Abstract
The hypersensitive response (HR), characterized by a rapid and localized cell death at the inoculation site, is one of the most efficient resistance reactions to pathogen attack in plants. The transcription factor AtMYB30 was identified as a positive regulator of the HR and resistance responses during interactions between Arabidopsis and bacteria. Here, we show that AtMYB30 and the secreted phospholipase AtsPLA2-α physically interact in vivo, following the AtMYB30-mediated specific relocalization of AtsPLA2-α from cytoplasmic vesicles to the plant cell nucleus. This protein interaction leads to repression of AtMYB30 transcriptional activity and negative regulation of plant HR. Moreover, Atspla2-α mutant plants are more resistant to bacterial inoculation, whereas AtsPLA2-α overexpression leads to decreased resistance, confirming that AtsPLA2-α is a negative regulator of AtMYB30-mediated defense. These data underline the importance of cellular dynamics and, particularly, protein translocation to the nucleus, for defense-associated gene regulation in plants.
Keywords: hypersensitive response, MYB transcription factor, secretory phospholipase, plant disease resistance
During evolution, plants have developed sophisticated mechanisms to recognize attacking organisms and to translate this perception into an adaptive response, thereby limiting spread of the pathogen within the plant. Plant defense reactions include programmed cell death (hypersensitive response; HR), modifications of cell walls, and the production of reactive oxygen species and antimicrobial proteins. The HR, rapidly induced in plant cells directly in contact with the pathogen, is thought to play an essential role in pathogen confinement and, consequently, in disease resistance (1).
Transcriptional reprogramming is a crucial step of plant defense in response to pathogen recognition, involving major changes in gene expression. Several families of transcription factors (TFs), including TGA-bZIP, ERF, MYB, WHRILY, and WRKY families, have been implicated in defense gene regulation in response to pathogen infection, pathogen-derived elicitors, and signaling molecules (2). Plant MYB proteins are involved in the control of plant-specific processes, including secondary metabolism, cell fate and development, as well as in plant responses to a variety of environmental stimuli and in mediating hormone actions (3, 4).
The MYB gene AtMYB30 was isolated on the basis of its early, transient, and specific expression in Arabidopsis after inoculation with avirulent bacteria (5). Plants overexpressing AtMYB30 show accelerated and stronger HR in response to avirulent pathogens, together with increased resistance and accumulation of HR molecular markers. These and other results identified AtMYB30 as a positive regulator of the signaling pathway controlling the establishment of cell death in response to pathogen attack (6). Putative AtMYB30 target genes are involved in the lipid biosynthesis pathway that leads to the production of very long chain fatty acids (VLCFAs), suggesting a role of this pathway in the control of the HR and plant defense responses (7).
Phospholipase A2s (PLA2s) hydrolyze membrane glycerophospholipids to yield lysophospholipids and free fatty acids in response to hormones and external stimuli. In animals, PLA2 enzymes and their enzymatic products are involved in diverse cellular responses, including bioactive lipid production, inflammation, host defense, and signal transduction (8). Evidence that phospholipid-derived molecules act as second messengers in plant signaling is also accumulating (9). Plant PLA2s activities have been implicated in plant growth, development, stress responses, and defense signaling (10, 11). In Arabidopsis, the secretory PLA2 family (AtsPLAs) consists of four isoforms denoted AtsPLA2-α, -β, -γ, and -δ (10). Plant sPLA2s contain N-terminal signal peptides and are of low molecular mass (13–18 kDa) after secretion. sPLA2s have a highly conserved Ca2+-binding loop and an active site motif with a conserved His/Asp dyad (12).
TFs interact with a variety of proteins that act as regulatory molecules to modulate transcriptional activity in both plant and animal cells. Here, we set out to identify proteins that interact with AtMYB30 by using a yeast two-hybrid (Y2H) screen. We describe the identification of AtsPLA2-α and its AtMYB30-mediated specific relocalization from cytoplasmic vesicles to the cell nucleus, where both proteins physically interact. Our results establish AtsPLA2-α as a negative regulator of AtMYB30-mediated plant defense responses.
Results
Identification of AtsPLA2-α.
To identify AtMYB30-interacting proteins, AtMYB30 was used as bait to screen a Y2H Arabidopsis cDNA library generated from mRNAs isolated from leaf tissue after bacterial inoculation. Different prey cDNA clones were identified. Here, we describe the identification of a partial cDNA clone encoding the last 119 amino acid residues of the secretory phospholipase A2-alpha (AtsPLA2-α; At2g06925). Fig. S1 shows the specificity of the interaction between AtsPLA2-α and AtMYB30 in yeast.
AtsPLA2-α Is Relocalized to the Cell Nucleus in the Presence of AtMYB30.
Agrobacterium-mediated transient expression of a 35S:AtMYB30-CFP fusion in leaf epidermal cells of Arabidopsis seedlings revealed a nuclear localization of AtMYB30 (Fig. S2A). As expected for a secreted phospholipase, a 35S:AtsPLA2-α-YFPv fusion was found to be located intracellularly in cytoplasmic vesicles and around the nucleus, early after agroinfiltration (Fig. S2C). At later time points, YFP fluorescence was detected outside the cells, indicating that AtsPLA2-α had been secreted (Fig. S2E). Although individual proteins were detected after transformation, the efficiency of single-cell cotransformation with both AtMYB30 and AtsPLA2-α was not high enough to allow colocalization studies. Therefore, transient protein expression was undertaken in Nicotiana benthamiana. AtMYB30 and AtsPLA2-α presented identical subcellular localization in N. benthamiana and Arabidopsis both 36 and 48 hours after inoculation (hpi) (Fig. S2), validating our strategy to use N. benthamiana as a heterologous system.
