Abstract
The Fas receptor (also known as CD95 and APO-1) is a member of the tumor necrosis factor α-family of death receptors that mediate T-cell responses. Here, we show that Fas receptor signaling requires a functional T-cell receptor (TCR) complex. Fas receptor directly binds to and activates TCR components in a stimulus-dependent manner. Fas receptor stimulation does not activate canonical downstream TCR pathways, but instead the TCR complex is required specifically for Fas-mediated calcium release. Importantly, null mutations in Lck, ZAP70, and the TCR α- and β-chains abrogate Fas signaling. Our results reveal a direct role for the TCR complex in mediating Fas-specific signaling events critical for T-cell homeostasis.
Keywords: apoptosis, calcium, Lck
One of the key aspects of Fas-dependent regulation of immune system homeostasis is the control of effector T-cell population in a process known as activation-induced cell death (AICD). This process serves to remove reactive T cells, preventing uncontrollable clonal expansion that would be deleterious for the host upon successful elimination of invading antigen (1). The importance of Fas receptor signaling in mediating AICD has been supported by mouse model,s as well as studies of human autoimmune lymphoproliferative syndrome (ALPS) patients, in which mutations of Fas receptor or its ligand cause ALPS and an increased risk of lymphoma (2–5).
AICD is initiated by repeated T-cell receptor (TCR) stimulation of previously activated T cells. TCR ligation in the absence of proper costimulation leads to sensitization of T cells toward the Fas-mediated apoptosis and rapid cell death upon engagement of Fas ligand (6). Importantly, TCR stimulation does not trigger immediate cell death; most antigen-specific T cells develop vulnerability to Fas-mediated apoptosis at least 48 to 72 h after TCR stimulation (7). The mechanisms underlying increased sensitivity of activated T cells to apoptosis are not fully understood; however, it might in part be explained by up-regulation of Fas receptor/ligand surface expression, reaching a maximum several days after TCR stimulation (8). Therefore, current models restrict the role of TCR in regulation of Fas-mediated apoptosis to a transcriptional regulation of T-cell sensitivity to the Fas-mediated cell death.
Our previous study demonstrated that Fas-induced death in T cells is strongly dependent on activation of phospholipase C-γ1 (PLC-γ1) (9). As PLC-γ1 activation is a key upstream event in TCR signaling, these observations suggest that activation of Fas and TCR pathways might not be triggered independently in different phases of AICD, but rather directly collaborate during initiation of Fas-mediated apoptosis.
Here, we investigated whether components of the TCR signaling cascade are involved in modulation of Fas-mediated cell death in Jurkat T-lymphoma cells. We found that Fas-mediated calcium release requires Lck, ZAP70, and the TCR β-chain, all canonical components of the TCR complex. Furthermore, we found that a lymphoma cell line with rearrangements at the TRA@ locus are completely deficient in Fas-mediated calcium release and cell death despite robust Fas-receptor expression levels. Our results suggest that the unengaged TCR complex directly controls the initiation of Fas-mediated signaling, providing a unique mechanism of T-cell resistance toward apoptosis and subsequent susceptibility to lymphoma.
Results
Lck Mediates Activation of the Fas Signaling Pathway.
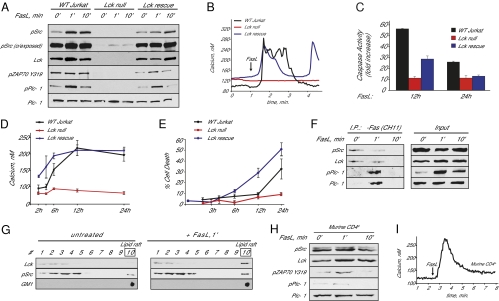
The most proximal event of TCR signal transduction is the activation of Src-family tyrosine kinases. T cells express two Src kinases: Lck and Fyn. TCR signaling is predominantly mediated by Lck activity (10). Thus, to examine the contribution of Src-family kinases to Fas-mediated cell death in Jurkat cells, we first determined whether Lck-null Jurkat cells were competent to activate PLC-γ1 in response to Fas receptor stimulation with Fas ligand. PLC-γ1 activation is mediated by phosphorylation of tyrosine residue 783 (Y783). In wild-type Jurkat cells, Fas ligand stimulation induced robust phosphorylation of PLC-γ1 on Y783, whereas phosphorylation of PLC-γ1 in Lck-null cells was abolished (Fig. 1A). Lck recruits and phosphorylates the Syk-family kinase ZAP70, which in turn phosphorylates T cell-specific adapters, ultimately resulting in the activation of PLC-γ1 (11). We found that in wild-type cells, ZAP70 was phosphorylated at Y319 in a Fas ligand-dependent manner, but in Lck-null cells, ZAP70 phosphorylation was eliminated (Fig. 1A). Weak Fyn activation after Fas ligand stimulation was evident in Lck-null cells (see Fig. 1A, pSrc overexposed). However, our results show that the presence of active Fyn was not sufficient to support PLC-γ1 and ZAP70 activation in response to Fas ligand stimulation. Importantly, stable expression of the Lck gene in Lck-null cells rescued all of these phenotypes (Fig. 1A).
