Abstract
Malaria transmission is strongly influenced by environmental temperature, but the biological drivers remain poorly quantified. Most studies analyzing malaria–temperature relations, including those investigating malaria risk and the possible impacts of climate change, are based solely on mean temperatures and extrapolate from functions determined under unrealistic laboratory conditions. Here, we present empirical evidence to show that, in addition to mean temperatures, daily fluctuations in temperature affect parasite infection, the rate of parasite development, and the essential elements of mosquito biology that combine to determine malaria transmission intensity. In general, we find that, compared with rates at equivalent constant mean temperatures, temperature fluctuation around low mean temperatures acts to speed up rate processes, whereas fluctuation around high mean temperatures acts to slow processes down. At the extremes (conditions representative of the fringes of malaria transmission, where range expansions or contractions will occur), fluctuation makes transmission possible at lower mean temperatures than currently predicted and can potentially block transmission at higher mean temperatures. If we are to optimize control efforts and develop appropriate adaptation or mitigation strategies for future climates, we need to incorporate into predictive models the effects of daily temperature variation and how that variation is altered by climate change.
Keywords: Anopheles mosquitoes, climate change, diurnal temperature variability, ectotherms, Plasmodium malaria
The basic reproductive number (R0), which defines the number of cases of a disease that arise from one case of the disease introduced into a population of susceptible hosts, is a key epidemiological metric providing essential information for understanding disease risk and for targeting resources for control. For malaria, R0 is commonly described by the formula R0 = ma2bce−pS/pr [note that this expression is also defined as (R0)2; ref. 1], where m is the vector:human ratio, a vector biting frequency, bc transmission coefficients defining vector competence, p daily vector survival rate, S the extrinsic incubation or development period of the parasite within the vector, and r the recovery rate of the vertebrate hosts from infection. Given that six of seven of these parameters relate in some way to mosquito abundance, biology, or physiology and that mosquitoes are small cold-blooded insects, it is clear that the transmission intensity of malaria will be strongly influenced by environmental temperature (2–6). Accordingly, the dynamics and distribution of malaria are expected to be extremely sensitive to climate change, although the nature and extent of the response remains highly controversial (7–15).
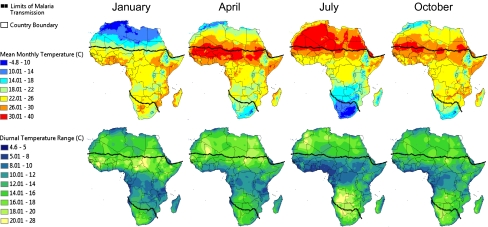
The standard relationships describing the effects of temperature on malaria parasite and mosquito life history derive largely from laboratory studies conducted under constant temperature conditions (e.g., ref. 2 and references therein) and tend to use measures such as average monthly temperature to characterize environmental conditions (2, 5, 7, 8, 14, 16). However, natural environments are highly dynamic; diurnal temperature ranges (DTRs, the difference between the minimum and maximum temperature) of 5 to >20 °C are common across many malaria transmission settings in Africa, including highland and lowland environments (17–19). We illustrate this further in Fig. 1, which shows mean temperatures and DTRs for 4 mo (one illustrative of each quarter) based on weather station data collected in Africa since the 1960s. The temperature surfaces demonstrate that both small (e.g., 5–10 °C) and large (e.g., 12–20 °C) DTRs occur around cool (16–18 °C) and warm (24–26 °C) mean temperatures within the malaria transmission range, depending on season and location. However, despite this clear variation, there exists little empirical understanding of the nonlinear ways in which the biology of the parasite and mosquito vector integrate with temperature fluctuations (18, 20).
Fig. 1.
Mean monthly temperature and mean monthly DTR throughout Africa for January, April, July, and October. Temperature surfaces were generated by interpolation using weather station data collected between 1960 and1990. For areas where data records were limited, such as in the Democratic Republic of the Congo, the time period was extended to 2000. The current geographical limits of malaria transmission are illustrated by the dotted lines.
