Abstract
Galanin receptors type 1 (GalR1) and/or type 2 (GalR2) represent unique pharmacological targets for treatment of seizures and epilepsy. Previous studies have shown that the endogenous peptide ligand galanin exerts powerful anticonvulsant effect through activation of these two G protein-coupled receptors, which are highly expressed in the temporal lobe of rodent brain. Here we report the characterization of a putative GalR2-positive allosteric modulator CYM2503. CYM2503 potentiated the galanin-stimulated IP1 accumulation in HEK293 cells stably expressing GalR2 receptor, whereas it exhibited no detectable affinity for the 125I galanin–binding site of GalR2 receptor, an effect consistent with that of a positive allosteric modulator. In the rat Li-pilocarpine status epilepticus model, CYM2503, injected intraperitoneally, increased the latency to first electrographic seizure and the latency to first stage 3 behavioral seizure, decreased the latency to the establishment of status epilepticus, and dramatically decreased the mortality. In a Li-pilocarpine seizure model in mice, CYM2503 increased the latency to first electrographic seizure and decreased the total time in seizure. CYM2503 also attenuated electroshock-induced seizures in mice. Thus, CYM2503 provides a starting point for the development of anticonvulsant therapy using the galanin R2 receptor as target.
Keywords: galanin, seizure, G protein-coupled receptor, status epilepticus
The neuropeptide galanin (1) is widely expressed in the central nervous system (2–4), in which it regulates a variety of physiological and pathological processes, including pain, learning and memory, mood, addiction, and food intake (5–16). In addition, converging lines of evidence suggest a critical role for galanin in seizure control. Galanin has been shown to exhibit potent anticonvulsant effect in both acute and chronic seizure models in rodents. Intrahippocampally injected galanin strongly and irreversibly attenuated the status epilepticus (SE) induced by perforant path stimulation in rats (17). Mice with null mutation of galanin were more susceptible to develop SE after perforant path stimulation or systemic kainic acid injection, and exhibited more severe seizures following pentylenetetrazol injection (18). In contrast, mice that overexpress galanin under a dopamine-β-hydroxylase promoter had increased resistance to seizure induction in these three models (18). A separate line of transgenic mice that overexpress galanin under the platelet-derived growth factor β promoter was found to exhibit delayed epileptogenesis induced by kindling stimulations (19). Moreover, galanin delivered to the rat brain via adenoassociated viral vectors has been shown to suppress kainic acid-induced seizures (20, 21), attenuate inferior collicular stimulation induced wild running seizure (21), and delay kindling development (22).
The aforementioned anticonvulsant effects of galanin were most likely mediated through galanin receptor type 1 (GalR1) and type 2 (GalR2). GalR1-knockout mice exhibited spontaneous seizures (23) and developed more severe seizures when subjected to perforant path stimulation and Li-pilocarpine injection (24). Down-regulation of GalR2 expression in the rat hippocampus with an anti-GalR2 peptide nucleic acid antisense significantly increased the severity of perforant path stimulation-induced SE (25). Both GalR1 and GalR2 are G protein–coupled receptors that are expressed at high levels in the hippocampus (26, 27). GalR1 receptor is predominantly coupled to Gi-mediating suppression of forskolin-stimulated cAMP accumulation. In addition to being weakly coupled to Gi, GalR2 is found to couple to Gq, resulting in membrane lipid turnover and inositol phosphate (IP) accumulation (28). GalR1 and GalR2 represent unique pharmacological targets for treatment of seizures and epilepsy.
Much effort has been made in recent years toward development of systemically active agonists/ modulators for GalR1 and GalR2 receptors and, as of today, two nonpeptide, systemically active agonists, Galnon (17) and Galmic (29), and a CNS-penetrating peptide, galanin analogue Gal-B2 (30–33), have been synthesized and characterized. All three galanin receptor agonists have been shown to exhibit potent anticonvulsant effect upon systemic application. However, Galnon and Galmic, the two nonpeptide agonists, were found to interact with multiple important targets in the CNS, raising concern of possible side effect profiles (34). The newly synthesized B-25 was estimated to have an impressive in vivo half-life of more than 10 h (33), but production of peptide drug might prove to be expensive. Therefore, there is a continuous need for selective, systemically active nonpeptide GalR1/R2 agonist or modulator.
Here we report the characterization of a putative GalR2-positive allosteric modulator CYM2503. CYM2503 potentiated the galanin stimulated IP1 accumulation in HEK293 cells stably expressing GalR2 receptors, whereas it exhibited no detectable affinity for the 125I galanin–binding site of GalR2 receptor, an effect consistent with that of a positive allosteric modulator. In the rat Li-pilocarpine SE model, CYM2503, injected intraperitoneally, increased the latency to first electrographic seizure and the latency to first stage 3 behavioral seizure, decreased the latency to the establishment of SE, and dramatically decreased mortality. In a Li-pilocarpine seizure model in mice, CYM2503 increased the latency to first electrographic seizure and decreased the total time in seizure. CYM2503 also attenuated electroshock-induced seizures in mice.
Results
CYM2503.