Fig. 1.
Lck is required for Fas ligand-mediated calcium release and cell death in Jurkat cells. (A) PLC-γ1, Lck/Fyn (pSrc), and ZAP70 activation in wild-type and Lck-null Jurkat cells after Fas ligand (FasL) vesicle stimulation as determined by phospho-specific antibodies. (B) Fas-dependent calcium release in wild-type, Lck-null, and Lck-null Jurkat cells stably expressing Lck (Lck rescue). Shown are single-cell responses representative of hundreds of determinations. (C) Caspase 3 activity in wild-type, Lck-null, and Lck rescue Jurkat cells 12 and 24 h after Fas ligand stimulation, relative to untreated cells. (D) Time-dependent increases in cytosolic calcium concentration in wild-type, Lck-null, and Lck rescued cells. (E) Cell death (propidium iodide–positive cells as a percentage of the total) in wild-type, Lck-null, and Lck rescued Jurkat cells. (F) Coimmunoprecipitation of PLC-γ1 and Lck with monoclonal anti-Fas receptor antibody CH11. Total lysates (1/5 load) are depicted as input. (G) Sucrose step gradient centrifugation of lysates prepared from Jurkat cells. The lipid raft fractions (9, 10) were confirmed by dot blotting with the GM1 ganglioside binding-protein cholera toxin-B subunit. (H) PLC-γ1, Lck/Fyn, and ZAP70 activation in primary murine CD4+ cells after Fas ligand stimulation. (I) Fas-dependent calcium release in primary murine CD4+ cells. Approximately 20% of primary CD4+ cells responded to Fas ligand stimulation. This variability in response to Fas ligand stimulation is likely the result of multiple subsets of CD4+ cells isolated (e.g., naive, memory, and effector cells).
Fas signaling is associated with a biphasic increase in cytoplasmic calcium concentration, with an initial fast phase characterized by oscillatory increases in cytoplasmic calcium mediated by PLC-γ1 activation (9). We next determined whether the absence of Lck affected Fas-mediated calcium release. We found that Lck-null Jurkat cells do not release calcium upon Fas ligand stimulation (Fig. 1B). Stable expression of wild-type Lck rescued the calcium-release phenotype.
Loss of Lck Impairs Fas-Dependent Apoptosis.
The second phase of Fas-mediated calcium signaling develops over the course of hours and is associated with persistent opening of IP3R channels, permeabilization of mitochondria, effector caspase activation, and ultimately cell death (9). To determine whether Lck is essential for mediating this second phase of Fas signaling, we monitored caspase 3 activation, late elevations in cytoplasmic calcium, and cell death in response to Fas ligand. We found that Lck-null cells were deficient in caspase-3 activation (Fig. 1C). Furthermore, late increases in cytoplasmic calcium and cell death were eliminated in Lck-null cells (Fig. 1 D and E). The calcium and cell-death phenotypes were fully rescued by stable expression of Lck (Fig. 1 D and E), and caspases activity was partially rescued by Lck expression (Fig. 1C). Partial rescue by stable Lck expression has been noted by other groups (12–14), and may be related to differences in expression level or the polyclonal nature of the cell population (15). Regardless, these results indicate that Lck is essential for Fas-mediated apoptotic calcium release, caspase activation, and cell death.
Lck and PLC-γ1 Are Recruited by Fas Receptor Complex.