In a recent theoretical study, we proposed that temperature fluctuation can substantially alter the incubation period of malaria parasites within the mosquito and hence influence malaria transmission rates (18). Specifically, using a temperature-development model of Plasmodium falciparum, we showed that diurnal temperature fluctuation around mean temperatures of <21 °C could speed parasite development, whereas fluctuation around means >21 °C could slow development, compared with constant temperatures. Here, using the rodent–malaria model Plasmodium chabaudi and the Asian malaria vector Anopheles stephensi, we confirm that not only the incubation period of malaria parasites but all of the essential mosquito and parasite parameters that determine R0 are strongly influenced by diurnal temperature fluctuations and that the impacts of climate on malaria cannot be fully understood based on mean conditions alone.
Results and Discussion
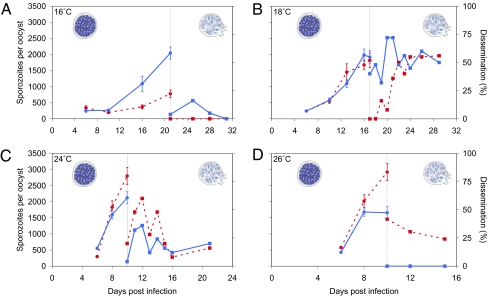
We first examined the development of malaria parasites within the mosquito, considering two low (16 and 18 °C) and two higher mean transmission temperatures (24 and 26 °C). For each baseline temperature, mosquitoes were held either at constant temperature (DTR of 0 °C) or a fluctuating temperature (±6 °C, giving an intermediate DTR of 12 °C). Mosquitoes were sampled over time, and parasite growth was quantified using a combination of microscopy and real-time quantitative PCR (qPCR) to determine infection prevalence and intensity (components of the transmission coefficients defining vector competence), rate of parasite replication, time of first sporozoite release (measures defining the extrinsic incubation period, S), and percentage of mosquitoes with disseminated sporozoites (a further component of vector competence). In pilot studies, we observed, as have others (21–23), the extreme temperature sensitivity of the early infection process. Therefore, we ensured in our first large-scale experiment that all treatments started at similar infection burdens (SI Materials and Methods) by maintaining infected mosquitoes at a constant 26 °C for the first 3 d following the infectious blood meal.
The growth kinetics of malaria parasites exposed to diurnal temperature fluctuation differed considerably to those kept at equivalent constant mean temperatures (Fig. 2). At 16 °C, fluctuation led to a significant increase in parasite growth rate, resulting in more sporozoites per oocyst, at the point of sporozoite release (F1,15 = 31.10, P < 0.001). Fluctuation also caused a significant increase in dissemination rate (χ2 = 5.94, df = 1, P = 0.02) to the extent that liberated sporozoites were observed only under fluctuating conditions. Conversely, at 26 °C, fluctuation significantly reduced the mean number of sporozoites/oocyst (F1,25 = 5.32, P = 0.03) and reduced dissemination to zero (χ2 = 26.34, df = 1, P < 0.001).
Fig. 2.
Growth rate and dissemination of P. chabaudi malaria in An. stephensi mosquitoes under constant and fluctuating temperature regimens. Mosquitoes were kept at either constant temperatures (dashed red lines) or temperatures with a diurnal temperature fluctuation of ±6 °C (DTR = 12 °C; solid blue lines). Baseline mean temperatures were (A) 16 °C, (B) 18 °C, (C) 24 °C, and (D) 26 °C. The number of sporozoites per oocyst (circles, Left) describes parasite growth kinetics up to the point of first sporozoite release. Dissemination (squares, Right) describes the percentage of mosquitoes that were observed with mature sporozoites circulating in the hemocoel. Error bars equal the SEM. Drawings of oocysts are generated by the Centers for Disease Control and Prevention (http://www.dpd.cdc.gov/dpdx/HTML/Malaria.htm).
Similar but slightly less marked effects were observed at 18 °C and 24 °C. Although the number of sporozoites/oocyst was not significantly affected by fluctuation (F1,32 = 0.23, P = 0.63 for 18 °C; F1,34 = 3.19, P = 0.08 for 24 °C), the number of mosquitoes with disseminated sporozoites was significantly increased by fluctuation at 18 °C (χ2 = 23.74, df = 1, P < 0.001) and significantly reduced by fluctuation at 24 °C (χ2 = 7.01, df = 1, P = 0.01).