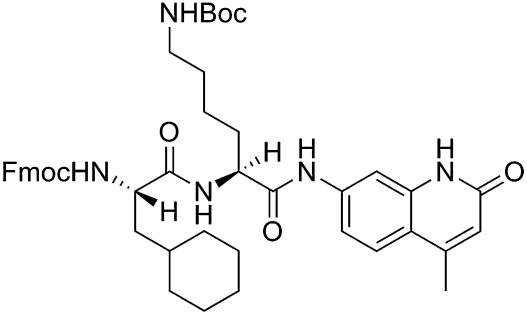
The chemical name of CYM2503 is (9H-fluoren-9-yl)methyl((S)-1(((S)-6(tert-butoxycarbonyl)amino-1-((4-methyl-2-oxo-1,2-dihydroquinolin-7-yl)amino)-1-oxohexan-2-yl)amino])-3-cyclohexyl-1-oxopropan-2-yl)carbamate (Fig. 1). The synthesis and structure–activity relationship will be published in due course.
Fig. 1.
Structure of CYM2503.
CYM2503 Potentiated GalR2 Signaling.
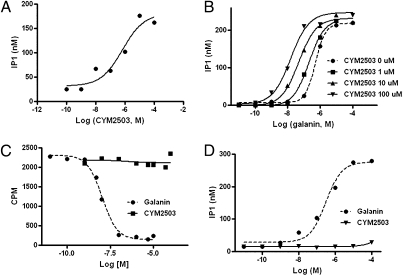
CYM2503 was first identified from our screen of a chemical library for modulators of the GalR2 receptor. The endogenous ligand galanin stimulated IP1 production in HEK293-GalR2 cells was used as a functional index of GalR2 receptor activation. In the absence of CYM2503, galanin stimulated IP1 accumulation in HEK293-GalR2 cells with an EC50 of 0.3 ± 0.6 μM (mean ± SEM; n = 10). The initial screen was carried out in the presence of 100 nM galanin, CYM2503 was found to concentration-dependently potentiate the effect of 100 nM galanin with an EC50 of 0.69 ± 2.7 μM (n = 2) and an efficacy of 9.7 ± 3.2-fold potentiation (Fig. 2A).
Fig. 2.
CYM2503 mediated potentiation of the agonist-stimulated GalR2 activation and the lack of affinity of CYM2503 for GalR2 orthosteric site in HEK293-GalR2 cells. IP1 accumulation was used as an index of GalR2 activation. (A) CYM2503 potentiated the effect of 100 nM galanin. (B) CYM2503 shifted the galanin dose–response curve to the left. (C) Iodine-125 porcine galanin, at 0.15 nM, was not displaced by CYM2503 at up to 100 μM concentration. For comparison, unlabeled rat galanin displaced 125I porcine galanin with an IC50 of 10.1 nM in the same experiment. (D) Lack of effect of CYM2503 on IP1 accumulation. CYM2503 at concentrations up to 100 μM did not change baseline IP1 level. As a comparison, rat galanin stimulated IP1 production in the same experiment. All data shown were from one experiment performed in triplicate and are typical of two to six independent experiments that yielded similar results.
We then tested the effect of CYM2503 on the galanin concentration–response curve. As shown in Fig. 2B, CYM2503 shifted the galanin concentration–response curve to the left. The EC50 was shifted by 50.4 ± 17.8-fold (mean ± SEM; n = 4), with 100 μM CYM2503. Smaller shifts of 12.5 ± 3.2-fold and 3.4 ± 0.7-fold were seen with 10 μM and 1 μM CYM2503, respectively. CYM2503 also increased the maximal galanin response by as much as 25 ± 9%.
In HEK293-GalR2 cells, CYM2503, by itself, did not stimulate IP1 production in the absence of galanin (Fig. 2D). In 125I-galanin competitive binding assay to membranes prepared from HEK293-GalR2 cells, CYM2503 at concentrations as high as 100 μM failed to displace 125I-galanin from its binding sites on GalR2 (Fig. 2C). The lack of affinity of CYM2503 at the orthosteric site of the GalR2 as well as the absence of baseline agonist-like activity suggests CYM2503 might be a putative GalR2 allosteric modulator with no intrinsic activity.
CYM2503 Had No Effect on GalR1 Signaling.
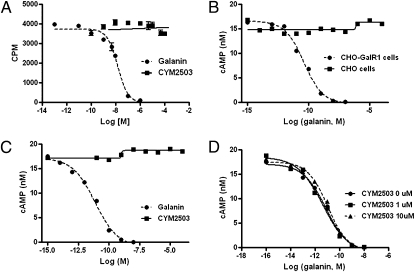
CYM2503 exhibited no affinity for the galanin binding site on GalR1 receptor (Fig. 3A) at up to 100 μM. A FRET-based cAMP assay was then used to test if CYM2503 modulates GalR1 signaling. Inhibition of forskolin-stimulated cAMP accumulation in CHO-GalR1 cells by the endogenous ligand galanin was used as a functional index of GalR1 receptor activation. In this assay, galanin suppressed forskolin-stimulated cAMP accumulation with an IC50 of 7.3 ± 6.8 pM (Fig. 3; mean ± SEM; n = 10), which is approximately two orders of magnitude lower than the observed affinity for GalR1 (Kd = 0.23 ± 0.45 nM). To ensure that the observed inhibitory effect of galanin on cAMP is indeed mediated by the GalR1 receptor, we compared the galanin response in CHO-GalR1 cells to that in the master CHO cell line, which was used to construct the CHO-GalR1 stable cell line. As shown in Fig. 3B, galanin had no effect on forskolin-stimulated cAMP production in CHO cells that are lacking the rat GalR1 transgene, confirming that the cAMP response to galanin in CHO-GalR1 cells is indeed mediated by GalR1 receptor. CYM2503 did not shift the galanin dose–response curve in cAMP assay (Fig. 3D), and it, by itself, had no effect on forskolin-stimulated cAMP production in CHO-GalR1 cells (Fig. 3C).