Fas signaling initiates with the formation of a large multiprotein complex known as the death-induced signaling complex (DISC) (16). To test whether members of the TCR signaling pathway found here to be required for Fas signaling are directly associated with the DISC, we immunopurified the DISC using a monoclonal antibody against the Fas receptor (CH11). Both Lck and PLC-γ1 copurified with the DISC in a stimulus-dependent manner (Fig. 1F). PLC-γ1 interaction with the Fas receptor was transient, and reflected its activation pattern in response to Fas receptor stimulation. As shown by others, Lck was found to be preassociated with the Fas signaling complex in untreated cells (17). Interestingly, Fas ligand stimulation rapidly decreased the amount of total and activated Lck copurifying with the Fas receptor (Fig. 1F). These experiments were performed with lysates that used Triton-X100 as a detergent, but Lck is known to be distributed into detergent-resistant membrane microdomains enriched in cholesterol and glycosphingolipid, which are referred to as “lipid rafts” (18). Therefore, we reasoned that Lck may be translocating into detergent-insoluble membranes in a stimulus-dependent manner. To address this point, we performed discontinuous sucrose-gradient separation of Brij98 solubilized lysates from unstimulated and stimulated cells. Fractions enriched in lipid rafts were identified by dot blotting with HRP-conjugated cholera toxin B, which recognizes GM1 ganglioside, a specific marker of lipid rafts (19). We found that total Lck distribution within lipid rafts was rapidly increased upon Fas receptor stimulation (Fig. 1G). This observation suggests that the loss of Lck immunoreactivity observed in Fig. 1F may be caused by rapid sequestration of Lck into lipid rafts after Fas stimulation. Similarly, TCR activation also leads to rapid accumulation of Lck into lipid rafts, and this is required for efficient TCR signaling (20).
Fas Ligand Stimulation Activates Components of the TCR Pathway in Primary CD4+ Cells.
We next wanted to confirm that the same pathways activated by Fas ligand stimulation in Jurkat cells are also conserved in primary T cells. Similar to Jurkat cells, Fas ligand stimulation led to rapid and transient activation of PLC-γ1, Lck, and ZAP70 in CD4+ murine T cells (Fig. 1H). We also observed calcium release in response to Fas ligand stimulation (Fig. 1I). Thus, signaling pathways activated by Fas in Jurkat cells are conserved in primary T cells.
Activation of Fas-Mediated Signaling Is Compromised in the Absence of ZAP70.
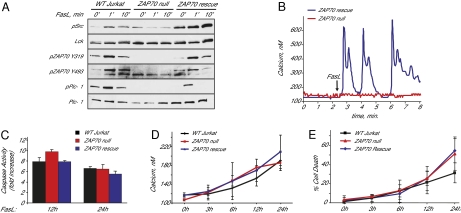
ZAP70 is activated immediately downstream of Lck following TCR stimulation. To determine whether ZAP70 is required for Fas-mediated calcium release and cell death, we used ZAP70-null Jurkat cells (21). As expected, we found that Fas ligand stimulation of ZAP70-null cells did not affect Lck phosphorylation (Fig. 2A). However, PLC-γ1 phosphorylation was abolished in ZAP70-null cells and could be rescued by stable expression of ZAP70 (Fig. 2A). Similarly, calcium release was abolished in ZAP70-null cells, and was restored by stable expression Zap70 (Fig. 2B). These results are consistent with ZAP70 being downstream of Lck after Fas stimulation. However, later stages of Fas-induced apoptosis were not significantly affected in absence of ZAP70, as ZAP70-null cells were similar to wild-type Jurkat cells with regards to caspase activation, late calcium elevations, and cell death (Fig. 2 C–E). Thus, although PLC-γ1 activation and calcium release are eliminated in ZAP70-null cells, alternative pathways mediate the cell-death response in Jurkat cells.
Fig. 2.
ZAP70 is required for Fas ligand-mediated PLC-γ1 activation and calcium release in Jurkat cells. (A) PLC-γ1, Lck/Fyn, and ZAP70 activation in wild-type and ZAP70-null Jurkat cells (B) Fas-dependent calcium release in wild-type, ZAP70-null, and ZAP70 rescued Jurkat cells. Red trace is ZAP70-null, blue trace is ZAP70 rescue. Wild-type Jurkat responses are not shown, but were performed in parallel as a positive control. (C) Caspase 3 activity in wild-type, ZAP70-null, and ZAP70 rescue Jurkat cells 12 and 24 h after Fas ligand stimulation, relative to untreated cells. (D) Time-dependent increases in cytosolic calcium concentration in wild-type, ZAP70-null, and ZAP70 rescue Jurkat cells. (E) Cell death in wild-type, ZAP70-null, and ZAP70 rescue Jurkat cells.
