK1 activity and destruction of PPTG1, which in turn allows full activation of Separase (extra spindle poles-like 1, ESPL1), a protease that cleaves REC8 and thus is required for proper chromosome segregation. Until all of the chromosomes are correctly attached to the mitotic spindle, APC–CDC20 function is inhibited by spindle assembly checkpoint (SAC) [reviewed in (van Leuken et al., 2008)].
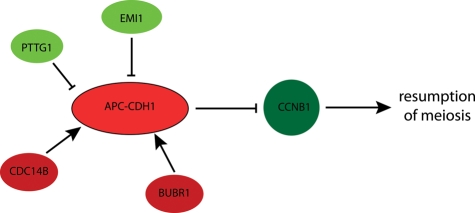
In prophase I oocytes APC–CDH1-mediated CCNB1 degradation prevents CCNB1 from accumulating and activating CDK1 (Fig. 3). When CDH1 is down-regulated by a morpholino oligonucleotide, about one-third of the oocytes resume meiosis in the presence of a PDE3A inhibitor (Reis et al., 2006). APC–CDH1 activity in prophase I arrested oocytes is stimulated by BUBR1, a protein kinase known mainly for its critical role in the SAC and in oocytes stabilizes the amount of CDH1 protein (Homer et al., 2009). Recently, it was discovered that in prophase I arrested oocytes APC–CDH1-mediated CCNB1 destruction is higher in the nucleus than in the cytoplasm. Nuclear APC–CDH1-dependent proteasomal activity would thereby prevent CCNB1 accumulation in the nucleus, a step that precedes and is essential for GVBD (Holt et al., 2010). APC–CDH1 activity is inhibited by CDK phosphorylation, which generates a positive feedback loop, i.e. an increase in CDK1 activity inhibits APC–CDH1 activity that results in CCNB1 accumulation and a concomitant increase in CDK1 activity. The CDC14B phosphatase expressed in mouse oocytes counteracts this phosphorylation and prevents premature CCNB1 accumulation and precocious resumption of meiosis by maintaining APC–CDH1 activity in GV-stage oocytes. Such a critical role for CDC14B in regulating maturation comes from the finding that RNAi-mediated CDC14B down-regulation induces resumption of meiosis of oocytes in the presence of PDE inhibitors and that over-expressing CDC14B delays spontaneous resumption of meiosis in vitro (Schindler and Schultz, 2009).
Figure 3.
Regulation of anaphase-promoting complex with CDH1 co-activator (APC–CDH1) during prophase I arrest. Molecules inhibiting resumption of meiosis are in red and those stimulating resumption of meiosis are green. PTTG1, pituitary tumour-transforming gene (securin); CDC14B, phosphatase; EMI1, early mitotic inhibitor 1; CCNB1, cyclin B; BUBR1, cell cycle spindle check point protein kinase.
Too high of a level of APC–CDH1 activity should inhibit resumption of meiosis even when the maturation-associated decrease in cAMP occurs or is experimentally induced because CCNB1 will not be able to accumulate and activate CDK1. Prophase I arrested oocytes express early mitotic inhibitor 1 (EMI1) that suppresses APC–CDH1 activity. As anticipated, EMI1 down-regulation results in a delay of GVBD onset whereas over-expression of EMI1 accelerates the onset of GVBD (Marangos et al., 2007). GV-stage oocytes also contain other APC–CDH1 substrates, such as PTTG1 and CDC20, whose stability is modulated by APC–CDH1 (Reis et al., 2006, 2007). Although PTTG1 is an ESPLl (separase) inhibitor and prevents premature chromosome segregation, PTTG1 also plays a role in prophase I arrest by serving as a substrate for—and thereby competing with CCNB1—APC-mediated degradation. For example, down-regulation of PTTG1 delays resumption of meiosis and over-expression of PTTG1 permits resumption of meiosis even in the presence of inhibitory concentrations of cAMP (Marangos and Carroll, 2008).
The APC–CDH1 complex clearly plays a role in maintaining meiotic arrest by preventing accumulation of CCNB1; it is well established that accumulation of CCNB1 following GVBD is essential for progression to metaphase I. Nevertheless, protein synthesis is not essential for the initial activation of CDK1 and GVBD, at least during in vitro maturation of mouse oocytes (Polanski et al., 1998; Ledan et al., 2001). An interesting but unanswered question is whether a decrease in APC–CDH1 activity precedes GVBD and whether modulation of activities of its regulators, e.g. CDC14B, occurs during this time. After GVBD the situation is clearer when CDC20, rather than CCNB1 and PTTG1, is the preferred substrate and thereby permits accumulation of CCNB1 and PTTG1. This finding, coupled with an increase in CDK1 and CDH1 degradation during this time, which attenuates APC–CDH1 destruction of CCNB1 (and PTTG1) ensures an irreversible progression into MI (Reis et al., 2007). Taken together, prophase I arrest is maintained by high cAMP levels that ensure inhibitory CDK1 phosphorylation and an active APC–CDH1 that prevents unwanted CCNB1 accumulation. Whether cAMP levels also control APC–CDH1 activity remains unanswered.