Fig. 3.
CYM2503 exhibited no affinity for GalR1 and it did not modulate GalR1 signaling. Agonist-induced inhibition of forskolin-stimulated cAMP production in CHO-GalR1 cells was used as an index of GalR1 activation. Forskolin was used at a concentration of 4 μM. (A) Iodine-125 porcine galanin, at 0.1 nM, was not displaced by CYM2503 at up to 100 μM concentration. For comparison, unlabeled rat galanin displaced 125I porcine galanin with an IC50 of 5.4 nM in the same experiment. (B) Rat galanin inhibited forskolin-stimulated cAMP accumulation with an IC50 of 7.8 pM in CHO-GalR1 cells, whereas it had no effect in the parent CHO cell line that does not express GalR1. (C) Lack of effect of CYM2503 on cAMP accumulation. CYM2503 at concentrations up to 100 μM had no effect on forskolin-stimulated cAMP production. (D) CYM2503 did not shift the galanin dose–response curve. The data shown here were from one experiment performed in triplicate and are typical of three or four independent experiments that yielded similar results.
Anticonvulsant Effect of CYM2503 in Mice.
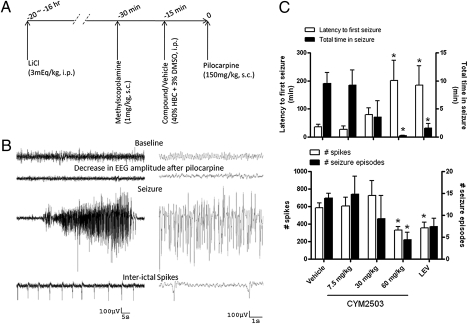
Pilocarpine at the dose of 150 mg/kg s.c. induced brief episodes of electrographic seizures with an average duration of 0.7 ± 0.4 min (mean ± SEM; n = 32) in LiCl (3 mEq/kg, i.p. 16–20 h before pilocarpine) and methylscopolamine (1 mg/kg, s.c., 30 min before pilocarpine) pretreated mice. Seizure started approximately 30 min after pilocarpine injection and, by 8 h, all mice were seizure-free. Only 10% of mice developed SE (3 of 30) at this pilocarpine dose. All EEG tracings were analyzed up to 8 h.
Pretreatment with CYM2503 (15 min before pilocarpine) attenuated Li-pilocarpine–induced seizures in mice (Fig. 4). The effects were significant at 60 mg/kg i.p. (Fig. 4C): the latency to first electrographic seizure was prolonged by approximately fivefold, from 36.7 ± 8.0 min in vehicle group to 180.7 ± 76.2 min (P < 0.05; mean ± SEM; n = 9); total time in seizures decreased from 9.5 ± 1.9 min in vehicle group to 0.2 ± 0.06 min (P < 0.05; n = 9); the total number of spikes decreased by 50%, from 614.4 ± 52.52 in vehicle group to 341.3 ± 34.2 (P < 0.05; n = 9); total number of seizure episodes decreased from 13.9 ± 1.1 in vehicle group to 4.4 ± 1.7 (P < 0.05; n = 9). Overall, the effects of CYM2503 (60 mg/kg, i.p.) were comparable to those of levetiracetam (50 mg/kg i.p.) in this seizure model.
Fig. 4.
Anticonvulsant effect of CYM2503 in the Li-pilocarpine model in mice. (A) Experimental paradigm. (B) Examples of electrographic seizure activity and spikes induced by Li-pilocarpine. (C) Statistic analysis of EEG. Data are mean ± SEM. (*P < 0.05, ANOVA followed by Dunnett multiple comparison with respective controls, n = 6–10.) LEV, levetiracetam 50 mg/kg i.p.
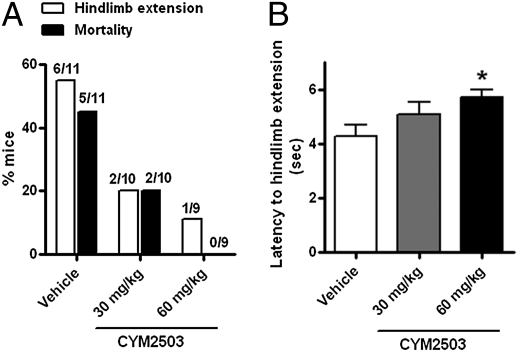
The electroshock seizure model is frequently used to predict anticonvulsant drug efficacy against generalized tonic-clonic seizures (35). Under the test conditions, 55% of mice in the vehicle-treated group (6 of 11) developed hindlimb extension and the mortality rate was approximately 45% (5 of 11; Fig. 5A). A 15-min pretreatment with CYM2503 at concentrations of 30 mg/kg i.p. and 60 mg/kg i.p. decreased the percentage of mice exhibited hindlimb extension from 55% (6 of 11) to 20% (2 of 10); and decreased the mortality rate from 45% (5 of 11) to 0% (0 of 9; Fig. 5A). The latency to hindlimb extension was prolonged by pretreatment with 60 mg/kg CYM2503, from 4.2 ± 0.42 s in vehicle group to 5.7 ± 0.27 s in CYM2503-treated group (Fig. 5B; P < 0.05; n = 9–11).