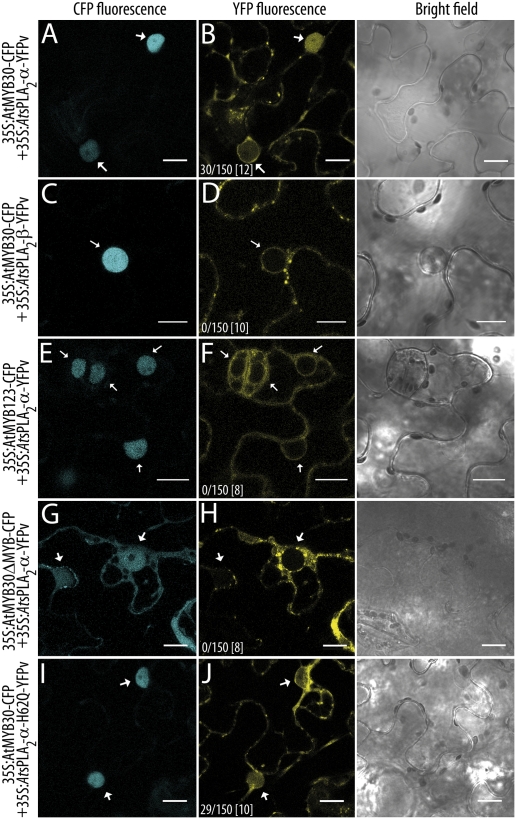
When AtsPLA2-α was coexpressed with AtMYB30, AtsPLA2-α was partially relocalized to the cell nucleus (20% of nuclei; Fig. 1 A and B). The three other Arabidopsis proteins of the AtsPLA2 family, AtsPLA2-β, -γ, and -δ, which present the same subcellular localization and expression level that AtsPLA2-α, were not relocalized to the nucleus in the presence of AtMYB30 (Fig. 1 C and D; Fig. S3). In addition, no relocalization of AtsPLA2-α was detected after coexpression with the unrelated MYB TF AtMYB123 (ref. 13; Fig. 1 E and F). Expression of an AtMYB30 version lacking its MYB domain (AtMYB30ΔMYB), which is not exclusively localized in the nucleus but ubiquitously distributed throughout the cell, is not sufficient to induce the nuclear targeting of AtsPLA2-α (Fig. 1 G and H), indicating that the MYB domain in AtMYB30 is necessary for AtsPLA2-α nuclear relocalization. In addition, AtsPLA2-α nuclear targeting is independent of its enzymatic activity because the inactive catalytic mutant AtsPLA2-α-H62Q (12) is also relocalized to the nucleus in the presence of AtMYB30 (Fig. 1 I and J). Finally, expression of full-length proteins fused to YFPvenus (YFPv) was confirmed by Western blot in all cases (Fig. S4), showing that nuclear detection of YFP fluorescence is due to nuclear relocalization of AtsPLA2-α and not passive diffusion of truncated YFPv.
Fig. 1.
AtsPLA2-α is specifically targeted to the nucleus by AtMYB30. Confocal images of epidermal cells of N. benthamiana leaves expressing the indicated constructs 36 hpi. (A and B) AtMYB30-CFP+AtsPLA2-α-YFPv; (C and D) AtMYB30-CFP+AtsPLA2-β-YFPv; (E and F) AtMYB123-CFP+ AtsPLA2-α-YFPv; (G and H) AtMYB30δMYB-CFP+AtsPLA2-α-YFPv; and (I and J) AtMYB30-CFP+AtsPLA2-α-H62Q-YFPv. Ratios indicate the average number of cells showing AtsPLA2 nuclear relocalization vs. the total number of cells screened per experiment. The total number of performed experiments is indicated between brackets. White arrows indicate cell nuclei. Bright field images are shown on the right. (Scale bars: 15 μm.)
Biochemical validation of the AtMYB30-mediated relocalization of AtsPLA2-α was obtained by purifying nuclei from cells expressing the different constructs. AtMYB30 was detected only in nuclear fractions, whereas HA-tagged AtsPLA2s were consistently found in the supernatant fraction, from which nuclei had been removed (Fig. S5A). We next tested the specificity of the weak signal observed in nuclear fractions only when coexpressing AtMYB30 and AtsPLA2-α or AtsPLA2-α-H62Q (Fig. S5A). All AtsPLA2s were detected after anti-HA immunoprecipitation of nuclei-depleted fractions (Fig. S5B), whereas only AtsPLA2-α and AtsPLA2-α-H62Q, but not AtsPLA2-β, -γ, or -δ, were detected in nuclear fractions when coexpressed with AtMYB30 but not AtMYB123 (Fig. S5C). Moreover, AtMYB30 was specifically detected after anti-HA immunoprecipitation only in nuclear fractions containing AtsPLA2-α or AtsPLA2-α-H62Q (Fig. S5 B and C).
Our data demonstrate the specificity of AtMYB30-mediated AtsPLA2-α nuclear targeting and strongly suggest that AtsPLA2-α is able to interact with AtMYB30 in the nucleus.
AtsPLA2-α Physically Interacts with AtMYB30 in the Cell Nucleus.
The physical interaction between AtsPLA2-α and AtMYB30 in the nucleus was further tested by using a quantitative noninvasive Fluorescence Lifetime Imaging (FLIM) approach to monitor the Förster resonance energy transfer (FRET) between the CFP (donor) and YFPv (acceptor) molecules fused to AtMYB30 and AtsPLA2-α, respectively. If the two proteins interact, the transfer of energy from the CFP to the YFP decreases the fluorescence lifetime of the CFP fluorophore. A significant reduction of the average CFP lifetime was measured in nuclei coexpressing AtMYB30-CFP and AtsPLA2-α-YFPv, or the inactive mutant AtsPLA2-α-H62Q-YFPv, as compared with nuclei expressing AtMYB30-CFP alone (Table 1). These data show that AtsPLA2-α interacts with AtMYB30 in the nucleus and that this protein interaction does not depend on the enzymatic activity of AtsPLA2-α, in agreement with data obtained in yeast (Fig. S1B). When AtMYB30-CFP was coexpressed with the nuclear TF AtMYB123-YFPv, no protein interaction could be detected, as shown by an average CFP lifetime that is not significantly different from that of AtMYB30-CFP alone, confirming that reduction of AtMYB30-CFP lifetime in the presence of AtsPLA2-α-YFPv is not due to nonspecific transfer of energy between the two fluorophores. Although AtsPLA2-α is not relocalized to the nucleus by AtMYB30ΔMYB, both proteins colocalize in the cytoplasm (Fig. 1 G and H). However, no physical interaction between the two proteins was detected in the cytoplasm, as shown by an average CFP lifetime similar to that of the CFP alone (Table 1), indicating that the MYB domain of AtMYB30 is necessary for the interaction with AtsPLA2-α. Finally, no evidence of protein interaction between AtMYB30-CFP and AtsPLA2-β-YFPv, AtsPLA2-γ-YFPv, or AtsPLA2-δ-YFPv could be obtained, in agreement with the observation that neither of these proteins was relocalized to the nucleus in the presence of AtMYB30 (Fig. 1 C and D; Fig. S3). Together, these data provide strong evidence of a specific physical interaction between AtMYB30 and AtsPLA2-α in planta, independently of the enzymatic activity of AtsPLA2-α.