Initiation of Fas-Mediated Apoptosis Is Directly Regulated by TCR.
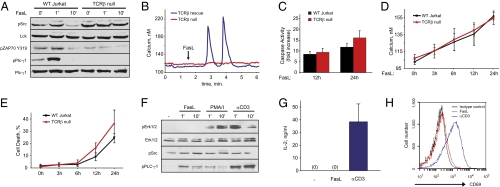
Next, we wished to test whether the Fas receptor directly engages the TCR complex. We employed a Jurkat cell-line derivative lacking expression of the TCR β-chain and, therefore, no functional TCR complex (22). Absence of a functional TCR complex prevented PLC-γ1 phosphorylation in response to Fas ligand stimulation (Fig. 3A). Importantly, these cells did not have compromised total PLC-γ1 or Lck protein levels (Fig. 3A). Fas ligand-mediated calcium release was abolished in TCR β-null cells, and could be rescued by transient expression of the Jurkat TCRB gene (Fig. 3B). We next assessed whether lack of the TCR β-chain would affect the progression of Fas-mediated apoptosis over the course of hours in Jurkat cells. Similar to ZAP70-null cells, TCR β-chain-null cells demonstrated equivalent caspase 3 activation and late elevations in cytosolic calcium and cell death as those of wild-type Jurkat cells (Fig. 3 C–E). This suggests that, at least in Jurkat cells, Fas receptor engagement of the TCR complex is important primarily for PLC-γ1 activation and calcium release after Fas ligand stimulation.
Fig. 3.
The TCR β-chain is required for Fas ligand-mediated PLC-γ1 activation and calcium release in Jurkat cells. (A) PLC-γ1, Lck/Fyn, and ZAP70 activation in wild-type and TCR-β-null Jurkat cells after Fas ligand stimulation. (B) Fas-dependent calcium release in TCR-β-null and TCR-β rescue Jurkat cells. Red trace is TCR-β-null, blue trace is TCR-β rescue. As in Fig. 2, wild-type Jurkat responses were performed in parallel but are not shown for clarity. (C) Caspase 3 activity in wild-type and TCR-β-null Jurkat cells 12 and 24 h after Fas ligand stimulation relative to untreated cells. (D) Time-dependent increases in cytosolic calcium concentration in wild-type and TCR-β-null Jurkat cells. (E) Cell death in wild-type and TCR-β-null Jurkat cells. (F) Erk1/2, Lck/Fyn, and PLC-γ1 activation upon Fas ligand, PMA/ionomycin and anti-CD3 antibody stimulation. (G) Production of IL-2 in Jurkat cells activated with anti-CD3 antibody or Fas ligand. IL-2 was measured by ELISA 12 h following cell stimulation. (H) CD69 expression flow-cytometry profile of Jurkat cells stimulated with Fas ligand or anti-CD3 antibody for 12 h.
The activation of signaling molecules normally associated with TCR signaling raises the possibility that Fas stimulation activates the canonical TCR pathway. TCR stimulation results in ERK pathway activation and, separately, the calcium-dependent translocation of nuclear factor of activated T cells to the nucleus. This process ultimately leads to increased IL-2 production (23). The TCR pathway can be activated in vitro in T cells using an anti-CD3 antibody or the combined addition of a calcium ionophore and a protein kinase-C activator. We thus compared Fas ligand stimulation to cells treated with either anti-CD3 or ionomycin/phorbol myristate acetate. Importantly, despite strong similarities in the initial response involving PLC-γ1 activation and rapid calcium release, stimulation of Fas or TCRs results in activation of different downstream effectors. In contrast to the classic TCR activators anti-CD3 or ionomycin/phorbol myristate acetate, Fas ligand did not induce ERK pathway activation (Fig. 3F) or the production of IL-2 (Fig. 3G). We next confirmed the distinct effects of TCR and Fas stimulation by analyzing the expression of early lymphocyte activation antigen (CD69). CD69 is known to be rapidly up-regulated upon T cell activation (24). As expected, anti-CD3 stimulation of Jurkat cells induced CD69 surface expression; however, no expression of CD69 was detectable after 12 h of treatment with Fas ligand (Fig. 3H).