Other mitotic kinases
Mitotic entry is regulated not only by CDK1 but also by other protein kinases such as AURKA and PLK1. In somatic cells AURKA activates PLK1 and promotes mitotic entry (Macurek et al., 2008; Seki et al., 2008). PLK1-catalyzed phosphorylation of WEE1 triggers WEE1 degradation (Watanabe et al., 2005) whereas PLK1-mediated phosphorylation of MYT1 inhibits MYT1 kinase activity (Nakajima et al., 2003). PLK1 phosphorylation of CDC25C (Toyoshima-Morimoto et al., 2002) or CDC25B (Lobjois et al., 2009) [reviewed in (Lindqvist et al., 2009)] promotes translocation of these protein phosphatases from the cytoplasm to nucleus. AURKA-dependent phosphorylation of centrosomally located CDC25B also contributes to entry into mitosis (Dutertre et al., 2004).
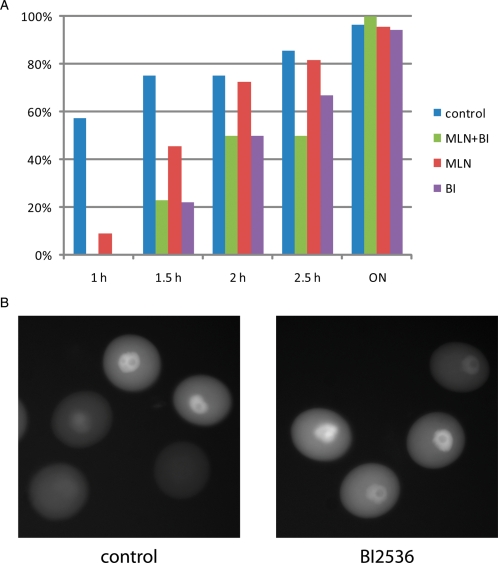
Because CDC25B plays a central role in resumption of meiosis and, AURKA and PLK1 potentiate CDC25B activity (see above), we explored whether AURKA or PLK1 are involved in the resumption of meiosis. Using an RNAi approach, we noted that down-regulation of AURKA protein blocks resumption of meiosis in a small but significant fraction of oocytes. On the other hand AURKA over-expression does not overcome the cAMP-mediated prophase I block (Saskova et al., 2008). Our recent data (Fig. 4A) show a delay of about 45 min in the onset of GVBD when AURKA is inhibited with MLN8054, a small-molecule inhibitor of AURKA (Hoar et al., 2007). Interestingly, pharmacological inhibition of PLK1 (Lenart et al., 2007) delays the onset of GVB by about 90 min. In contrast to somatic cells (Lobjois et al., 2009), PLK1 inhibition does not block CDC25B nuclear translocation (Fig. 4B). Inhibiting both AURKA and PLK1 has the same effect on the kinetics of GVBD as inhibiting PLK1 alone. These data demonstrate that although both AURKA and PLK1 are involved in the signalling pathway that drives the initial activation of CDK1 and resumption of meiosis, their function is not absolutely essential for resumption of meiosis and that it is highly unlikely that AURKA triggers entry into meiosis I in mouse oocytes.
Figure 4.
Role of AURKA and PLK1 during resumption of meiosis. (A) Effect of pharmacological inhibition of AURKA by MLN8054 (Hoar et al., 2007) and PLK1 by BI2536 (Lenart et al., 2007) on the kinetics of GVBD. The indicated times are in hours, and ON indicates that the oocytes were cultured overnight for 18 h. (B) PLK1 is not responsible for nuclear CDC25B translocation. Shown are oocytes expressing a small amount of exogenous GFP-CDC25B. In the control group and experimental group, in which PLK1 is inhibited with BI2536, shortly before GVBD there is a clear translocation of CDC25B to the nucleus. Note that in prophase I arrested oocytes with a high cAMP level that CDC25B is strictly cytoplasmic (Fig. 1B).