Table 1.
FLIM analysis shows that AtMYB30 interacts with AtsPLA2-α in the nucleus of N. benthamiana epidermal cells
| Donor | Acceptor | Lifetime* | SD | n† | E‡ | P value§ |
| AtMYB30-CFP | — | 2.306 | 0.030 | 60 | — | — |
| AtMYB30-CFP | AtsPLA2-α-YFPv | 2.067 | 0.024 | 46 | 10.4 | 4.0 x 10−8 |
| AtMYB30-CFP | AtsPLA2-α-H62Q-YFPv | 2.053 | 0.031 | 20 | 10.9 | 2.1 x 10−5 |
| AtMYB30-CFP | AtMYB123-YFPv | 2.278 | 0.041 | 31 | — | 0.58 |
| AtMYB30ΔMYB-CFP | — | 2.345 | 0.064 | 30 | — | — |
| AtMYB30ΔMYB-CFP | AtsPLA2-α-YFPv | 2.323¶ | 0.071 | 20 | — | 0.82 |
*Mean lifetime in nanoseconds.
†Total number of measured nuclei.
‡FRET efficiency percentage (E = 1 − τDA/τD) was calculated by comparing the lifetime of the donor in the presence of the acceptor (τDA) with its lifetime in the absence of the acceptor (τD).
§P value of the difference between the donor lifetimes in the presence and in the absence of the acceptor (Student's t test).
¶Values were measured in areas outside the nucleus where AtsPLA2-α-YFPv and AtMYB30ΔMYB colocalize.
AtsPLA2-α Negatively Regulates AtMYB30 Transcriptional Activity and HR Control in N. benthamiana.
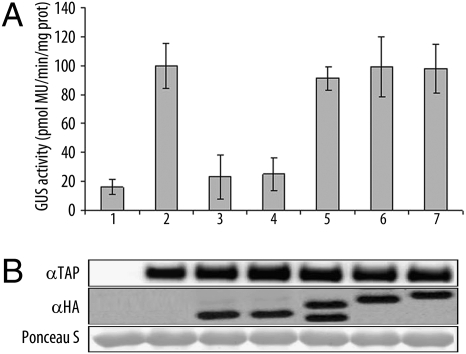
KCS1 (β-keto acyl-CoA synthase), involved in the biosynthesis of VLCFAs (14), was proposed as one of the target genes of AtMYB30 (7). We next tested the effect of AtsPLA2-α on AtMYB30-mediated transcriptional activation of the KCS1 promoter (KCS1p) fused to a GUS reporter gene. As reported, overexpression of AtMYB30 induced KCS1p transcriptional activation (Fig. 2A). Interestingly, when coexpressed with AtsPLA2-α, or the catalytic mutant AtsPLA2-α-H62Q, AtMYB30-mediated transcriptional activation of KCS1p was significantly reduced (Fig. 2A), indicating that the observed effect does not depend on AtsPLA2-α enzymatic activity. In contrast, no effect on KCS1p activation was observed when the other AtsPLA2 isoforms were coexpressed with AtMYB30, underlining the specific effect of AtsPLA2-α in the control of AtMYB30 transcriptional activity. Western blot confirmed equal protein expression (Fig. 2B).
Fig. 2.
AtsPLA2-α represses AtMYB30 transcriptional activity. Transient transactivation of the KCS1p by AtMYB30 in N. benthamiana leaves. Lanes: 1,KCS1p:GUS reporter alone or with: 2, AtMYB30-TAP; 3, AtMYB30-TAP+AtsPLA2-α-HA; 4, AtMYB30-TAP+AtsPLA2-α-H62Q-HA; 5, AtMYB30-TAP+AtsPLA2-β-HA; 6, AtMYB30-TAP+AtsPLA2-γ-HA; 7, AtMYB30-TAP+AtsPLA2-δ-HA. (A) Fluorimetric GUS assays in leaf discs 36 hpi. Mean and SEM values are calculated from eight independent experiments (two to four replicates per experiment). MU, methylumbelliferone. (B) Western blot showing expression of AtMYB30-TAP and HA-tagged AtsPLA2 constructs. Ponceau S staining confirms equal loading.