Resistance to Fas-Mediated Apoptosis Is Associated with Loss of Functional TCR Complex.
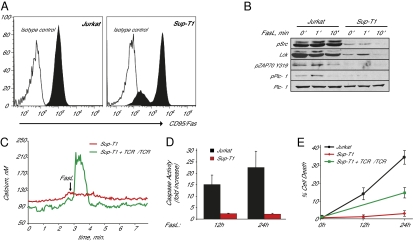
Rearrangements at the TCR locus are common in T-cell lymphomas (25). To further confirm the role of TCR in the initiation of Fas-mediated apoptosis, we have taken advantage of a human T-cell acute lymphoblastic leukemia line (Sup-T1), which has undergone a chromosomal inversion disrupting the TRA@ locus (26). Despite higher levels of surface Fas receptor expression in the majority of the SupT1 cell population (Fig. 4A), Fas ligand stimulation was associated with markedly reduced phosphorylation of PLC-γ1 in Sup-T1 cells compared with wild-type Jurkat cells. Both cell lines express similar levels of total PLC-γ1 protein (Fig. 4B). Furthermore, Sup-T1 cells did not release calcium in response to Fas stimulation. Importantly, this phenotype could be rescued by transient expression of Jurkat TCRB gene (Fig. 4C). As these cells lack TCR-α expression, rescue of the calcium-release phenotype is likely mediated by TCR-β homodimers (27). Sup-T1 cells exhibited dramatically reduced caspase 3 activation and cell death in response to Fas ligand stimulation (Fig. 4 D and E). Importantly, deficiency in Fas-mediated apoptosis of SupT1 cells can be rescued by transient expression of the TCR α- and β-chains (Fig. 4E).
Fig. 4.
Rearrangements at the TRA@ locus associated with acute lymphoblastic leukemia (T-ALL) leads to defective Fas ligand-mediated calcium release and cell death. (A) Fas receptor expression by flow cytometry of Jurkat and T-ALL cell line Sup-T1. The majority of Sup-T1 cells express higher levels of Fas receptor, A small population (∼15%) express lower levels of Fas receptor. (B) PLC-γ1, Lck/Fyn, and ZAP70 activation in Jurkat and Sup-T1 cells. (C) Fas ligand-dependent calcium release in Sup-T1 cells and Sup-T1 cells expressing TCR α- and β-chains. (D) Caspase 3 activity in Jurkat and Sup-T1 cells 12 and 24 h after Fas ligand stimulation relative to untreated cells. (E) Cell death in Jurkat, Sup-T1 cells, and Sup-T1 cells expressing TCR α- and β-chains 12 and 24 h after Fas ligand stimulation.
Thus, the loss of the TCR α-chain in Sup-T1 cells is directly correlated with defective Fas ligand-mediated calcium release and cell death. Importantly, and in contrast to Jurkat cells, a functional TCR complex in Sup-T1 cells is absolutely required for Fas-mediated calcium release, caspase activation, and cell death.
Discussion
The effects of calcium release into cytoplasm through IP3R channels have been shown to trigger a complex proapoptotic program involving increased mitochondrial permeabilization and activation of caspases (28). However, a mechanism for the activation of lymphocyte IP3R channels during apoptosis is largely unknown. Our previous observations demonstrated a critical role of PLC-γ1 in Fas-mediated calcium release from endoplasmic reticulum stores, providing a possible link between Fas stimulation and engagement of the TCR signaling pathway (9). In the present study we identified Lck and ZAP70 protein tyrosine kinases as upstream activators of PLC-γ1 in response to Fas ligation. We found that Lck-null cells were completely resistant to Fas-induced apoptosis, blocking both initiation of Fas signaling and its ultimate progression to apoptotic cell death. Further experiments confirmed role of ZAP70 as a downstream target of Lck mediating activation of PLC-γ1.