Resumption of meiosis versus checkpoint recovery
As described above, resumption of meiosis is often compared with the G2-M transition in somatic cells. Nevertheless, it is essential to distinguish the molecular mechanisms that govern mitotic entry of continuously proliferating cells and checkpoint recovery (cell cycle resumption) from the G2-arrest induced by DNA damage (Lindqvist et al., 2009). Cells exposed to genotoxic stress (e.g. double strand DNA breaks, DSBs) prior to entry into mitosis activate a G2-checkpoint that delays mitotic entry in the presence of unrepaired DNA lesions. The critical components of checkpoint signalling are activation of phosphoinositide 3-kinase related kinases ATM and ATR. These sensor kinases then activate the effector kinases CHK2 and CHK1, respectively (Bartek and Lukas, 2007). Among the CHK1 and CHK2 substates are the CDC25 phosphatases, whose phosphorylation by CHK1/2 leads to CDC25A degradation and inhibition of CDC25B and C (Donzelli and Draetta, 2003). CDC14B activation is also a critical component of the G2-checkpoint. CDC14B activates APC–CDH1 that leads to degradation of PLK1 that would otherwise target Skp, Cullin, F-box containing complex (SCF)-dependent WEE1 and Claspin (CHK1 co-activator) destruction (Bassermann et al., 2008).
G2-arrest in somatic cells induced by DSBs is associated with activation of the kinase ATM in the presence of damaged DNA. During development in the fetal ovary, recombination naturally leads to dsDNA breaks in oocytes at early stages of prophase I. Processing and repairing these natural DNA lesions also requires ATM; ATM-deficient female mice are completely infertile due to meiotic problems at prophase I that results in severe oocyte loss as a response to unrepaired DSBs (Barlow et al., 1998; Di Giacomo et al., 2005).
Meiotic dsDNA breaks are initiated by SPO11. Although oocytes from SPO11-deficient mice exhibit prophase I arrest they die during postnatal development (Di Giacomo et al., 2005). This finding suggests that normal oocyte development and reproduction are intimately connected with dsDNA breaks and DNA repair but uncoupled temporally with when the phenotype becomes manifest (Jackson and Bartek, 2009). Moreover, both prophase I arrested oocytes and G2-arrested somatic cells must resume the cell cycle at an appropriate time for future development.
G2-arrest in somatic cells depends on the action of CDC14B and APC–CDH1, both of which are essential for the prophase I arrest of mouse oocytes (Reis et al., 2006; Schindler and Schultz, 2009). Interestingly, in normal somatic cells that have not suffered DNA damage CDC14B is sequestered in the nucleolus, but following DNA damage CDC14B leaves the nucleolus and is presumably active (Bassermann et al., 2008). In prophase I arrested oocytes CDC14B is not localized in the nucleus suggesting that it may be active (Schindler and Schultz, 2009). Moreover, after DNA damage repair cell cycle resumption of somatic cells requires PLK1 and CDC25B (Lincoln et al., 2002). For normal mitotic entry CDC25B (Lincoln et al., 2002; van Vugt et al., 2004; Lindqvist et al., 2005) or PLK1 (van Vugt et al., 2004) are dispensable, whereas resumption of meiosis requires CDC25B (Lincoln et al., 2002).
Protein phosphatase 1 (PPP1) and protein phosphatase 2A (PPP2) are involved in resumption of meiosis and G2-checkpoint recovery. Okadaic acid (OA), a PPP1 and PPP2 inhibitor, induces resumption of meiosis of oocytes cultured in the presence of a PDE inhibitor (Alexandre et al., 1991; Gavin et al., 1991; Schwartz and Schultz, 1991). OA also overrides G2-checkpoint and induces premature mitotic entry in human cancer cells (Ghosh et al., 1996). Exogenous HOX11, an orphan homeobox gene, which can interact with and inhibit PPP1 and PPP2, induces resumption of meiosis of Xenopus oocytes and overrides infra-red radiation induced G2-checkpoint in Jurkat T-cells (Roberts et al., 1994). How PPPs are regulated during resumption of meiosis and checkpoint recovery is not well understood. Around the time of GVBD CDK1 catalyzes an inhibitory phosphorylation of PPP1 on T320. Nevertheless, inhibiting PPP1 overcomes the GV-stage block by the CDK inhibitor roscovitine, demonstrating an ability of PPP1 to induce GVBD in the absence of CDK1 activity (Swain et al., 2003). Similarly, OA can induce premature mitotic entry independent of CDK1 activity in HeLa cells (Ghosh et al., 1998). Greatwall kinase (GWL) inactivates PPP2 in Xenopus egg extracts (Castilho et al., 2009) but activates CDC25 phosphates in Xenopus oocytes (Zhao et al., 2008). These data implicate GWL for initiation of M-phase entry by inhibiting PPP2 and inducing CDC25 activities that lead to CDK1 activation. Recently published whole genome screening (Neumann et al., 2010) identified microtubule associated serine/threonine kinase-like (MASTL) protein, a mammalian GWL orthologue, as a critical player for normal mitosis progression in HeLa cells. Whether MASTL is involved in resumption of meiosis and G2-checkpoint recovery remains to be established.