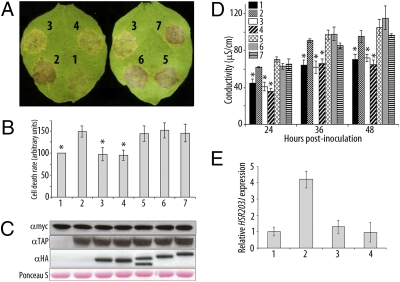
As shown in Arabidopsis and tobacco plants overexpressing AtMYB30 (6), AtMYB30-TAP overexpression in N. benthamiana accelerates and enhances the HR induced by the expression of the Pseudomonas effector AvrRpt2 (ref. 15; Fig. 3A), showing that TAP-tagged AtMYB30 retains its function as a positive regulator of the HR in N. benthamiana. Coexpression of AtsPLA2-α, or AtsPLA2-α-H62Q, with AtMYB30 has a negative effect on the development of the AvrRpt2-triggered HR, whereas the other AtsPLA2s do not affect HR progress, demonstrating that the negative effect of AtsPLA2-α on HR development is specific and independent of its enzymatic activity (Fig. 3A). These results were confirmed by quantification of the uptake of Evans blue and ion leakage measurements in leaf disk assays. In the presence of AvrRpt2, both the accumulation of Evans blue and conductivity values were significantly lower in leaves coexpressing AtMYB30 and AtsPLA2-α, or AtsPLA2-α-H62Q, but not AtsPLA2-β, -γ or -δ, as compared with leaves where AtMYB30 was expressed alone (Fig. 3 B and D). Western blot confirmed expression of all proteins (Fig. 3C). Finally, accumulation of HSR203J transcript, a well-known marker of HR cell death in tobacco (16), was significantly higher in N. benthamiana leaves coexpressing AvrRpt2 and AtMYB30 compared with those expressing AvrRpt2 (Fig. 3E). However, the AtMYB30-mediated overaccumulation of HSR203J was lost when AtsPLA2-α, or AtsPLA2-α-H62Q, were coexpressed with AtMYB30, providing molecular confirmation of the negative role played by AtsPLA2-α on the AtMYB30-mediated HR in N. benthamiana.
Fig. 3.
AtsPLA2-α negatively regulates HR development. Lanes: 1, AvrRpt2-myc alone or with: 2, AtMYB30-TAP; 3, AtMYB30-TAP+AtsPLA2-α-HA; 4, AtMYB30-TAP+AtsPLA2-α-H62Q-HA; 5, AtMYB30-TAP+AtsPLA2-β-HA; 6, AtMYB30-TAP+AtsPLA2-γ-HA; 7, AtMYB30-TAP+AtsPLA2-δ-HA. (A) HR development for each combination 3 dpi. Cell death was quantified by measuring the uptake of Evans blue 48 hpi (values are related to that of AvrRpt2-myc, which is set at 100%) (B) or electrolyte leakage at the indicated time points (D). Mean and SEM values are calculated from four independent experiments (three replicates per experiment). Statistical significance according to a Student's t test P value <10−7 is indicated by asterisks. (C) Western blot showing expression of AvrRpt2-myc, AtMYB30-TAP, and HA-tagged AtsPLA2 constructs. Ponceau S staining of the membrane confirms equal loading. (E) HSR203J transcript levels determined by Q-RT-PCR 48 hpi. Expression values are normalized by using the expression level of β-tubulin 4 and related to the value of HSR203J expression in leaves expressing AvrRpt2-myc, which is set at 1. Mean and SEM values are calculated from three independent experiments (three to five replicates per experiment).
To determine the functional role of AtsPLA2-α nuclear targeting, AtsPLA2-α was fused to a nuclear export signal (NES; ref. 17). As AtsPLA2-α, AtsPLA2-α-YFP-NES is localized in cytoplasmic vesicles 36 hpi (Fig. S6A). However, AtsPLA2-α-YFP-NES is not relocalized to the nucleus in the presence of AtMYB30, whereas a nonfunctional nes fusion (17) restored AtMYB30-mediated AtsPLA2-α nuclear targeting. Importantly, conductivity assays showed that, unlike the nes fusion, AtsPLA2-α fused to NES is not able to repress AvrRpt2-mediated HR progress in the presence of AtMYB30 (Fig. S6B), demonstrating that AtsPLA2-α nuclear relocalization is necessary to mediate negative regulation of AtMYB30-mediated HR.
AtsPLA2-α Negatively Regulates AtMYB30 HR and Defense Responses in Arabidopsis.
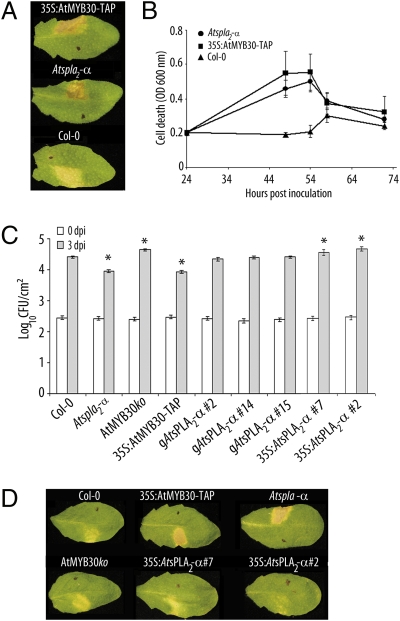
Genetic characterization of four candidate mutant lines of the SALK collection (http://signal.salk.edu) allowed us to identify one homozygous Atspla2-α knockout line (SALK_099415) containing a T-DNA insertion at position -43 of the predicted ORF that completely abolished AtsPLA2-α expression (Fig. S7 A and B). We first tested HR development in Atspla2-α mutant plants after inoculation with the bacterial strain Pseudomonas syringae pv tomato DC3000 carrying the avirulence gene AvrRpm1 (Pst AvrRpm1). In these assays, we used a bacterial inoculum of 2 × 106 cfu/mL to delay the timing of HR appearance and the magnitude of the response and allow clearer visualization of differential HR symptoms. Consistent with the results obtained in N. benthamiana, mutant Atspla2-α and 35S:AtMYB30-TAP plants overexpressing AtMYB30 showed clear HR development 64 hpi, whereas Col-0 only showed chlorosis symptoms when using these low inoculum conditions (Fig. 4A). This phenotype was quantified by measuring the uptake of Evans blue in leaf disk assays. The Evans blue coloration peaked earlier and reached higher values in the Atspla2-α and 35S:AtMYB30-TAP lines than in Col-0 (Fig. 4B), confirming the role of AtsPLA2-α as a negative regulator of cell death development in Arabidopsis. In addition, mutant Atspla2-α and 35S:AtMYB30-TAP lines, but not an AtMYB30ko mutant (18), were more resistant to inoculation with two bacterial strains, Pst AvrRpm1 and Pst AvrRps4, as shown by reduced in planta bacterial growth compared with Col-0 plants (Fig. 4C; Fig. S8).