Because Lck and ZAP70 are key regulators of proximal TCR signaling, our results suggest that Fas engagement recruits the TCR signaling machinery to promote initiation of Fas-mediated cell death. This hypothesis is in part supported by our findings demonstrating physical association of both Lck and its downstream target PLC-γ1 with Fas signaling complex in a stimulus-dependent manner. PLC-γ1 recruitment to DISC is transient, correlating well with its activation status. However, the amount of Lck captured by anti-Fas antibodies appears to decrease upon Fas engagement compared with unstimulated cells. Because the detection of coimmunoprecipitated proteins is limited to detergent-soluble cell fractions, we proposed that Lck association with Fas complex may be promoted in lipid rafts. This model is favored by previous studies demonstrating that Fas-mediated apoptosis involves Fas translocation in lipid rafts (29), as well as our data showing rapid accumulation of Lck protein in Brij98-resistant membrane subdomains after Fas cross-linking. Alternatively, Lck may be sequestered from the DISC during activation of Fas-mediated signaling. A previous study reported a direct association between Lck and the TCR signaling complex (30), providing a possibility that initiation of the Fas signaling pathway would be dependent on presence of and functional interaction with the TCR complex. Consistent with this hypothesis, Jurkat T cells deficient in functional TCR failed to induce PLC-γ1 activation and calcium release from endoplasmic reticulum stores in response to Fas stimulation. (See Fig. S1 for a model illustrating the functional crosstalk between Fas and TCR signaling complexes.)
To further validate the model of TCR-dependent initiation of the Fas apoptotic pathway, we took the advantage of Sup-T1 cells. This cell line is characterized by a chromosomal translocation that prevents efficient surface expression of the both α- and β-chains of TCR (31). In our experiments, we found that TCR-deficient Jurkat cells, although deficient in calcium release, still underwent apoptosis in response to Fas stimulation. In contrast, Sup-T1 cells, which are also deficient in TCR expression, were completely refractory to Fas-mediated calcium release and cell death, and this phenotype could be rescued with exogenous TCR expression. Differences in the response of TCR-deficient Jurkats versus Sup-T1 cells is difficult to reconcile at this stage, but may be a result of shunting down the “type I” (mitochondrial independent pathway) in the absence of the TCR in Jurkats but not in Sup-T1 cells. Further work will be necessary to elucidate the differences between Jurkat and Sup-T1 cells. Regardless, our studies clearly show that the TCR is a potent regulator of Fas signaling. Our findings also raise the possibility that regulation of TCR expression levels could regulate the sensitivity of lymphocytes to Fas-mediated apoptosis in vivo.
Taken together, our findings provide unique evidence of concurrent crosstalk between Fas- and TCR signaling machineries, suggesting that the unengaged TCR complex might act a direct regulator of Fas signaling in the mitochondria-dependent apoptotic pathway. Furthermore, as malignant transformation of T cells is often associated with loss of TCR expression, our observations suggest that defective TCR machinery may be responsible to Fas-mediated apoptotic resistance and tumor expansion.
Materials and Methods
Antibodies and Reagents.
Antibodies against the phospho-Src, Lck, phospho-ZAP70 (Tyr319), phospho-ZAP70 (Tyr493), PLC-γ1, phospho-PLC-γ1, Erk1/2 and phospho-Erk1/2 (Thr202/Tyr204) were obtained from Cell Signaling Technology. Anti-human CD3 antibody (OKT3) was obtained from Ebioscience. Anti-human Fas antibody (CH11) was obtained form Millipore. Anti-human antibodies CD95-PE, CD69-PE and isotype controls were obtained from BD Bioscience. Fas ligand vesicles were purchased from Pierce Biotechnology. Phorbol 12- myristate 13-acetate (PMA), ionomycin, and HRP-conjugated-cholera toxin-B subunit were purchased from Sigma. Propidium iodide was purchased from Molecular Probes.
Cell Culture.
Sup-T1, Jurkat T cell leukemia (clone E6-1) and Jurkat derivatives J.CaM1.6, P116, P116.cl39, and J.RT3-T3.5 were obtained and cultured according to the guidelines of American Type Culture Collection. Jurkat Lck-rescue cells were kindly provided by Arthur Weiss (University of California, San Francisco, CA) and were cultured in RPMI 1640 supplemented with 10% FBS, 50 μM 2-mercaptoethanol, 250 μg/mL hygromycin B, 1 mM glutamine, and 1% penicillin-streptomycin. Primary murine CD4+ cells were purified by autoMACS separation using anti-CD4 microbeads (Miltenyi Biotec).
Cloning of the Jurkat TCRB Gene.