Fig. 5.
CYM2503 protected mice in the electroshock induced seizure model. A 15-min pretreatment with 60 mg/kg i.p. (A) decreased the percent of mice that developed hindlimb extension and decreased the mortality. The number of animals in each group is shown above the bar graph. (B) Latency to hindlimb extension was also increased. Data are mean ± SEM. (*P < 0.05, one-way ANOVA followed by Dunnett multiple comparison with vehicle, n = 9–11.)
Anticonvulsant Effect of CYM2503 in Li-Pilocarpine SE Model in Rats.
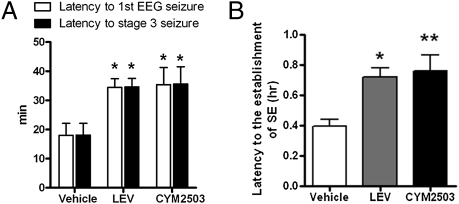
An i.p. injection of CYM2503 (60 mg/kg), similarly to levetiracetam (50 mg/kg i.p.), increased the latency to first electrographic seizure by twofold (Fig. 6A), from 18.0 ± 4.1 min in the vehicle group to 34.4 ± 3.0 min (mean ± SEM; P < 0.05; n = 5); increased the latency to stage 3 seizure by twofold (Fig. 6A), from 18.1 ± 4.1 min in vehicle group to 35.6 ± 5.9 min (P < 0.05); and increased the latency to the establishment of SE twofold (Fig. 6B), from 0.40 ± 0.05 h in vehicle group to 0.72 ± 0.06 h (P < 0.01). In the vehicle-treated group, 100% of rats (5 of 5) developed SE, and in the CYM2503-treated group, 80% (4 of 5) developed SE (Table 1). There appeared to be a great survival benefit of CYM2503, as the 24-h mortality rate was 0% (0 of 5) in CYM2503-treated rats compared with 100% (5 of 5) in vehicle-treated rats.
Fig. 6.
Anticonvulsant effect of CYM2503 in the Li-pilocarpine model in rats. (A) Latency to first EEG seizure and latency to first stage 3 or higher behavioral seizure. (B) Latency to the establishment of SE. Data are mean ± SEM. (*P < 0.05 and **P < 0.01, ANOVA followed by Dunnett multiple comparison vs. respective controls, n = 4–5.) LEV, levetiracetam 50 mg/kg i.p. CYM2503 dose was 60 mg/kg i.p.
Table 1.
Effects of CYM2503 on the establishment of SE and on mortality in rat Li-pilocarpine SE model
| Group | i.p. Dose, mg/kg | Rats tested | Rats with SE | Mortality |
|
| 4 h | 24 h | ||||
| Vehicle | – | 5 | 5 (100%) | 2 (40%) | 5 (100%) |
| Levetiracetam | 50 | 4 | 4 (100%) | 1 (25%) | 4 (100%) |
| CYM2503 | 60 | 5 | 4 (80%) | 0 | 0 |
Discussion
In the present study, we showed that CYM2503 potentiated galanin response at GalR2 receptor by increasing the potency of galanin, as well as maximal response in HEK293 cells stably expressing GalR2 receptors. The lack of the affinity of CYM2503 for orthosteric site of GalR2 and the lack of effect of CYM2503 on baseline IP turnover suggest CYM2503 is a positive allosteric modulator of GalR2 without intrinsic activity. The specificity of the effect of CYM2503 was evidenced by its lack of effect on galanin response at GalR1 receptor and its lack of GalR1-like signaling and affinity for GalR1 in CHO cells stably expressing GalR1 receptors. Furthermore, we showed that CYM2503 was systemically active in attenuating seizures in Li-pilocarpine model in mice and rats, and in electroshock-induced seizures in mice.
GalR2 signaling represents a potent anticonvulsant and antiepileptic mechanism. In addition to attenuating acute seizures (25), intrahippocampal infusion of the GalR2 preferring ligand galanin (2–11) was recently shown to completely prevent the occurrence of full kindled seizures (36). It is known that GalR2 is expressed at the highest level in the dentate gyrus of hippocampus and it has been shown that heightened neuronal activity and seizure trigger the release of galanin into hippocampus from projections originating from septal basal forebrain complex, locus coeruleus, and hypothalamus (18, 26, 37–39). At the same time, seizure activity induces de novo galanin expression in the entire hippocampus, as well as in the interneurons of the dentate gyrus (22, 39). Thus, the presence of intact GalR2 signaling during ictal and periictal period lends a theoretic support for developing GalR2-positive allosteric modulators to treat seizures. In addition to an anticonvulsant effect, enhanced GalR2 signaling by positive allosteric modulators such as CYM2503 might provide the added benefit of neuroprotection during seizures (25).
The Li-pilocarpine model is a widely used animal model for temporal lobe seizure/epilepsy. In rats, it is a very robust model for SE (39, 40). Our data showed that, although CYM2503 did not seem to block the establishment of SE, it increased the latency to seizure and to SE (Fig. 6). More importantly, it appeared to decrease the mortality dramatically (Table 1). Centrally or peripherally mediated cardiovascular and respiratory failure is a significant cause of death in Li-pilocarpine–induced SE. As GalR2 is expressed in the heart (41) and galanin has been shown to regulate cardiac function via peripheral mechanisms (42, 43), it would be of interest to explore the direct effect of CYM2503 and/or GalR2 agonist on parameters of cardiac function in the future.