Fig. 4.
Altered AtsPLA2-α expression modifies Arabidopsis HR and resistance to bacteria. (A and D) Symptoms developed by the indicated Arabidopsis lines 64 (A) or 50 (D) hpi with Pst AvrRpm1 at 2 × 106 cfu/mL; 1 cm2 area of the half leaf opposite to the black dot was infiltrated. Pictures are representative of three independent experiments in which five plants of each line were infiltrated. (B) Quantification of cell death by measuring the uptake of Evans blue in the indicated lines, after inoculation with Pst AvrRpm1 as indicated in A. Mean and SEM values are calculated from five replicates of one representative experiment out of three. (C) Growth of Pst AvrRpm1 in the indicated Arabidopsis lines 0 (white bars) and 3 dpi (gray bars) with a bacterial suspension of 5 × 105 cfu/mL. Mean bacterial densities are calculated from three independent experiments (six individual plants per experiment). *, statistical significant difference using multiple factor ANOVA (P < 0.05).
Genetic complementation of the Atspla2-α mutant was performed by using a genomic AtsPLA2-α clone (gAtsPLA2-α). Three independent homozygous gAtsPLA2-α complemented T2 lines displayed AtsPLA2-α gene expression and bacterial growth rates similar to those of wild-type plants after inoculation with Pst AvrRpm1 (Fig. S7; Fig. 4C), showing that increased resistance in Atspla2-α plants is causally related to loss of function of AtsPLA2-α.
To confirm the negative role played by AtsPLA2-α on AtMYB30-mediated response, Arabidopsis transgenic plants overexpressing a 35S:AtsPLA2-α−HA construct were generated. Expression of AtsPLA2-α was monitored by RT-PCR and Western blot in two T3 homozygous independent lines (Fig. S7). To better discriminate the phenotypes shown by the different lines, plants were inoculated by using Pst AvrRpm1 with a bacterial density of 2 × 106 cfu/mL. As shown before, Atspla2-α and 35S:AtMYB30-TAP plants developed stronger HR as compared with Col-0, whereas 35S:AtsPLA2-α-HA plants exhibited chlorosis symptoms similar to those developed by AtMYB30ko plants (Fig. 4D). Consistent with this observation, 35S:AtsPLA2-α-HA and AtMYB30ko plants were more susceptible to inoculation with Pst AvrRpm1, as shown by increased in planta bacterial growth compared with Col-0 (Fig. 4C), strongly indicating that the HA-tagged AtsPLA2-α construct is functional.
Together, our results demonstrate that AtsPLA2-α acts as a negative regulator of Arabidopsis HR and resistance.
Temporal and Spatial Analysis of AtsPLA2-α and AtMYB30 Expression.
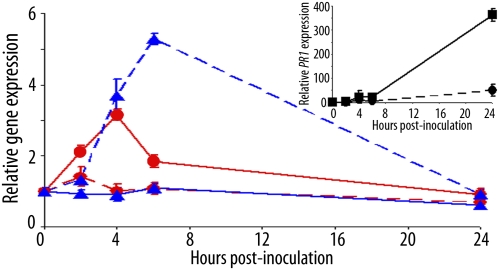
To gain further knowledge on the mode of action of AtsPLA2-α, wild-type Col-0 plants were inoculated with Pst AvrRpm1and leaf samples were harvested from areas both inside and immediately neighboring the infiltrated zone at different times after inoculation. AtMYB30 expression is specifically induced in the inoculated zone 4 hpi, whereas AtsPLA2-α expression peaks 6 hpi in the area immediately neighboring the inoculated zone (Fig. 5). In contrast, no modification of AtMYB30 or AtsPLA2-α expression was observed in the adjacent or inoculated zone, respectively. As expected for a marker of the plant defense response, the expression level of PR1 increased 24 hpi in the infiltrated area (Fig. 5 Inset). Our data indicate that the complementary character of AtsPLA2-α and AtMYB30 expression, from both a spatial and a temporal perspective, together with the negative control exerted by AtsPLA2-α on AtMYB30 activity, provide an efficient molecular mechanism that contributes to the necessary attenuation of the plant defense response.
Fig. 5.
AtMYB30 and AtsPLA2-α gene expression is spatially and temporally regulated. Col-0 plants were inoculated with Pst DC3000 (AvrRpm1) (5 × 107 cfu/mL) on a 0.4 cm2 leaf area. Leaf samples were harvested from areas both inside and immediately neighboring the infiltrated zone at the indicated time points. Relative expression of AtMYB30 (red circles) and AtsPLA2-α (blue triangles) in the inoculated (solid line) or in the adjacent (dashed line) zone was determined by Q-RT-PCR. (Inset) Relative PR1 gene expression in the infiltrated zone (■, solid) and in the adjacent zone (●, dashed) was also determined. The expression values were normalized by using SAND family and β-tubulin 4 genes as internal standards, and related to the value of each gene at time 0, which is set at 1. Mean and SEM values were calculated from three replicates of one representative experiment out of three.
Discussion
AtsPLA2-α, a Protein Partner for a Plant Transcription Factor, Negatively Regulates Plant Defense.
Physical or functional interactions between cytoplasmic PLA2s (cPLA2) and proteins involved in transcriptional regulation have been suggested in animal cells (19, 20). In contrast, besides the more classic examples of plant MYB TFs interacting with bHLH and WD40 repeat proteins to regulate secondary metabolism and epidermal cell fate identity (21), here we report the interaction between a phospholipase and a MYB protein.
