Abstract
It has been known for many decades that nonmammalian vertebrates detect light by deep brain photoreceptors that lie outside the retina and pineal organ to regulate seasonal cycle of reproduction. However, the identity of these photoreceptors has so far remained unclear. Here we report that Opsin 5 is a deep brain photoreceptive molecule in the quail brain. Expression analysis of members of the opsin superfamily identified as Opsin 5 (OPN5; also known as Gpr136, Neuropsin, PGR12, and TMEM13) mRNA in the paraventricular organ (PVO), an area long believed to be capable of phototransduction. Immunohistochemistry identified Opsin 5 in neurons that contact the cerebrospinal fluid in the PVO, as well as fibers extending to the external zone of the median eminence adjacent to the pars tuberalis of the pituitary gland, which translates photoperiodic information into neuroendocrine responses. Heterologous expression of Opsin 5 in Xenopus oocytes resulted in light-dependent activation of membrane currents, the action spectrum of which showed peak sensitivity (λmax) at ∼420 nm. We also found that short-wavelength light, i.e., between UV-B and blue light, induced photoperiodic responses in eye-patched, pinealectomized quail. Thus, Opsin 5 appears to be one of the deep brain photoreceptive molecules that regulates seasonal reproduction in birds.
Keywords: circadian rhythms, Japanese quail, photoperiodism, paraventricular organ, cerebrospinal fluid-contacting neuron
Animals living outside the tropics use changes in the photoperiod to adapt to seasonal changes in the environment. In addition to the photoreceptive retina and pineal organ, nonmammalian vertebrates are known to have additional photoreceptors that mediate seasonal changes in physiology and behavior (1). In 1911, Karl von Frisch first reported evidence for the existence of deep brain photoreceptors that mediate skin color changes in minnows (Phoxinus laevis) (2). Subsequently, Benoit demonstrated that blind ducks showed normal testicular growth in response to a long-day stimulus, whereas a black cap placed on the duck's head abolished this response (3). Menaker et al. demonstrated that injection of India ink under the scalp of a sparrow also abolishes testicular recrudescence, whereas pinealectomy does not interfere with this response (4). The Japanese quail (Coturnix japonica) is an excellent model to study these processes because of its rapid and robust response to changes in the photoperiod (5, 6). Removing the eyes and/or pineal gland from quail does not influence the photoperiodic response (7). In contrast, local illumination of the mediobasal hypothalamus (MBH) or septal region of the telencephalon leads to gonadal growth, suggesting the existence of deep brain photoreceptors in these regions (1, 8). In 1985, Foster et al. and Foster and Follett reported an action spectrum for the photoperiodic responses of quail and suggested the possible involvement of rhodopsin-like photoreceptors (9, 10). Indeed, gene expression and immunoreactivity for rhodopsin have been reported in the septal region of the pigeon (11, 12). Surprisingly, in several studies, rhodopsin-like immunoreactivity has not been successfully detected in the quail (13, 14). Recently, mRNA expression of melanopsin (Opn4) in the chick septal region and VA opsin-like immunoreactivity in the chicken and quail anterior hypothalamus have been reported, and these opsins are suggested to be candidate deep brain photoreceptors (15, 16). However, the functional significance of these candidate photopigments remains unknown, along with the identity of the deep brain photoreceptors.
In this study, we identified expression of a novel mammalian neural tissue opsin (Opsin 5) within the quail MBH. Functional characterization demonstrated that Opsin 5 is a violet-sensitive photopigment. Furthermore, short-wavelength light induced testicular growth in eye-patched, pinealectomized quail.
Results
Localization of Opsin 5 in the PVO and Its Projections to the External Zone of the Median Eminence.