The protein encoding the Jurkat TCRB gene was amplified by PCR from a Jurkat random hexamer primed cDNA library and cloned into the EcoR1/NotI sites of pcDNA3.1 expression vector (Invitrogen) using following primers: TCRB forward primer 5′-AAGAATTCGCCACCATGGACTCCTGGACCTTC-3′ and TCRB reverse primer 5′-TTTGCGGCCGCTCAGAAATCCTTTCTCTT-3′. The sequence of the obtained Jurkat TCRB transcript was deposited in GenBank (accession number GU32633).
Sucrose Density Gradient Centrifugation.
Jurkat cells were resuspended in Buffer B [150 mM NaCl, 50 mM Tris, pH 7.8, 1% Brij98, 1 mM EDTA, supplemented with a protease inhibitor mixture and phosphatase inhibitor mixture 2 (Sigma)], briefly sonicated, and incubated for 10 min at 37 °C. Postnuclear lysates were obtained by 10 min centrifugation at 10,000 × g and adjusted to 40% final concentration of sucrose. A discontinuous sucrose gradient is then formed by sequentially layering 35 and 5% sucrose, and the tubes were subject to ultracentrifugation at 260,000 × g for 15 h in a Beckman SW 41 Ti rotor at 4 °C. Ten 1-mL fractions were collected and equal volume of each fraction was analyzed by Western blotting. The fractions containing GM1 ganglioside (lipid rafts) were determined by dot blotting with HRP-conjugated cholera toxin B.
Caspase Activity.
Caspase activity was determined fluorometrically, as previously described (32).
Calcium Imaging.
Calcium measurements were performed as previously described (9, 33). Cells with spontaneous release activity in the absence of Fas ligand were identified by imaging at least 100 s before Fas ligand addition and were eliminated from analysis. A cell population was considered to be responsive to Fas treatment if calcium oscillations were detected in more than 20% of cells in a field. In cell populations considered to be resistant to Fas stimulation, calcium oscillations after addition of Fas ligand were detected in less than 1% of cells examined. In experiments where cDNA was transfected into Jurkat or Sup-T1 cells, expressing cells were identified by cotransfecting YFP (at ratio 1:4:4). Nonexpressing cells were imaged simultaneously with expressing cells as internal controls. Each experiment was repeated a minimum of three times, comprising hundreds of single-cell traces. Traces of 8 to 15 of YFP-positive cells were observed in experiments where cDNA was transfected into Jurkat or Sup-T1 cells. For measurement of cytosolic calcium after prolonged Fas treatment, cells treated for 2, 3, 4, 6, 12, or 24 h with Fas ligand were loaded with Fura-2 at each time point and cytoplasmic calcium imaged in three separate fields, each comprising 50 to 100 cells. The mean of the three fields comprised one datapoint. Each experiment was repeated an additional two times and presented as the SEM of three experiments.
Cell Death.
Cell death was quantified as previously described (9, 33). In experiments where cDNA was transfected into Sup-T1 cells, expressing cells were identified by cotransfecting YFP and TCR α- and β-chains (at ratio 1:4:4).
Cytokine Expression Measurement.
Jurkat cells were stimulated with 5 μg/mL of anti-human CD3 antibody or 1 ng/mL Fas ligand vesicles for 12 h. IL-2 expression level in supernatants was measured using human IL-2 ELISA KIT II (BD Bioscience). Each experiment was repeated a minimum three times and presented as the SEM of three experiments.
Supplementary Material
Acknowledgments
We thank Arthur Weiss (University of California, San Francisco) for providing the Lck rescue cell line (J.CaM1.6WT), Suresh K. Joseph for critical reading of the manuscript and José M. Barral for helpful suggestions and critical reading of the manuscript. We would also like to thank the members of the D.B., Barral, and Epstein laboratories at the University of Texas Medical Branch for critical discussions. This work was supported by Grants GM081685 (to D.B.) and AI069142 (to J.S.) from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: TCRB transcript was deposited in GenBankTM (accession number GU326331)
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005419107/-/DCSupplemental.