Cellular functions of the four Arabidopsis sPLA2s are poorly characterized, although AtsPLA2-α and AtsPLA2-β have been respectively implicated in the control of auxin transport protein trafficking to the plasma membrane and cell elongation and shoot gravitropism (22, 23). The amino acid sequence, enzymatic properties, and expression pattern of AtsPLA2-α are distinguishable from those of the other three AtsPLA2 isoforms, suggesting that it may play a distinct role that differs from that of the other AtsPLA2s (24). Here, we show that the Arabidopsis TF AtMYB30 partially modifies the targeting of AtsPLA2-α from cytoplasmic vesicles to the nucleus, where both proteins interact. The observation that (i) AtMYB30 is not able to interact with the other three isoforms of the AtsPLA2 subfamily, which are expressed in the same cellular compartment and at similar levels than AtsPLA2-α, and (ii) AtsPLA2-α does not interact with the unrelated nuclear MYB TF AtMYB123 strongly suggest that the detected interaction between AtMYB30 and AtsPLA2-α is specific and not related to protein overexpression. Interestingly, our results are reminiscent of those obtained for a cytosolic animal PLA2 protein, which is relocalized to the nucleus upon interaction with the B-MYB TF, where it inhibits B-MYB-dependent target gene expression (20). Moreover, the authors postulated that cPLA2 would play a role in the inhibition of proliferation and cell death in the nucleus. Consistent with this hypothesis, we show that down-regulation of AtMYB30 transcriptional activity by AtsPLA2-α correlates with the inhibition of the plant hypersensitive cell death. The resemblance between both processes represents an example of the still poorly documented conservation of the mechanisms controlling cell death in plants and animals.
Participation of sPLA2s in host defense, either as major antibacterial factors or as biologically active molecules modulating immune or inflammatory responses, has been documented in animal cells (25, 26). Experimental evidence of the involvement of sPLA2s in plant defense is, in contrast, more limited. Similar to the eicosanoid pathway in animals, the plant octadecanoid pathway leads to the production of oxylipins and jasmonic acid, which play important roles in cell death and plant defense signaling against pathogens and herbivores (27, 28). Elicitor-induced PLA activity has been reported in a variety of plant pathogen interactions (29, 30). These and other studies suggest that PLA activity is involved in the plant response to pathogen elicitors, but the nature of its involvement remains unclear.
To our knowledge, this is the first report of direct involvement of a sPLA2 in the regulation of cell death and plant-resistance responses. Based on our data, we propose that AtsPLA2-α may control AtMYB30-mediated response through the physical interaction between the two proteins rather than through a lipid signal produced by AtsPLA2-α. In agreement with this assumption, new functions have been attributed to sPLA2s that do not require enzymatic activity in animal cells. For example, it has been reported that sPLA2s may serve as high-affinity ligands for cell surface receptors (31) or be able to bind proteoglycans with high affinity because of their overall positive charge (32). Although our knowledge of the cellular functions of plant PLAs is limited, our work suggests that, similar to animal PLAs, their functions may extend beyond those related to their catalytic activities.
Nuclear Translocation of AtsPLA2-α as Another Example of Protein Trafficking Toward the Nucleus for Defense-Associated Gene Regulation.
Accumulating evidence indicates that spatial restriction of defense regulators by the nuclear envelope and stimulus-induced nuclear translocation constitutes an important level of defense-associated gene regulation in plants. Some R proteins, defense regulators, and essential components of the Arabidopsis immune response traffic between the cytoplasm and the nucleus as part of a key process in plant innate immunity that provides a possible framework for defense signal trafficking between the two compartments (33, 34). Finally, cytoplasmic retention of inactive TFs or transcriptional regulators, and their activation and signal-dependent translocation into the nucleus, allows plants to rapidly connect signal perception at the cell surface, and cytoplasmic signal transduction, to defense gene activation in a stimulus-dependent manner (35).
At least one chloroplastic and two endomembrane proteins have been reported as being relocalized to the nucleus. NRIP1, a tobacco rhodanase sulfurtransferase that normally localizes to the stroma of choroplasts, is recruited to the cytoplasm and nucleus by the tobacco mosaic virus p50 effector in a process that, similar to AtsPLA2-α, is independent of the enzymatic activity of NRIP1 and important for plant cell death and defense responses (36). The Ralstonia solanacearum effector PopP2 induces nuclear targeting of the cysteine protease RESPONSIVE TO DEHYDRATATION19 (RD19), otherwise localized to mobile vacuole-associated vesicles and destined to the lytic vacuole (37). The tomato LeCp vacuolar protease acts as a nuclear TF following treatment with the EIX elicitor (38). Although, as in our case, details of the molecular mechanism behind the nuclear relocalization of these proteins remain unknown, these important findings strengthen the biological significance of our data. It has been proposed that EIX and PopP2 might trigger a vacuole membrane collapse leading to the respective release of LeCp and RD19 from vacuole-associated compartments into the cytoplasm, where they may become available for SUMOylation, thereby allowing their nuclear targeting. Similarly, AtMYB30 might release AtsPLA2-α from vesicle-associated compartments to the nucleus via an unknown mechanism that may or may not involve SUMOylation. Intriguingly, the SUMOplot program (www.abgent.com/tools/sumoplot) predicts two lysine residues in AtsPLA2-α (K81, K146) with high probability of being SUMOylated. The export of AtsPLA2-α may be similar to the release of procell death signals from the mitochondria, such as cytochrome c, during the initial steps of apoptosis in animal cells (39). Binding of AtMYB30 to AtsPLA2-α may mask an AtsPLA2-α vesicle targeting signal, or AtMYB30 may indirectly disrupt global vesicle sorting affecting AtsPLA2-α translocation. Alternatively, we cannot rule out that AtMYB30 may intercept AtsPLA2-α on its way to the extracellular space via retrograde transport from the endomembrane system, which has some continuity with the nuclear envelope.
AtsPLA2-α Regulates HR Development via Temporal and Spatial Control of AtMYB30 Activity.