We then extended our approach to include the effects of temperature fluctuation immediately following the infectious blood meal, selecting the intermediate temperatures of 18 and 24 °C at which full parasite development and dissemination were shown to be completed. With this more extended exposure, fluctuation at 24 °C now caused a significant reduction in the number of sporozoites/oocyst (F1,12 = 6.11, P = 0.03), whereas fluctuation at 18 °C caused a significant increase (F1,21 = 19.54, P < 0.001; Table S1).
In addition, fluctuation at 24 °C led to a 3.9-fold reduction of the average number of oocysts per midgut compared with the constant temperature (Table S1; χ2 = 18.29, df = 1, P < 0.001). No such effects were observed for the 18 °C treatments (χ2 = 0.20, df = 1, P = 0.66), highlighting the extreme sensitivity of malaria parasites to high rather than low temperatures during early sporogonic development (24).
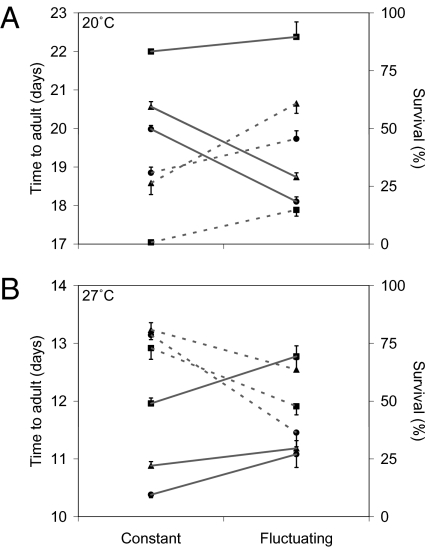
Next, we examined effects of daily temperature dynamics on three key mosquito life history parameters affecting vectorial capacity. The approach followed the basic methodology of comparing traits under cool and warm conditions, with and without a DTR of 12 °C. First, we examined immature mosquito development and survival (traits affecting mosquito population dynamics and the vector:host ratio, m), considering three larval densities and mean temperatures of 20 °C and 27 °C. These slightly elevated means were selected to reflect the fact that temperatures in the aquatic breeding habitats of many anopheline vectors tend to be warmer than ambient air temperatures (25).
Consistent with the patterns for parasite development, fluctuation around the cooler temperature acted to speed mosquito development compared with constant conditions (F1,36 = 261.96; P < 0.001), whereas fluctuation around the warmer temperature caused a relative slowing in development rate (F1,66 = 27.75; P < 0.001; Fig. 3). Larval density generally acted as a scaling factor with no consistent interaction effects. Furthermore, daily fluctuation increased relative survival at 20 °C (F1,64 = 68.91; P < 0.001) but reduced relative survival at 27 °C (F1,64 = 54.45; P < 0.001; Fig. 3). At the lowest larval density, fluctuation around 20 °C made the difference between 14.8% of larvae (i.e., 40 of the original 270) surviving through to adulthood compared with just 0.7% (i.e., 2 of 270) in the constant temperature treatment.
Fig. 3.
Interaction plot of the development time and survival of the immature stages of An. stephensi under constant compared with fluctuating temperature regimens. (A) Development time (days, solid lines) and survival (percentage, dashed lines) of mosquito immatures until they reached the adult stage at a constant 20 °C and at a mean temperature of 20 °C but with a diurnal temperature fluctuation of ±6 °C (DTR = 12 °C). (B) Data from an equivalent experiment at 27 °C. Results are given for three different larval densities (■, 0.5, ▲, 1, and •, 2 larvae/cm2). Error bars equal the SEM.
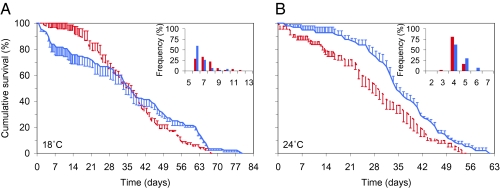
Second, returning to the mean temperatures of 18 °C and 24 °C, we examined the effect of temperature fluctuation on adult survival (Fig. 4). Survival analysis revealed no effect of fluctuation on overall cumulative survival at 18 °C (log-rank statistic = 3.18, P = 0.08). However, analysis of the first 21 d, during which an estimated 95% of mosquitoes will have died in nature (26), showed fluctuation reduced initial survival (log-rank statistic = 11.96, P = 0.001). In the 24 °C treatments, fluctuation resulted in a significant increase in survival overall (log-rank statistic = 7.80, P = 0.01). The results confirm once again that realistic daily temperature variation impacts a key parameter (p) determining transmission intensity, with effects differing between warm and cool conditions.