We propose that prophase I arrest and resumption of meiosis are more similar to G2-arrest induced by DSBs in somatic cells and the checkpoint recovery than to a normal G2-M transition that occurs in proliferating cells, a view that differs markedly from what has been previously proposed. If correct, the molecular details that are unmasked to govern meiotic prophase I arrest may be directly relevant to understanding the mitotic G2-checkpoint, and vice-versa, despite some differences between G2-arrest in somatic cells and prophase I arrest in oocytes. For example, the principle target for CDC14B-APC/CDH1 in somatic cells is PLK1 and not CCNB1 (Bassermann et al., 2008), whereas in mouse oocytes the primary target appears to be CCNB1 (Reis et al., 2006; Schneider and Wolf, 2009). On the other hand, PLK1 is essential for G2-checkpoint recovery (van Vugt et al., 2004; Macurek et al., 2008) but for resumption of meiosis PLK1 is dispensable but can enhance the process.
Epidermal growth factor-like signalling and resumption of meiosis in vivo
Resumption of meiosis is triggered in vivo by LH surge (Neal and Baker, 1975; Lei et al., 2001). LH receptor (LHR) expression, however, is restricted to mural granulosa and thecal-interstitial cells, and neither oocytes nor their associated cumulus cells express detectable LHR (Peng et al., 1991). Therefore, a signalling pathway must exist in which a signal(s) originating in LHR-expressing cells acts on non-LHR-expressing cells.
LH induces expression of epidermal growth factor (EGF) like factors AREG, epiregulin (EREG) and betacellulin (BTC) in LHR-bearing mural granulosa cells. AREG and EREG mRNAs expression proceeds GVBD in vivo, whereas BTC expression is detected 3 h after the LH surge at which time the majority of oocytes have already undergone GVBD. These results, coupled with the observation that either AREG or EREG is more effective than BTC in inducing meiotic maturation in cultured intact follicles strongly implies that BTC is not the primary signalling molecule synthesized in response to LH, but rather this role is executed by AREG/EREG (Park et al., 2004). Consistent with this latter proposal is that oocytes from both AREG and EREG KO mice display a significant delay in the onset of meiotic maturation after the LH/hCG surge in vivo (Hsieh et al., 2007).
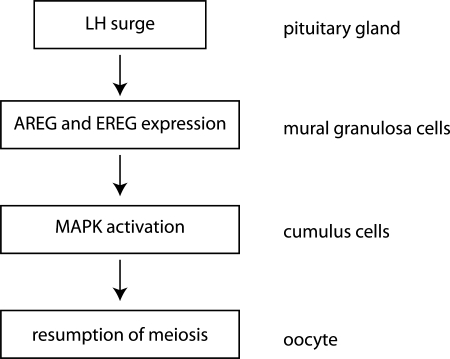
EGF-like growth factors are produced as transmembrane precursors that are cleaved at the cell surface of expressing cells by extracellular proteases. The released soluble growth factors then act in an endocrine, paracrine or autocrine fashion. These factors bind to tyrosine-kinase epidermal growth factor receptor (EGFR) on the target cells and activate multiple intracellular signalling pathways (Schneider and Wolf, 2009). Further evidence for involvement of this signalling pathway in resumption of meiosis in vivo is that LH/hCG-induced resumption of meiosis is strongly inhibited in AREG−/− EGFRwa2/wa2 mice. Furthermore, the observation that oocytes isolated from AREG−/− EGFRwa2/wa2 mice resume meiosis spontaneously in vitro with the same kinetics as controls indicates that they are meiotically competent (Hsieh et al., 2007). Taken together, these data provide the foundation for the model (Fig. 5) in which LH induces expression of AREG and EREG in mural granulosa cells and these diffusible factors trigger EGFR signalling in the cumulus cells leading to resumption of meiosis.
Figure 5.
Schematic diagram depicting signalling pathway in the follicle after LH peak leading to the resumption of meiosis in oocytes. AREG, amphiregulin; EREG, epiregulin; MAPK, mitogen-activated protein kinase.
Mitogen-activated protein kinase (MAPK) signalling in granulosa cells is also an essential part of the events downstream from activation of the EGFR (Fan et al., 2009). Mice deficient in MAPKs (ERK1 and ERK2, ERK1/2 gc−/−) only in granulosa cells are completely infertile because oocytes do not resume meiosis and ovulate in vivo, even after exogenous hormone treatment. Nevertheless, oocytes from these mice undergo GVBD and reach metaphase II when cultured in vitro. Further evidence that EGF-like signalling in cumulus cells relies on ERK1 and ERK2 is derived from experiments in which addition of AREG overcomes hypoxanthine-mediated inhibition of maturation of oocytes in oocyte–cumulus cell complexes from wild-type, but not from ERK1/2 gc−/− mice (Fan et al., 2009).