Plant resistance to disease is a costly response, intimately connected to general plant developmental processes, which reduces the fitness of the host in the absence of disease (40). Mutants with constitutively active defense responses, or with HR lesion-mimic phenotypes, often show stunted growth and low fertility. Therefore, defense responses need to be tightly regulated to be not only efficient but also beneficial to the plant. A model that illustrates the complementary character of the expression pattern of AtsPLA2-α and AtMYB30 is presented in Fig. S9. In this model, AtsPLA2-α low expression level in the inoculated zone allows only weak repression of the activity of AtMYB30. In contrast, in the leaf area immediately neighboring the inoculated zone, AtsPLA2-α expression is high enough to act as an efficient negative regulator of AtMYB30 action by sequestering AtMYB30 and preventing it from activating its targets. According to this model, it is tempting to speculate that the complementary character of AtsPLA2-α and AtMYB30 expression, together with the negative control exerted by AtsPLA2-α on AtMYB30 activity, provide an efficient mechanism to restrict HR development to the inoculated zone, thereby preventing spreading of cell death throughout the leaf. However, Atspla2-α mutant plants do not show spreading HR after pathogen challenge, showing that the negative regulation exerted by AtsPLA2-α on AtMYB30 action is not the only factor controlling HR restriction to the inoculated zone. We thus propose that AtsPLA2-α represents an additional element contributing to the negative regulation of defense as part of the necessary to the fine-tuning of plant responses to pathogen attack to avoid deleterious effects to the host.
Materials and Methods
Y2H Screening.
An Arabidopsis thaliana Gal4 Y2H cDNA prey library (MatchMaker; Clontech) was generated from mRNA isolated from leaves of 4-wk-old Ws-4 plants infiltrated with the Xanthomonas campestris pv campestris 147 strain (7). An AtMYB30 version deleted from its C-terminal activation domain (amino acids 1–234) was used as bait for screening 2 × 106 independent transformants exhibiting His auxotrophy on selective plates.
Constructs.
Details of cloning procedures are provided as SI Materials and Methods.
Plant and Bacterial Materials.
Arabidopsis lines used in this study were in the Columbia background and grown in Jiffy pots under controlled conditions (18). The AtMYB30ko line was described (18). Transient expression in N. benthamiana leaves or Arabidopsis seedlings was performed as described (7, 41). Plant inoculation with Pst AvrRpm1 or Pst AvrRps4 and in planta bacterial growth analysis was performed as described (18). Data were submitted to a statistical analysis by using Statgraphics Centurion XV.II Professional Software (Statpoint Technologies). Normality of residues was verified by the Kolmogorov–Smirmov test. The effect of the genotype was tested by Multiple Factor ANOVA (P < 0.05).
RNA Extraction and Quantitative Real-Time-PCR (Q-RT-PCR) Analysis.
Q-RT-PCR was performed according to ref. 7. Primers used for Q-RT-PCR and further details are provided as SI Materials and Methods.
Protein Gel Blot Analysis.
Protein extracts were prepared as described (7). Details of the nuclear fractionation performed according to ref. 34 are provided as SI Materials and Methods. For detection of TAP-, YFP-, myc-, and HA-tagged proteins, blots were respectively incubated with rabbit PAP soluble complex (Sigma), mouse monoclonal anti-GFP IgG1 K (clones 7.1 and 13.1; Roche), mouse monoclonal anti-c-myc (clone 9E10; Roche), and rat monoclonal anti-HA (clone EF10; Roche) antibodies, linked to horseradish peroxidase (dilution 1:5,000). Anti-Hsc70 (plant ER, BIP) mouse monoclonal (1D9; Stressgen) was used at 1:2,000.
Fluorescence Microscopy and FLIM Analysis.
CFP and YFPv fluorescence was analyzed with a confocal laser scanning microscope as detailed in SI Materials and Methods. FLIM assays were performed as described (37). Statistical comparisons between control (donor) and assay (donor + acceptor) lifetime values were performed by using Student's t test.
Fluorimetric GUS Assays.
GUS activity was measured 36 hpi by using the substrate 4-methylumbelliferyl-β-d-glucuronide as described (7). After protein extraction, 1 μg of total protein was used in replicates to measure enzymatic activities of individual samples.
Quantification of Cell Death.
Cell death was quantified by monitoring the uptake of Evans blue (0.25%) by leaf discs from N. benthamiana leaves 48 hpi, following a described protocol (18). For Arabidopsis assays, five leaf discs (5-mm diameter) from each infiltrated plant were harvested at the indicated times points after infiltration of Pst AvrRpm1 at 2.106 cfu/mL For electrolyte leakage measurements, eight leaf discs (6-mm diameter) were harvested 24 hpi, washed, and incubated at room temperature in 10 mL of distilled water before measuring conductivity.
Supplementary Material
Acknowledgments
We thank Céline Remblière for help with plant transformation and Laurent Deslandes for helpful discussions. S.F. and J.C. were respectively funded by a grant from the Centre National de la Recherche Scientifique (Bourse Docteur-Ingénieur) and the French Ministry of National Education and Research. X.D. was funded by VILMORIN and Cie and Biogemma.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009056107/-/DCSupplemental.