The Li-pilocarpine model in mice has also been used as a model for SE by several researchers (35), but great variations have been observed with this model, perhaps because of its high sensitivity to strain differences and to subtle changes in experimental condition (35). In our hands, 150 mg/kg pilocarpine in mice pretreated with LiCl and methylscopolamine induced single seizures rather than SE; only three of 30 mice developed SE and the mortality was also low at 3.3%. When the pilocarpine dose was increased to 200 mg/kg, still, only a small percentage of mice developed SE (n = 2 of 8), but mortality rate increased to 42% (n = 3 of 8) and most animals died within the first 6 h, confounding the data analysis. We therefore used a Li-pilocarpine (pilocarpine 150 mg/kg s.c.) seizure model to test the anticonvulsant efficacy of the CYM2503 in mice and found that CYM2503 at 60 mg/kg was comparable to levetiracetam (50 mg/kg) in increasing latency to first electrographic seizure and decreasing total time in seizure. The absence of SE following 150 mg/kg pilocarpine injection is in contrast to a previous report by Mazarati et al. and our group (24), but appears to be in line with a report by Muller et al. (35), which showed that none of five mice developed behavioral SE after 150 mg/kg and 200 mg/kg pilocarpine in the setting of pretreatment with LiCl. It is worth noting that the study of Muller et al. (35) used NMRI mice, the study of Mazarati et al. (24) was carried out in a KO strain on C57BL/6 background, and the present study was performed with C57BL/6 mice obtained from the Scripps Research Institute local colony.
The pharmacological potential of GalR1 and GalR2 agonists has been well recognized during the past 20 years but there have been great difficulties in finding receptor subtype–selective, systemically active, nonpeptide-type galanin receptor ligands. Two systemically active nonpeptide galanin receptor agonists, Galnon and Galmic, from the Langel and Rebek groups (17, 29), became important pharmacological tools for studying the pharmacology of galanin signaling and represented prototypic compounds for design of improved nonpeptide ligands. Peptide analogues shorter than the endogenous galanin with receptor subtype selectivity were synthesized and characterized (36, 44, 45). Efforts were also made to improve the CNS bioavailability of galanin peptide. Cationization and lipidization of galanin (1–13) provided two galanin analogues, Gal-B2 and [B-Me, des-Sar]Gal-B2, which are metabolically stable and CNS-penetrating. Both analogues are highly active in 6-Hz model of pharmacoresistant seizures upon systemic administration (30–33). In addition, Gal-B2 is active in the Frings audiogenic seizure-susceptible mouse and in the corneal kindling model of partial epilepsy. Now, we show that CYM2503, a nonpeptide-type, positive allosteric modulator of GalR2, exhibited potent anticonvulsant activity upon systemic administration in acute seizure models in both rats and mice. This opens up a development strategy for anticonvulsant therapy targeting GalR2. However, CYM2503 did not completely block all acute seizures in the models tested, and before it is demonstrated to block spontaneous seizures in chronic models, one cannot make predictions about the potential of this compound as an antiepileptic agent.
Development of allosteric modulators for ionotropic and G protein receptor–coupled receptors has enjoyed great success in recent years. Compared with a full agonist, an allosteric modulator might be less susceptible to desensitization mechanism and it may be associated with higher receptor subtype selectivity. Because an allosteric modulator is effective only when the endogenous agonist is also present, it is anticipated to be associated with a more benign side effect profile than a tonically active exogenous agonist. CYM2503 represents a starting point for developing positive allosteric modulator of GalR2.
Materials and Methods
Animals.
All mice used in the experiments were 8-week-old male C57BL/6 mice obtained from The Scripps Research Institute. Male Wistar weighing 240 to 260 g were obtained from Simonsen Laboratories. The animals were housed with a 12-h light/dark cycle with ad libitum access to water and food. Animal care, maintenance, and experimental procedures were according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Materials.
All cell culture supplies were from Invitrogen. LiCl and pilocarpine HCl were from Sigma-Aldrich. Methylscopolamine was from Pfaltz and Bauer. 2-Hydroxypropyl-β-cyclodextrin (HBC) was purchased from Acros Organics. The 125I porcine galanin and rat galanin were from PerkinElmer and Biopeptide, respectively.
Cell Lines.
The cDNA encoding rat GalR1 and rat GalR2 receptor was generated by RT-PCR with total RNA prepared from rat hypothalamus and cloned into a TA vector (Invitrogen). The entire coding sequence of each receptor was subcloned into pCMV vector (Clontech) and verified by sequencing. After transfecting CHO cells and HEK293 cells with the pCMV vectors containing rat GalR1 and rat GalR2 receptor coding sequences, respectively, cell lines were selected by the antibiotics G418, followed by limited dilution to obtain single clones. Ten clones from each cell line were screened with 125I porcine galanin binding (125I porcine galanin 0.1 nM; 5 μM cold rat galanin as competitor) and clones with highest binding (one for each cell type) were expanded and used in subsequent experiments. The 125I galanin saturation binding assays were performed on the CHO-GalR1 and HEK293-GalR2 cell lines selected by the screening. Nonlinear regression analysis of specific binding data indicated a Bmax of 1411 ± 267 fmol/mg membrane protein and a Kd value of 0.23 ± 0.45 nM in the CHO-GalR1 cells; a Bmax of 497 ± 60 fmol/mg membrane protein and a Kd of 2.0 ± 1.2 nM in the HEK293-GalR2 cells (mean ± SEM; n = 3).