The remaining question is the nature of the link between EGFR and MAPK activation in the cumulus cells and the meiotic resumption in oocytes. The answer appears to be that cGMP from the somatic compartment (cumulus cells) diffuses into the oocyte via the heterologous gap junctions coupling the two cell types where it inhibits PDE3A activity (Norris et al., 2009; Vaccari et al., 2009). It seems unlikely that cGMP works by activating PKG because pharmacological inhibition of PKG has no effect on oocyte maturation (Wang et al., 2008). After the LH peak, cGMP level decreases in both the somatic compartment and oocyte (Norris et al., 2009; Vaccari et al., 2009). The cGMP decrease in oocytes, which may be in response to MAPK-dependent closure of gap-junction communication between cumulus cells via phosphorylation of connexin 43, would then result in an increase in PDE3 activity in oocytes (Norris et al., 2009). Because LH-induced signalling does not terminate GPR3-Gs-AC signalling (Norris et al., 2007), i.e. the ability of the oocyte to generate endogenous cAMP, the increase in PDE3A activity is likely sufficient to promote the maturation-associated decrease in oocyte cAMP that occurs during the period of time when oocytes with an intact GV become committed to resume meiosis (Schultz et al., 1983). The proposed model is consistent with the observation that pharmacologically inducing gap-junction closure results in spontaneous resumption of meiosis of follicle-enclosed oocytes both matured in vitro or in vivo (Sela-Abramovich et al., 2006).
In light of this new model, there are conflicting results obtained using 8-Br-cGMP, a membrane-permeable cGMP analog or Sildenafil, an inhibitor of phosphodiesterase 5 that is probably responsible for cGMP destruction in cumulus cells (Vaccari et al., 2009). Bu et al. (2004) suggest that 8-Br-cGMP significantly inhibits resumption of meiosis in both denuded oocytes or in cumulus cell-enclosed oocytes. On the other hand, Hubbard and Terranova (1982) demonstrated an inhibitory effect of 8-Br-cGMP only on cumulus cell-enclosed oocytes suggesting that this effect is mediated indirectly by the cumulus cells. Recently, one report documented an inhibitory effect of Sildenafil on LH-induced resumption of meiosis of in vitro cultured follicle-enclosed oocytes, but no effect was observed on spontaneous resumption of meiosis of either denuded oocytes or cumulus cell-enclosed oocytes (Vaccari et al., 2009). In contrast, a recent study noted an inhibitory reversible effect of Sildenafil on spontaneous resumption of meiosis of cumulus cell-enclosed oocytes (Wang et al., 2008).
Despite these apparently conflicting data, cGMP is most likely a critical component required to maintain prophase I arrest and does so by interacting with the cAMP signalling pathway. Future work using genetic models will be essential to dissect out precisely the relationships between cGMP, cAMP and EGF-like signalling during resumption of meiosis.
Future directions
Meiotic prophase I arrest and resumption of meiosis are functionally similar to the G2-checkpoint arrest and recovery from it in somatic cells but many questions remain, including whether changes in APC–CDH1 activity are connected to resumption of meiosis, whether the cAMP decrease is directly responsible for activation of CDC25 phosphatases, and whether these phosphatases are also directly activated by others kinases, such as PKB, AURKA or PLK1. These questions are challenging, but given the remarkable progress that has been made in the past decade, there is much hope that answers will be forthcoming.
Funding
Work on meiotic maturation in the context of IRP IAPG No. AV0Z50450515 is supported by grants 301/09/J036 (Czech Science Foundation) and ME 08030 (Ministry of Education, Youth and Sports) to J.M. R.M.S. was supported by a grant from the NIH (HD22681).