Fig. 4.
Cumulative percent survival and gonotrophic cycle length of female An. stephensi mosquitoes under constant and fluctuating temperature regimens. (A) Survival at constant 18 °C (dashed red line) compared with survival at a mean temperature of 18 °C but with a diurnal temperature fluctuation of ±6 °C (DTR = 12 °C; closed blue line). (Inset) The percentage of mosquitoes that completed the gonotrophic cycle on a given day (on the x axis) at constant 18 °C (red bars) compared with completion at a mean temperature of 18 °C but with a diurnal temperature fluctuation of ±6 °C (DTR = 12 °C; blue bars). (B) Data from equivalent experiments at 24 °C. Error bars equal the SEM.
Finally, we examined the effect of daily temperature dynamics on the length of the gonotrophic cycle [time between blood meal and oviposition, which is inversely related to vector biting frequency (a)]. Again, fluctuation around the cool temperature shortened the mean length of the first gonotrophic cycle from 7.4 to 6.7 d (Mann–Whitney U test, P < 0.01; Fig. 4A), whereas fluctuation around the warm temperature increased the length of the cycle from 4.1 to 4.5 d (P = 0.02; Fig. 4B).
Overall, our results provide empirical evidence that the key mosquito-related traits that combine to determine malaria transmission intensity (i.e., parasite infection, parasite growth and development, immature mosquito development and survival, length of the gonotrophic cycle, and adult survival) are all sensitive to daily variation in temperature. Given that certain of these traits affect transmission as quadratic or exponential terms, even small changes could have large biological significance. Consistent with recent theoretical predictions (18), we find that, in general, fluctuation increases relative rate processes under cool conditions and slows rate processes under warm conditions; effects that, on average, will tend to lessen the impact of increases in mean temperature in the real world (18).
Our experiments used a rodent malaria and one species of mosquito, and there is clearly a need to extend investigations to human malaria species and to other important vectors (27). However, there is no reason to believe that the sensitivity of P. chabaudi and An. stephensi to moderate diurnal temperature variation is unique among malaria parasites and their mosquito vectors. Moreover, the effects will apply whether considering aquatic, terrestrial, indoor, or outdoor environments because all can exhibit variable diurnal temperatures (17, 25, 28) (Fig. 1).
These findings caution against standard practice in studies estimating mosquito– and/or malaria–climate relations and strengthen arguments for greater ecological understanding of how infectious organisms respond to the natural environment (29). We find that neither the essential transmission parameters nor the upper or lower temperature thresholds for transmission can be derived reliably from experiments conducted at constant temperatures or from monthly, weekly, or even daily environmental means.
Despite substantial research interest and clear social and political importance, few (if any) climate change and/or malaria transmission models have considered daily temperature dynamics or how DTRs will shift in addition to means. Accordingly, the relevance of many current policy syntheses (9, 30, 31) remain unclear, especially when considering issues of range expansion or contraction because, for malaria, edges of range are characterized by extreme temperatures and large DTRs. More broadly, although the focus of the current study is malaria, the observed impact of daily temperature fluctuation on basic aspects of insect and parasite life histories suggest the need to consider the role of temperature variation for many ectotherms (other insects, amphibians, reptiles, etc.) and their parasites and pathogens, both for understanding current biology and the likely impacts of climate change (e.g., 32, 33).
Materials and Methods
Temperature Surface Maps of Africa.
Minimum and maximum mean monthly temperature surfaces were obtained from WorldClim, version 1.4 (release 3) (http://www.worldclim.org; ref. 34). The WorldClim surfaces were created using the thin-plate smoothing spline interpolation method in ANUSPLIN (35). Mean monthly minimum and maximum temperature surfaces were generated using weather station records containing at least 10 y of data from the period 1960 to1990. For areas where recent data records were limited, such as in the Democratic Republic of the Congo, the time period was extended to include 1960–2000. These surfaces were imported into ESRI ArcGIS ArcView 9.3 and used to generate mean monthly diurnal temperature ranges for Africa. Limits of malaria transmission zones were obtained from the Africa world and regional level maps (http://www.map.ox.ac.uk/data; ref. 36). An image of the map was brought into ArcView 9.3 and georeferenced. Once georeferenced, the malaria transmission limits were digitized.