References
- 1.Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, et al. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 3.Alderson MR, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher GH, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RM, Muppidi J, Roberts M, Porter M, Wu Z. Death receptor signaling and autoimmunity. Immunol Res. 2003;27:499–512. doi: 10.1385/IR:27:2-3:499. [DOI] [PubMed] [Google Scholar]
- 6.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 7.Inaba M, et al. Primed T cells are more resistant to Fas-mediated activation-induced cell death than naive T cells. J Immunol. 1999;163:1315–1320. [PubMed] [Google Scholar]
- 8.Miyawaki T, et al. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–3758. [PubMed] [Google Scholar]
- 9.Wozniak AL, et al. Requirement of biphasic calcium release from the endoplasmic reticulum for Fas-mediated apoptosis. J Cell Biol. 2006;175:709–714. doi: 10.1083/jcb.200608035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 11.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotzer VL, Mabardy AS, Weiss A, Brodsky FM. T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J Exp Med. 2004;199:981–991. doi: 10.1084/jem.20031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr VA, et al. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: Puncta and distal caps. Mol Biol Cell. 2008;19:2802–2817. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson S, August A, Branch D, Dupont B, Mills GM. Functional LCK Is required for optimal CD28-mediated activation of the TEC family tyrosine kinase EMT/ITK. J Biol Chem. 1996;271:7079–7083. doi: 10.1074/jbc.271.12.7079. [DOI] [PubMed] [Google Scholar]
- 15.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 16.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharif-Askari E, et al. p56Lck tyrosine kinase enhances the assembly of death-inducing signaling complex during Fas-mediated apoptosis. J Biol Chem. 2007;282:36048–36056. doi: 10.1074/jbc.M706007200. [DOI] [PubMed] [Google Scholar]
- 18.Resh MD. Fatty acylation of proteins: New insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim Biophys Acta. 1999;1451(1):1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 19.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bijlmakers MJ. Protein acylation and localization in T cell signaling (Review) Mol Membr Biol. 2009;26:93–103. doi: 10.1080/09687680802650481. [DOI] [PubMed] [Google Scholar]
- 21.Williams BL, et al. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: Reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitmarsh AJ, Davis RJ. Analyzing mitogen-activated protein kinase (MAPK) activities in T cells. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im1108s58. Chapter 11:Unit 11.18. [DOI] [PubMed] [Google Scholar]
- 24.Zola H. Markers of cell lineage, differentiation and activation. J Biol Regul Homeost Agents. 2000;14:218–219. [PubMed] [Google Scholar]
- 25.Dadi S, Le Noir S, Asnafi V, Beldjord K, Macintyre EA. Normal and pathological V(D)J recombination: Contribution to the understanding of human lymphoid malignancies. Adv Exp Med Biol. 2009;650:180–194. doi: 10.1007/978-1-4419-0296-2_15. [DOI] [PubMed] [Google Scholar]
- 26.Baer R, Forster A, Rabbitts TH. The mechanism of chromosome 14 inversion in a human T cell lymphoma. Cell. 1987;50:97–105. doi: 10.1016/0092-8674(87)90666-0. [DOI] [PubMed] [Google Scholar]
- 27.Groettrup M, Baron A, Griffiths G, Palacios R, von Boehmer H. T cell receptor (TCR) beta chain homodimers on the surface of immature but not mature alpha, gamma, delta chain deficient T cell lines. EMBO J. 1992;11:2735–2745. doi: 10.1002/j.1460-2075.1992.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollinedo F, Gajate C. Fas/CD95 death receptor and lipid rafts: New targets for apoptosis-directed cancer therapy. Drug Resist Updat. 2006;9:51–73. doi: 10.1016/j.drup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 30.August A, Dupont B. Association between mitogen-activated protein kinase and the zeta chain of the T cell receptor (TcR) with the SH2,3 domain of p56lck. Differential regulation by TcR cross-linking. J Biol Chem. 1996;271:10054–10059. doi: 10.1074/jbc.271.17.10054. [DOI] [PubMed] [Google Scholar]
- 31.Jones S, et al. Lentiviral vector design for optimal T cell receptor gene expression in the transduction of peripheral blood lymphocytes and tumor-infiltrating lymphocytes. Hum Gene Ther. 2009;20:630–640. doi: 10.1089/hum.2008.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boehning D, et al. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 33.Boehning D, van Rossum DB, Patterson RL, Snyder SH. A peptide inhibitor of cytochrome c/inositol 1,4,5-trisphosphate receptor binding blocks intrinsic and extrinsic cell death pathways. Proc Natl Acad Sci USA. 2005;102:1466–1471. doi: 10.1073/pnas.0409650102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.