References
- Alexandre H, Van Cauwenberge A, Tsukitani Y, Mulnard J. Pleiotropic effect of okadaic acid on maturing mouse oocytes. Development. 1991;112:971–980. doi: 10.1242/dev.112.4.971. [DOI] [PubMed] [Google Scholar]
- Barlow C, Liyanage M, Moens PB, Tarsounas M, Nagashima K, Brown K, Rottinghaus S, Jackson SP, Tagle D, Ried T, et al. Atm deficiency results in severe meiotic disruption as early as leptonema of prophase I. Development. 1998;125:4007–4017. doi: 10.1242/dev.125.20.4007. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. doi: 10.1016/j.ceb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell. 2008;134:256–267. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Mattei P, Schultz RM. Involvement of cAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev Biol. 1986;114:453–462. doi: 10.1016/0012-1606(86)90209-5. [DOI] [PubMed] [Google Scholar]
- Bornslaeger EA, Mattei PM, Schultz RM. Protein phosphorylation in meiotically competent and incompetent mouse oocytes. Mol Reprod Dev. 1988;1:19–25. doi: 10.1002/mrd.1080010105. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maro B. Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction. 2005;130:801–811. doi: 10.1530/rep.1.00364. [DOI] [PubMed] [Google Scholar]
- Bu S, Xie H, Tao Y, Wang J, Xia G. Nitric oxide influences the maturation of cumulus cell-enclosed mouse oocytes cultured in spontaneous maturation medium and hypoxanthine-supplemented medium through different signaling pathways. Mol Cell Endocrinol. 2004;223:85–93. doi: 10.1016/j.mce.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Downs SM. AMP-activated protein kinase is involved in hormone-induced mouse oocyte meiotic maturation in vitro. Dev Biol. 2008;313:47–57. doi: 10.1016/j.ydbio.2007.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Hurov J, White LS, Woodford-Thomas T, Piwnica-Worms H. Absence of apparent phenotype in mice lacking Cdc25C protein phosphatase. Mol Cell Biol. 2001;21:3853–3861. doi: 10.1128/MCB.21.12.3853-3861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hudson E, Chi MM, Chang AS, Moley KH, Hardie DG, Downs SM. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol. 2006;291:227–238. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Dekel N, Lawrence TS, Gilula NB, Beers WH. Modulation of cell-to-cell communication in the cumulus-oocyte complex and the regulation of oocyte maturation by LH. Dev Biol. 1981;86:356–362. doi: 10.1016/0012-1606(81)90193-7. [DOI] [PubMed] [Google Scholar]
- Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M. Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci USA. 2005;102:737–742. doi: 10.1073/pnas.0406212102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli M, Draetta GF. Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Rep. 2003;4:671–677. doi: 10.1038/sj.embor.embor887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Cazales M, Quaranta M, Froment C, Trabut V, Dozier C, Mirey G, Bouche JP, Theis-Febvre N, Schmitt E, et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2-M transition. J Cell Sci. 2004;117:2523–2531. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin AC, Tsukitani Y, Schorderet-Slatkine S. Induction of M-phase entry of prophase-blocked mouse oocytes through microinjection of okadaic acid, a specific phosphatase inhibitor. Exp Cell Res. 1991;192:75–81. doi: 10.1016/0014-4827(91)90159-r. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Schroeter D, Paweletz N. Okadaic acid overrides the S-phase check point and accelerates progression of G2-phase to induce premature mitosis in HeLa cells. Exp Cell Res. 1996;227:165–169. doi: 10.1006/excr.1996.0262. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Paweletz N, Schroeter D. Cdc2-independent induction of premature mitosis by okadaic acid in HeLa cells. Exp Cell Res. 1998;242:1–9. doi: 10.1006/excr.1998.4115. [DOI] [PubMed] [Google Scholar]
- Hagting A, Karlsson C, Clute P, Jackman M, Pines J. MPF localization is controlled by nuclear export. EMBO J. 1998;17:4127–4138. doi: 10.1093/emboj/17.14.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol. 2005;15:1670–1676. doi: 10.1016/j.cub.2005.07.056. [DOI] [PubMed] [Google Scholar]
- Han SJ, Vaccari S, Nedachi T, Andersen CB, Kovacina KS, Roth RA, Conti M. Protein kinase B/Akt phosphorylation of PDE3A and its role in mammalian oocyte maturation. EMBO J. 2006;25:5716–5725. doi: 10.1038/sj.emboj.7601431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol. 2005;287:249–261. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hoar K, Chakravarty A, Rabino C, Wysong D, Bowman D, Roy N, Ecsedy JA. MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007;27:4513–4525. doi: 10.1128/MCB.02364-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JE, Weaver J, Jones KT. Spatial regulation of APCCdh1-induced cyclin B1 degradation maintains G2 arrest in mouse oocytes. Development. 2010;137:1297–1304. doi: 10.1242/dev.047555. [DOI] [PubMed] [Google Scholar]