Radioligand Binding Assay.
Ligand competition binding of 125I porcine galanin to the membrane preparations (29) was performed in a volume of 150 μL in a 96-well plate. Cell membranes were diluted in Hepes buffer containing 25 mM Hepes, pH 7.4, 10 mM MgCl2, 1 mM CaCl2, 0.5% BSA, and 1× protease inhibitor. The 125I porcine galanin was diluted in Tris buffer (50 mM Tris-HCl, pH 7.4, 14 mM MgCl2, 2.45% BSA). Compounds and rat galanin were diluted in 20% DMSO. Eighty microliters cell membrane (2.5 μg for GalR1 and 5 μg for GalR2 binding), 55 μL 125I porcine galanin, and 15 μL compound/galanin preparations were combined and incubations were carried out at room temperature for 1 h. The 125I porcine galanin was used at 0.1 nM and 0.15 nM for GalR1 and GalR2 binding, respectively. The reactions were terminated by rapid vacuum filtration through glass fiber filters (Packard Bioscience), which had been pretreated with 0.3% polyethylenimine. After three washes with cold PBS solution (pH 7.4) containing 0.01% (vol/vol) Triton X-100, the filter was counted with Cobra II auto-γ-counting systems (Packard Bioscience). All data were analyzed by nonlinear regression (Prism; GraphPad).
cAMP Assay and IP1 Assay.
cAMP and IP1 levels were measured with a cAMP dynamic 2 kit and a IP-One Tb kit (both from Cisbio), respectively, following the manufacturer’s instructions. Assays were performed in 384-well plates and 2,500 cells/well and 7,500 cells/well were used in cAMP and IP1 assays, respectively. The signal was quantified with Tecan Infinite F500. Assays were performed in triplicates and the results were analyzed by nonlinear regression (GraphPad).
Li-Pilocarpine Seizure Experiment in Mice.
Under isoflurane anesthesia, mice were surgically implanted with recording electrodes according to stereotaxic guidance as previously described in detail (46, 47). Electrodes were fabricated with Teflon-insulated, 0.25-mm outside diameter wire (Plastics One). Two small holes were drilled on the skull over right and left parietal lobes for implantation of recording electrode and reference electrode, respectively. The biopotential lead wires were attached to small stainless steel screws (no. 80 × 0.125 in; Small Parts). The electrodes were then connected to a multipin socket and secured to the skull by acrylic dental cement. Mice were allowed to recover for 3 to 5 d before the experiment.
Mice were injected with LiCl (3 mEq/kg, i.p.), followed 16 to 20 h later with pilocarpine HCl (150 mg/kg, s.c.) to induce seizure. Methylscopolamine (1 mg/kg, s.c.) was injected 30 min before pilocarpine to protect mice from peripheral cholinergic effects. Vehicle or compound (vehicle was 40% HBC + 2% DMSO, i.p.) was injected 15 min before pilocarpine. Reference drug was levetiracetam (50 mg/kg). Levetiracetam is a newer-generation anticonvulsant/antiepileptic drug with a unique mechanism of action and with fewer side effects compared with the older generations of anticonvulsants. Similarly to the GalR1 and GalR2 signaling–mediated anticonvulsant action, levetiracetam might exert its anticonvulsant effects by inhibiting the release of excitatory neurotransmitters (48). Furthermore, levetiracetam was previously shown to suppress Li-pilocarpine–induced seizure in mice and rats (48, 49), thus it is a suitable comparator in this seizure model. EEG was recorded with Biopac recording system (Biopac Systems) with a sampling frequency of 200 Hz and a filter setting of 0.5 Hz high pass and 100 Hz low pass. Seizures were defined as clusters of spikes with the frequency of 3 Hz or more and duration of 3 s or longer.
Mouse Electroshock Seizure Model.
Mice were administered vehicle (40% HBS + 2% DMSO) or CYM2503 i.p. 15 min before electroshock. The electroshock was delivered through auricular electrodes [(unidirectional rectangular pulse 0.5 ms, 100 pulses/s, 3 s, 35 mA; Pulse Generator 57800–001 (UGO Basile)] while holding the mouse in supine position in a researcher’s hand. Full tonic extension of both hind limbs was taken as the endpoint. The experiment was performed between 2 and 5 PM. All mice were videotaped and the latency to hindlimb extension was scored by a person who was blind to the treatment. If extension did not occur within 6 s, a value of 6 s was recorded (50).
Li-Pilocarpine Seizures in Rats.
Male Wistar rats weighing 240 to 260 g were implanted under isoflurane anesthesia with stainless steel skull screws to serve as epidural EEG electrodes. A ground screw was placed in the rostrum and two others, one on each side, were placed approximately 1 mm anterior to λ and 1 mm from the midline. A tripolar electrode was connected to skull screws and anchored with dental cement. After surgery, animals were placed in an observation chamber on a temperature pad until they recovered. Animals were allowed to recover for 7 to 10 d and were then given 3 mEq/kg LiCl s.c. 16 h before initiating of seizures. For recordings, which began 15 min before pilocarpine injection, EEG electrodes were connected to tethered cables with swivel mounts (Plastics One), which fed the amplified signal to a monitoring and recording system. Seizures were initiated by the s.c. injection of 60 mg/kg pilocarpine and 1 mg/kg methylscopolamine. Test compounds and vehicle were injected i.p. immediately before pilocarpine. Vehicle consisted of 2.5% DMSO in 40% HBC. Levetiracetam was used at 50 mg/kg. EEG was continuously recorded for 18 to 24 h using a MP100 workstation (Biopac Systems). Seizures were defined as a discharge lasting at least 3 s, with a mean frequency higher than 3 Hz, coefficient of variation of at least 65, and amplitude 2.7 times higher than baseline. Scoring of behavioral seizures used a modified Racine scale (51). Other measurements were performed by inspection or by computer analysis of the recordings using Stellate Harmonie software, as previously described (52).