References
- 1.Mur LA, et al. The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot. 2008;59:501–520. doi: 10.1093/jxb/erm239. [DOI] [PubMed] [Google Scholar]
- 2.Eulgem T. Regulation of the Arabidopsis defence transcriptome. Trends Plant Sci. 2005;10:71–77. doi: 10.1016/j.tplants.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Martin C, Paz-Ares J. MYB transcription factors in plants. Trends Genet. 1997;13:67–73. doi: 10.1016/s0168-9525(96)10049-4. [DOI] [PubMed] [Google Scholar]
- 4.Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol. 2001;4:447–456. doi: 10.1016/s1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- 5.Daniel X, Lacomme C, Morel JB, Roby D. A novel myb oncogene homologue in Arabidopsis thaliana related to hypersensitive cell death. Plant J. 1999;20:57–66. doi: 10.1046/j.1365-313x.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 6.Vailleau F, et al. A R2R3-MYB gene, AtMYB30, acts as a positive regulator of the hypersensitive cell death program in plants in response to pathogen attack. Proc Natl Acad Sci USA. 2002;99:10179–10184. doi: 10.1073/pnas.152047199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raffaele S, et al. A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis. Plant Cell. 2008;20:752–767. doi: 10.1105/tpc.107.054858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Six DA, Dennis EA. The expanding superfamily of phospholipase A(2) enzymes: Classification and characterization. Biochim Biophys Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 9.Munnik T, Testerink C. Plant phospholipid: “In a nutshell. J Lipid Res. 2009;50:S260–S265. doi: 10.1194/jlr.R800098-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu SB. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends Plant Sci. 2004;9:229–235. doi: 10.1016/j.tplants.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang X. Lipid signaling. Curr Opin Plant Biol. 2004;7:329–336. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Mansfeld J, Gebauer S, Dathe K, Ulbrich-Hofmann R. Secretory phospholipase A2 from Arabidopsis thaliana: Insights into the three-dimensional structure and the amino acids involved in catalysis. Biochemistry. 2006;45:5687–5694. doi: 10.1021/bi052563z. [DOI] [PubMed] [Google Scholar]
- 13.Nesi N, et al. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for Proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13:2099–2114. doi: 10.1105/TPC.010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruitt RE, et al. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proc Natl Acad Sci USA. 2000;97:1311–1316. doi: 10.1073/pnas.97.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mudgett MB, Staskawicz BJ. Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: Demonstration of secretion and processing during bacterial pathogenesis. Mol Microbiol. 1999;32:927–941. doi: 10.1046/j.1365-2958.1999.01403.x. [DOI] [PubMed] [Google Scholar]
- 16.Pontier D, et al. Identification of a novel pathogen-responsive element in the promoter of the tobacco gehe HSR203J, a molecular marker of the hypersensitive response. Plant J. 2001;26:495–507. doi: 10.1046/j.1365-313x.2001.01049.x. [DOI] [PubMed] [Google Scholar]
- 17.Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 18.Raffaele S, Rivas S, Roby D. An essential role for salicylic acid in AtMYB30-mediated control of the hypersensitive cell death program in Arabidopsis. FEBS Lett. 2006;580:3498–3504. doi: 10.1016/j.febslet.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Pawliczak R, et al. 85-kDa cytosolic phospholipase A2 mediates peroxisome proliferator-activated receptor gamma activation in human lung epithelial cells. J Biol Chem. 2002;277:33153–33163. doi: 10.1074/jbc.M200246200. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro S, et al. B-Myb-dependent regulation of c-Myc expression by cytosolic phospholipase A2. J Biol Chem. 2004;279:17715–17722. doi: 10.1074/jbc.M310561200. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay NA, Glover BJ. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, et al. Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell. 2003;15:1990–2002. doi: 10.1105/tpc.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee O, et al. Phospholipase A2 is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell. June 4, 2010 doi: 10.1105/tpc.110.074211. tpc.110.074211v1-tpc.110.074211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu SB, Lee HY, Doelling JH, Palta JP. Characterization of a cDNA encoding Arabidopsis secretory phospholipase A2-alpha, an enzyme that generates bioactive lysophospholipids and free fatty acids. Biochim Biophys Acta. 2005;1736:144–151. doi: 10.1016/j.bbalip.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Gronroos JO, et al. Roles of group IIA phospholipase A2 and complement in killing of bacteria by acute phase serum. Scand J Immunol. 2005;62:413–419. doi: 10.1111/j.1365-3083.2005.01678.x. [DOI] [PubMed] [Google Scholar]
- 26.Triggiani M, Granata F, Frattini A, Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta. 2006;1761:1289–1300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Blee E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002;7:315–322. doi: 10.1016/s1360-1385(02)02290-2. [DOI] [PubMed] [Google Scholar]
- 28.Katsir L, Chung HS, Koo AJ, Howe GA. Jasmonate signaling: A conserved mechanism of hormone sensing. Curr Opin Plant Biol. 2008;11:428–435. doi: 10.1016/j.pbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra S, Heinstein PF, Low PS. Activation of Phospholipase A by plant defense elicitors. Plant Physiol. 1996;110:979–986. doi: 10.1104/pp.110.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gobel C, et al. Oxylipin profiling reveals the preferential stimulation of the 9-lipoxygenase pathway in elicitor-treated potato cells. J Biol Chem. 2001;276:6267–6273. doi: 10.1074/jbc.M008606200. [DOI] [PubMed] [Google Scholar]
- 31.Boyanovsky BB, Webb NR. Biology of secretory phospholipase A2. Cardiovasc Drugs Ther. 2009;23:61–72. doi: 10.1007/s10557-008-6134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosengren B, et al. Secretory phospholipase A2 group V: Lesion distribution, activation by arterial proteoglycans, and induction in aorta by a Western diet. Arterioscler Thromb Vasc Biol. 2006;26:1579–1585. doi: 10.1161/01.ATV.0000221231.56617.67. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Coaker G. Nuclear trafficking during plant innate immunity. Mol Plant. 2008;1:411–422. doi: 10.1093/mp/ssn010. [DOI] [PubMed] [Google Scholar]
- 34.Feys BJ, et al. Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell. 2005;17:2601–2613. doi: 10.1105/tpc.105.033910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong X. NPR1, all things considered. Curr Opin Plant Biol. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Caplan JL, et al. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell. 2008;132:449–462. doi: 10.1016/j.cell.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernoux M, et al. RD19, an Arabidopsis cysteine protease required for RRS1-R-mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector. Plant Cell. 2008;20:2252–2264. doi: 10.1105/tpc.108.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matarasso N, Schuster S, Avni A. A novel plant cysteine protease has a dual function as a regulator of 1-aminocyclopropane-1-carboxylic Acid synthase gene expression. Plant Cell. 2005;17:1205–1216. doi: 10.1105/tpc.105.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Heil M. Ecological costs of induced resistance. Curr Opin Plant Biol. 2002;5:345–350. doi: 10.1016/s1369-5266(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 41.Marion J, et al. Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. Plant J. 2008;56:169–179. doi: 10.1111/j.1365-313X.2008.03596.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.