- Homer H, Gui L, Carroll J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science. 2009;326:991–994. doi: 10.1126/science.1175326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner K, Livera G, Hinckley M, Trinh K, Storm D, Conti M. Rodent oocytes express an active adenylyl cyclase required for meiotic arrest. Dev Biol. 2003;258:385–396. doi: 10.1016/s0012-1606(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard CJ, Terranova PF. Inhibitory action of cyclic guanosine 5′-phosphoric acid (GMP) on oocyte maturation: dependence on an intact cumulus. Biol Reprod. 1982;26:628–632. doi: 10.1095/biolreprod26.4.628. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol Cell. 2006;98:111–123. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Schultz RM, Kopf GS. Acquisition of meiotic competence in mouse oocytes: absolute amounts of p34(cdc2), cyclin B1, cdc25C, and wee1 in meiotically incompetent and competent oocytes. Biol Reprod. 2000;63:1610–1616. doi: 10.1095/biolreprod63.6.1610. [DOI] [PubMed] [Google Scholar]
- Kim S, Jee K, Kim D, Koh H, Chung J. Cyclic AMP inhibits Akt activity by blocking the membrane localization of PDK1. J Biol Chem. 2001;276:12864–12870. doi: 10.1074/jbc.M001492200. [DOI] [PubMed] [Google Scholar]
- Ledan E, Polanski Z, Terret ME, Maro B. Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev Biol. 2001;232:400–413. doi: 10.1006/dbio.2001.0188. [DOI] [PubMed] [Google Scholar]
- Lei ZM, Mishra S, Zou W, Xu B, Foltz M, Li X, Rao CV. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol. 2001;15:184–200. doi: 10.1210/mend.15.1.0586. [DOI] [PubMed] [Google Scholar]
- Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- Li M, Yin S, Yuan J, Wei L, Ai JS, Hou Y, Chen DY, Sun QY. Cdc25A promotes G2/M transition in oocytes. Cell Cycle. 2008;7:1301–1302. doi: 10.4161/cc.7.9.5958. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–449. doi: 10.1038/ng856. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Kallstrom H, Lundgren A, Barsoum E, Rosenthal CK. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome. J Cell Biol. 2005;171:35–45. doi: 10.1083/jcb.200503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobjois V, Jullien D, Bouche JP, Ducommun B. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim Biophys Acta. 2009;1793:462–468. doi: 10.1016/j.bbamcr.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;455:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- Marangos P, Carroll J. The dynamics of cyclin B1 distribution during meiosis I in mouse oocytes. Reproduction. 2004;128:153–162. doi: 10.1530/rep.1.00192. [DOI] [PubMed] [Google Scholar]
- Marangos P, Carroll J. Securin regulates entry into M-phase by modulating the stability of cyclin B. Nat Cell Biol. 2008;10:445–451. doi: 10.1038/ncb1707. [DOI] [PubMed] [Google Scholar]
- Marangos P, Verschuren EW, Chen R, Jackson PK, Carroll J. Prophase I arrest and progression to metaphase I in mouse oocytes are controlled by Emi1-dependent regulation of APC(Cdh1) J Cell Biol. 2007;176:65–75. doi: 10.1083/jcb.200607070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest. 2004;114:196–205. doi: 10.1172/JCI21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Jones TL, Jaffe LA. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science. 2002;297:1343–1345. doi: 10.1126/science.1073978. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–1950. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- Mitra J, Schultz RM. Regulation of the acquisition of meiotic competence in the mouse: changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J Cell Sci. 1996;109:2407–2415. doi: 10.1242/jcs.109.9.2407. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Toyoshima-Morimoto F, Taniguchi E, Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J Biol Chem. 2003;278:25277–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- Neal P, Baker TG. Response of mouse graafian follicles in organ culture to varying doses of follicle-stimulating hormone and luteinizing hormone. J Endocrinol. 1975;65:27–32. doi: 10.1677/joe.0.0650027. [DOI] [PubMed] [Google Scholar]
- Nedachi T, Conti M. Potential role of protein tyrosine phosphatase nonreceptor type 13 in the control of oocyte meiotic maturation. Development. 2004;131:4987–4998. doi: 10.1242/dev.01368. [DOI] [PubMed] [Google Scholar]
- Neumann B, Walter T, Heriche JK, Bulkescher J, Erfle H, Conrad C, Rogers P, Poser I, Held M, Liebel U, et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature. 2010;464:721–727. doi: 10.1038/nature08869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKnight GS. Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol. 2006;16:321–327. doi: 10.1016/j.cub.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Freudzon L, Freudzon M, Hand AR, Mehlmann LM, Jaffe LA. A G(s)-linked receptor maintains meiotic arrest in mouse oocytes, but luteinizing hormone does not cause meiotic resumption by terminating receptor-G(s) signaling. Dev Biol. 2007;310:240–249. doi: 10.1016/j.ydbio.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–1878. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Han SJ, Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol. 2010;188:199–207. doi: 10.1083/jcb.200907161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. doi: 10.1210/endo-129-6-3200. [DOI] [PubMed] [Google Scholar]