Statistical Analysis.
Seizure data were analyzed by one-way ANOVA followed by Dunnett post hoc comparison of the test compounds to vehicle.
Acknowledgments
We thank Viktor Zhukov for technical assistance. The study was supported by National Institute of Health Grants NS063560 and R01MH074055 (to T.B.). The present study is Scripps Research Institute Manuscript 20798.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 14943.
References
- 1.Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.Melander T, Hökfelt T, Rökaeus A. Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol. 1986;248:475–517. doi: 10.1002/cne.902480404. [DOI] [PubMed] [Google Scholar]
- 3.Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7:609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 4.Vrontakis ME, Peden LM, Duckworth ML, Friesen HG. Isolation and characterization of a complementary DNA (galanin) clone from estrogen-induced pituitary tumor messenger RNA. J Biol Chem. 1987;262:16755–16758. [PubMed] [Google Scholar]
- 5.Counts SE, Perez SE, Mufson EJ. Galanin in Alzheimer’s disease: Neuroinhibitory or neuroprotective? Cell Mol Life Sci. 2008;65:1842–1853. doi: 10.1007/s00018-008-8159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawley JN. Galanin impairs cognitive abilities in rodents: Relevance to Alzheimer’s disease. Cell Mol Life Sci. 2008;65:1836–1841. doi: 10.1007/s00018-008-8158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes A, Picciotto MR. Galanin: A novel therapeutic target for depression, anxiety disorders and drug addiction? CNS Neurol Disord Drug Targets. 2006;5:225–232. doi: 10.2174/187152706776359600. [DOI] [PubMed] [Google Scholar]
- 8.Kalra SP, Horvath TL. Neuroendocrine interactions between galanin, opioids, and neuropeptide Y in the control of reproduction and appetite. Ann N Y Acad Sci. 1998;863:236–240. doi: 10.1111/j.1749-6632.1998.tb10698.x. [DOI] [PubMed] [Google Scholar]
- 9.Leibowitz SF. Regulation and effects of hypothalamic galanin: Relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis. Neuropeptides. 2005;39:327–332. doi: 10.1016/j.npep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Liu HX, Hökfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol Sci. 2002;23:468–474. doi: 10.1016/s0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Sharkey L, Bartfai T. The brain galanin receptors: Targets for novel antidepressant drugs. CNS Neurol Disord Drug Targets. 2007;6:183–192. doi: 10.2174/187152707780619335. [DOI] [PubMed] [Google Scholar]
- 12.Ogren SO, Kuteeva E, Elvander-Tottie E, Hökfelt T. Neuropeptides in learning and memory processes with focus on galanin. Eur J Pharmacol. 2010;626:9–17. doi: 10.1016/j.ejphar.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 13.Picciotto MR, Brabant C, Einstein EB, Kamens HM, Neugebauer NM. Effects of galanin on monoaminergic systems and HPA axis: Potential mechanisms underlying the effects of galanin on addiction- and stress-related behaviors. Brain Res. 2010;1314:206–218. doi: 10.1016/j.brainres.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss JM, Bonsall RW, Demetrikopoulos MK, Emery MS, West CH. Galanin: A significant role in depression? Ann N Y Acad Sci. 1998;863:364–382. doi: 10.1111/j.1749-6632.1998.tb10707.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundström L, Elmquist A, Bartfai T, Langel U. Galanin and its receptors in neurological disorders. Neuromolecular Med. 2005;7:157–180. doi: 10.1385/NMM:7:1-2:157. [DOI] [PubMed] [Google Scholar]
- 16.Bartfai T, Hökfelt T, Langel U. Galanin—a neuroendocrine peptide. Crit Rev Neurobiol. 1993;7:229–274. [PubMed] [Google Scholar]
- 17.Saar K, et al. Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci USA. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazarati AM, et al. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokaia M, et al. Suppressed kindling epileptogenesis in mice with ectopic overexpression of galanin. Proc Natl Acad Sci USA. 2001;98:14006–14011. doi: 10.1073/pnas.231496298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin EJ, et al. Recombinant AAV-mediated expression of galanin in rat hippocampus suppresses seizure development. Eur J Neurosci. 2003;18:2087–2092. doi: 10.1046/j.1460-9568.2003.02926.x. [DOI] [PubMed] [Google Scholar]
- 21.McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of galanin suppresses limbic seizure activity in vivo. Mol Ther. 2006;14:63–68. doi: 10.1016/j.ymthe.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Kanter-Schlifke I, et al. Galanin gene transfer curtails generalized seizures in kindled rats without altering hippocampal synaptic plasticity. Neuroscience. 2007;150:984–992. doi: 10.1016/j.neuroscience.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 23.Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP. Critical role for GALR1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res. 2002;107:195–200. doi: 10.1016/s0169-328x(02)00451-5. [DOI] [PubMed] [Google Scholar]