Bioassays.
The effects of temperature on the various mosquito and malaria life history traits were assessed using laboratory incubators. Incubators were programmed to maintain either constant temperature or to fluctuate around mean temperatures equivalent to the constant temperature treatments with a diurnal temperature fluctuation of ±6 °C (DTR = 12 °C) using a realistic minimum–maximum temperature model (37) (Fig. S1). Relative humidity within the incubators was maintained at 90 ± 5%.
Parasite Development.
Mosquitoes were allowed to feed on infectious mice (day 0), with fully engorged mosquitoes pooled and then divided across temperature treatments. Mosquitoes were maintained in cardboard cups on glucose water with 25–30 mosquitoes per cup. Mosquitoes were sampled over time by dissecting 20–25 mosquitoes from one cup per time point per treatment. Parasite growth was quantified by scoring the number of oocysts on the midgut and by counting the number of sporozoites per oocyst (by qPCR). Dissemination was quantified by scoring the number of mosquitoes with disseminated sporozoites.
Immature Mosquito Development and Survival.
First-instar larvae (<24 h) were transferred to transparent plastic cups containing 3 cm of distilled water at three different densities (0.5, 1, and 2 larvae/cm2 water surface area). Larvae and pupae were counted daily, with water and food replaced to maintain constant food conditions per larva per day. Between 10 and 15 replicate cups were used per treatment. The day of adult mosquito emergence was recorded.
Mosquito Survival.
Mosquitoes were blood-fed, and fully engorged mosquitoes were divided into cardboard cups (25 mosquitoes per cup), with four replicates per temperature treatment. Mosquitoes were maintained on glucose water and mortality was scored daily.
Mosquito Feeding Frequency.
Mosquitoes were allowed to blood feed, and fully engorged females were placed individually in plastic 5-mL oviposition tubes and maintained on glucose solution. Tubes (50 replicates per temperature treatment) were monitored daily for eggs. The length of the gonotrophic cycle was determined as the time between the blood feed and oviposition.
Full methods and associated references are available in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank B. Chan, K. Glunt, S. Huijben, and D. Kroczynski for their assistance during experiments. This study benefited from discussions in working groups of the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health, and was supported by a Netherlands Organisation for Scientific Research grant to K.P.P., a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds, and a National Science Foundation EID program grant (EF-0914384).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006422107/-/DCSupplemental.
References
- 1.Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2:23–41. doi: 10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- 2.Craig MH, Snow RW, Le Sueur D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today. 1999;15:105–111. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 3.Rogers DJ, Randolph SE. In: Global Mapping of Infectious Diseases: Methods, Examples and Emerging Applications (Advances in Parasitology) Hay SI, Graham A, Rogers DJ, editors. San Diego: Elsevier; 2006. pp. 345–381. [DOI] [PubMed] [Google Scholar]
- 4.Harvell CD, et al. Climate warming and disease risks for terrestrial and marine biota. Science. 2002;296:2158–2162. doi: 10.1126/science.1063699. [DOI] [PubMed] [Google Scholar]
- 5.Guerra CA, et al. The limits and intensity of Plasmodium falciparum transmission: Implications for malaria control and elimination worldwide. PLoS Med. 2008;5:300–311. doi: 10.1371/journal.pmed.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patz JA, Olson SH. Malaria risk and temperature: Influences from global climate change and local land use practices. Proc Natl Acad Sci USA. 2006;103:5635–5636. doi: 10.1073/pnas.0601493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pascual M, Ahumada JA, Chaves LF, Rodó X, Bouma M. Malaria resurgence in the East African highlands: Temperature trends revisited. Proc Natl Acad Sci USA. 2006;103:5829–5834. doi: 10.1073/pnas.0508929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay SI, et al. Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–909. doi: 10.1038/415905a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Confalonieri U, et al. In: Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Parry ML, Canziani OF, Palutikof JP, Linden PJvd, Hanson CE, editors. Cambridge UK: Cambridge Univ Press; 2007. pp. 391–431. [Google Scholar]