- Pirino G, Wescott MP, Donovan PJ. Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle. 2009;8:665–670. doi: 10.4161/cc.8.4.7846. [DOI] [PubMed] [Google Scholar]
- Polanski Z, Ledan E, Brunet S, Louvet S, Verlhac MH, Kubiak JZ, Maro B. Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development. 1998;125:4989–4997. doi: 10.1242/dev.125.24.4989. [DOI] [PubMed] [Google Scholar]
- Reis A, Chang HY, Levasseur M, Jones KT. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol. 2006;8:539–540. doi: 10.1038/ncb1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis A, Madgwick S, Chang HY, Nabti I, Levasseur M, Jones KT. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol. 2007;9:1192–1198. doi: 10.1038/ncb1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Shutter JR, Korsmeyer SJ. Hox11 controls the genesis of the spleen. Nature. 1994;368:747–749. doi: 10.1038/368747a0. [DOI] [PubMed] [Google Scholar]
- Rodrigues P, Limback D, McGinnis LK, Plancha CE, Albertini DF. Oogenesis: prospects and challenges for the future. J Cell Physiol. 2008;216:355–365. doi: 10.1002/jcp.21473. [DOI] [PubMed] [Google Scholar]
- Saskova A, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. Aurora kinase A controls meiosis I progression in mouse oocytes. Cell Cycle. 2008;7:2368–2376. doi: 10.4161/cc.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Koide SS. Forskolin and mouse oocyte maturation in vitro. J Exp Zool. 1984;230:125–129. doi: 10.1002/jez.1402300116. [DOI] [PubMed] [Google Scholar]
- Schindler K, Schultz RM. CDC14B acts through FZR1 (CDH1) to prevent meiotic maturation of mouse oocytes. Biol Reprod. 2009;80:795–803. doi: 10.1095/biolreprod.108.074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Schultz RM. Stimulatory effect of okadaic acid, an inhibitor of protein phosphatases, on nuclear envelope breakdown and protein phosphorylation in mouse oocytes and one-cell embryos. Dev Biol. 1991;145:119–127. doi: 10.1016/0012-1606(91)90218-r. [DOI] [PubMed] [Google Scholar]
- Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology. 2006;147:2280–2286. doi: 10.1210/en.2005-1011. [DOI] [PubMed] [Google Scholar]
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol. 2008;317:260–269. doi: 10.1016/j.ydbio.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Wang X, Saunders TL, Dunn R, Smith GD. Specific inhibition of mouse oocyte nuclear protein phosphatase-1 stimulates germinal vesicle breakdown. Mol Reprod Dev. 2003;65:96–103. doi: 10.1002/mrd.10258. [DOI] [PubMed] [Google Scholar]
- Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima-Morimoto F, Taniguchi E, Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Horner K, Mehlmann LM, Conti M. Generation of mouse oocytes defective in cAMP synthesis and degradation: endogenous cyclic AMP is essential for meiotic arrest. Dev Biol. 2008;316:124–134. doi: 10.1016/j.ydbio.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL, 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod. 2009;81:595–604. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leuken R, Clijsters L, Wolthuis R. To cell cycle, swing the APC/C. Biochim Biophys Acta. 2008;1786:49–59. doi: 10.1016/j.bbcan.2008.05.002. [DOI] [PubMed] [Google Scholar]
- van Vugt MA, Bras A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Wang S, Ning G, Chen X, Yang J, Ouyang H, Zhang H, Tai P, Mu X, Zhou B, Zhang M, et al. PDE5 modulates oocyte spontaneous maturation via cGMP-cAMP but not cGMP-PKG signaling. Front Biosci. 2008;13:7087–7095. doi: 10.2741/3212. [DOI] [PubMed] [Google Scholar]
- Wansink DG, Peters W, Schaafsma I, Sutmuller RP, Oerlemans F, Adema GJ, Wieringa B, van der Zee CE, Hendriks W. Mild impairment of motor nerve repair in mice lacking PTP-BL tyrosine phosphatase activity. Physiol Genomics. 2004;19:50–60. doi: 10.1152/physiolgenomics.00079.2004. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc Natl Acad Sci USA. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb RJ, Tinworth L, Thomas GM, Zaccolo M, Carroll J. Developmentally acquired PKA localisation in mouse oocytes and embryos. Dev Biol. 2008;317:36–45. doi: 10.1016/j.ydbio.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Yang J, Bardes ES, Moore JD, Brennan J, Powers MA, Kornbluth S. Control of cyclin B1 localization through regulated binding of the nuclear export factor CRM1. Genes Dev. 1998;12:2131–2143. doi: 10.1101/gad.12.14.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Xu XY, Li XS, Yu M, Yu AM, Zong ZH, Yu BZ. Protein kinase A modulates Cdc25B activity during meiotic resumption of mouse oocytes. Dev Dyn. 2008;237:3777–3786. doi: 10.1002/dvdy.21799. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Haccard O, Wang R, Yu J, Kuang J, Jessus C, Goldberg ML. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]