- 24.Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience. 2004;128:431–441. doi: 10.1016/j.neuroscience.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazarati A, Lu X. Regulation of limbic status epilepticus by hippocampal galanin type 1 and type 2 receptors. Neuropeptides. 2005;39:277–280. doi: 10.1016/j.npep.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 26.O’Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: Distinct distribution from GALR1. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 27.Hohmann JG, et al. Distribution and regulation of galanin receptor 1 messenger RNA in the forebrain of wild type and galanin-transgenic mice. Neuroscience. 2003;117:105–117. doi: 10.1016/s0306-4522(02)00798-4. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- 29.Bartfai T, et al. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. Proc Natl Acad Sci USA. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson CR, et al. Engineering galanin analogues that discriminate between GalR1 and GalR2 receptor subtypes and exhibit anticonvulsant activity following systemic delivery. J Med Chem. 2010;53:1871–1875. doi: 10.1021/jm9018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White HS, et al. Developing novel antiepileptic drugs: Characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics. 2009;6:372–380. doi: 10.1016/j.nurt.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, et al. Structural requirements for a lipoamino acid in modulating the anticonvulsant activities of systemically active galanin analogues. J Med Chem. 2009;52:1310–1316. doi: 10.1021/jm801397w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulaj G, et al. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem. 2008;51:8038–8047. doi: 10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- 34.Lu X, Lundström L, Langel U, Bartfai T. Galanin receptor ligands. Neuropeptides. 2005;39:143–146. doi: 10.1016/j.npep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Müller CJ, Bankstahl M, Gröticke I, Löscher W. Pilocarpine vs. lithium-pilocarpine for induction of status epilepticus in mice: Development of spontaneous seizures, behavioral alterations and neuronal damage. Eur J Pharmacol. 2009;619:15–24. doi: 10.1016/j.ejphar.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Mazarati A, et al. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- 37.Melander T, Staines WA, Rökaeus A. Galanin-like immunoreactivity in hippocampal afferents in the rat, with special reference to cholinergic and noradrenergic inputs. Neuroscience. 1986;19:223–240. doi: 10.1016/0306-4522(86)90017-5. [DOI] [PubMed] [Google Scholar]
- 38.Hökfelt T, Xu ZQ, Shi TJ, Holmberg K, Zhang X. Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann N Y Acad Sci. 1998;863:252–263. doi: 10.1111/j.1749-6632.1998.tb10700.x. [DOI] [PubMed] [Google Scholar]
- 39.Mazarati AM, et al. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia. 2008;49(suppl 9):63–73. doi: 10.1111/j.1528-1167.2008.01928.x. [DOI] [PubMed] [Google Scholar]
- 41.Borowsky B, et al. Cloning and characterization of the human galanin GALR2 receptor. Peptides. 1998;19:1771–1781. doi: 10.1016/s0196-9781(98)00133-8. [DOI] [PubMed] [Google Scholar]
- 42.Ewert TJ, Gritman KR, Bader M, Habecker BA. Post-infarct cardiac sympathetic hyperactivity regulates galanin expression. Neurosci Lett. 2008;436:163–166. doi: 10.1016/j.neulet.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons RL, Merriam LA. Galanin and bethanechol appear to activate the same inwardly rectifying potassium current in mudpuppy parasympathetic neurons. Neurosci Lett. 1992;140:33–36. doi: 10.1016/0304-3940(92)90675-w. [DOI] [PubMed] [Google Scholar]
- 44.Runesson J, Saar I, Lundström L, Järv J, Langel U. A novel GalR2-specific peptide agonist. Neuropeptides. 2009;43:187–192. doi: 10.1016/j.npep.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Liu HX, et al. Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA. 2001;98:9960–9964. doi: 10.1073/pnas.161293598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balosso S, et al. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann Neurol. 2005;57:804–812. doi: 10.1002/ana.20480. [DOI] [PubMed] [Google Scholar]
- 47.El Bahh B, et al. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y and not Y receptors. Eur J Neurosci. 2005;22:1417–1430. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- 48.Klitgaard H, Matagne A, Grimee R, Vanneste-Goemaere J, Margineanu DG. Electrophysiological, neurochemical and regional effects of levetiracetam in the rat pilocarpine model of temporal lobe epilepsy. Seizure. 2003;12:92–100. doi: 10.1016/s1059131102001930. [DOI] [PubMed] [Google Scholar]
- 49.Klitgaard H, Matagne A, Gobert J, Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 50.Wickenden AD, et al. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): A novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol. 2008;73:977–986. doi: 10.1124/mol.107.043216. [DOI] [PubMed] [Google Scholar]
- 51.Haas KZ, Sperber EF, Moshé SL. Kindling in developing animals: Expression of severe seizures and enhanced development of bilateral foci. Brain Res Dev Brain Res. 1990;56:275–280. doi: 10.1016/0165-3806(90)90093-e. [DOI] [PubMed] [Google Scholar]
- 52.Suchomelova L, et al. Treatment of experimental status epilepticus in immature rats: Dissociation between anticonvulsant and antiepileptogenic effects. Pediatr Res. 2006;59:237–243. doi: 10.1203/01.pdr.0000196333.16608.30. [DOI] [PubMed] [Google Scholar]