- 10.Reiter P, et al. Global warming and malaria: A call for accuracy. Lancet Infect Dis. 2004;4:323–324. doi: 10.1016/S1473-3099(04)01038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randolph SE. Perspectives on climate change impacts on infectious diseases. Ecology. 2009;90:927–931. doi: 10.1890/08-0506.1. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 13.Pascual M, Bouma MJ. Do rising temperatures matter. Ecology. 2009;90:906–912. doi: 10.1890/08-0730.1. [DOI] [PubMed] [Google Scholar]
- 14.Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- 15.Gething PW, et al. Climate change and the global malaria recession. Nature. 2010;465:342–346. doi: 10.1038/nature09098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martens P, et al. Climate change and future populations at risk of malaria. Glob Environ Change. 1999;9:S89–S107. [Google Scholar]
- 17.Geerts B. Empirical estimation of the monthly-mean daily temperature range. Theor Appl Climatol. 2003;74:145–165. [Google Scholar]
- 18.Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proc Natl Acad Sci USA. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minakawa N, Omukunda E, Zhou G, Githeko A, Yan G. Malaria vector productivity in relation to the highland environment in Kenya. Am J Trop Med Hyg. 2006;75:448–453. [PubMed] [Google Scholar]
- 20.Pascual M, Dobson AP, Bouma MJ. Underestimating malaria risk under variable temperatures. Proc Natl Acad Sci USA. 2009;106:13645–13646. doi: 10.1073/pnas.0906909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noden BH, Kent MD, Beier JC. The impact of variations in temperature on early Plasmodium falciparum development in Anopheles stephensi. Parasitology. 1995;111:539–545. doi: 10.1017/s0031182000077003. [DOI] [PubMed] [Google Scholar]
- 22.Vanderberg J, Yoeli M. Effects of temperature on sporogonic development of Plasmodium berghei. J Parasitol. 1966;52:559–564. [PubMed] [Google Scholar]
- 23.Eling W, Hooghof J, van de Vegte-Bolmer M, Sauerwein R, van Gemert G-J. Tropical temperatures can inhibit development of the human malaria parasite Plasmodium falciparum in the mosquito. Proc Sect Exp Appl Entomol Neth Entomol Soc (N.E.V.) 2001;12:151–156. [Google Scholar]
- 24.Sinden RE, Butcher GA, Billker O, Fleck SL. Advanced Parasitology. San Diego: Elsevier; 1996. pp. 53–117. [DOI] [PubMed] [Google Scholar]
- 25.Paaijmans KP, et al. Observations and model estimates of diurnal water temperature dynamics in mosquito breeding sites in western Kenya. Hydrol Proc. 2008;22:4789–4801. [Google Scholar]
- 26.Kiszewski A, et al. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70:486–498. [PubMed] [Google Scholar]
- 27.Cohuet A, et al. Anopheles and Plasmodium: From laboratory models to natural systems in the field. EMBO Rep. 2006;7:1285–1289. doi: 10.1038/sj.embor.7400831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2006;74:772–778. [PubMed] [Google Scholar]
- 29.Lafferty KD. Calling for an ecological approach to studying climate change and infectious diseases. Ecology. 2009;90:932–933. doi: 10.1890/08-1767.1. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Protecting Health from Climate Change: Connecting Science, Policy and People. Geneva: World Health Organization; 2009. [Google Scholar]
- 31.Costello A, et al. Managing the health effects of climate change. Lancet. 2009;373:1693–1733. doi: 10.1016/S0140-6736(09)60935-1. [DOI] [PubMed] [Google Scholar]
- 32.Deutsch CA, et al. Impacts of climate warming on terrestrial ectotherms across latitude. Proc Natl Acad Sci USA. 2008;105:6668–6672. doi: 10.1073/pnas.0709472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci USA. 2010;107:8269–8274. doi: 10.1073/pnas.0912883107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 35.Hutchinson M. Anusplin Version 4.3. Canberra, Australia: Centre for Resource and Environmental Studies. The Australian National University; 2004. [Google Scholar]
- 36.Hay SI, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:17. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parton WJ, Logan JA. A model for diurnal variation in soil and air temperature. Agric Meterol. 1981;23:205–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.