The recently available chicken genome sequence has facilitated comprehensive expression analyses in quail, because these species are both galliformes with high interspecies DNA sequence conservation (17). To identify opsin genes that are expressed in the MBH and/or the septal region, we first performed a database search to identify DNA sequences of opsin superfamily members; these sequences were used to create 33P-labeled oligonucleotide probes for in situ hybridizations (Table S1). Among the 12 opsin genes examined, the expression of only OPN5 (Opsin 5: also known as Gpr136, Neuropsin, PGR12 and TMEM13) was detected in the PVO (Fig. 1A and Fig. S1). We did not detect the expression of any of the examined genes in the septal region (Fig. S1). The PVO is composed of three layers of neurons arranged in parallel to the surface of the third ventricle (18). The neuronal perikarya of the first layer are located either in the ependyma or just underneath it (i.e., the subependyma). The perikarya of the second and third neuronal layer are located farther away from the ependyma. OPN5 expression in the PVO was further confirmed using a digoxigenin (DIG)–labeled riboprobe, which produced a strong signal in the subependymal layer of the PVO (Fig. 1B and Fig. S2).
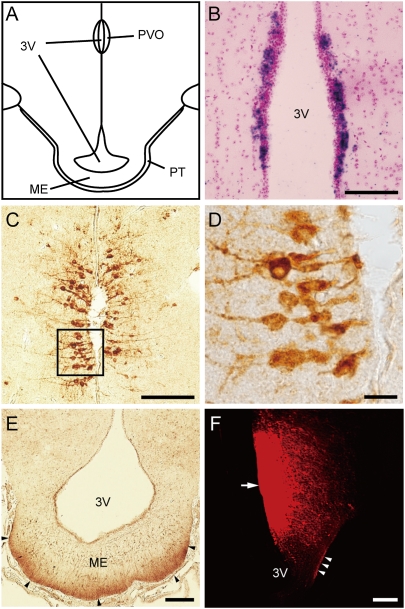
Fig. 1.
Localization of Opsin 5 in the PVO and its projections to the external zone of the median eminence. (A) Schematic drawing of the quail mediobasal hypothalamus. (B) In situ hybridization of OPN5 mRNA in the PVO. (C–E) Representative image of Opsin 5-like immunoreactivity in PVO neurons that contact the CSF (C and D) and fibers in the external zone of median eminence (ME) (arrowhead) adjacent to the pars tuberalis (PT) of the pituitary gland (E). Note that the angles of the sections are slightly different between B and C (Fig. S7). (F) The neural tracer DiI was applied to the PVO (arrow) and labeled fibers in the external zone of the ME (arrowheads). (Scale bars: 10 μm, D; 100 μm, B, C, and E; and 200 μm, F.) 3V, third ventricle.
Of note, bipolar subependymal neurons in the PVO that contact the cerebrospinal fluid (CSF) have been considered as candidate deep brain photoreceptors for several decades, because these neurons structurally resemble modified pineal photoreceptors and photoreceptor cells in the developing retina (19). To further detail the localization of Opsin 5, we generated various anti-quail Opsin 5 antibodies. Among them, one that recognized a C-terminal region of Opsin 5 was highly specific for quail Opsin 5. Western blot analysis of quail MBH extracts revealed an immunoreactive band of the expected size (∼42 kDa) (Fig. S3A). The ∼1.9-kDa synthetic Opsin 5 peptide used to produce the antibody was also detected (Fig. S3A). Most of the cells labeled with this antibody were CSF-contacting neurons in the first layer (i.e., neurons with a cell body localized in the subependymal layer and dendritic process with a knob-like terminal that penetrates the ependyma) and a few immunopositive cells were also observed in the second layer (Fig. 1 C and D and Fig. S3C). These immunopositive signals were eliminated after the antibody was preincubated with the synthetic Opsin 5 peptide (Figs. S3 B and D). We also observed Opsin 5-positive fibers in the external layer of the median eminence adjacent to the pars tuberalis of the pituitary gland (Fig. 1E). Recently, long-day induction of TSH expression in the pars tuberalis was shown to trigger the photoperiodic response, and the pars tuberalis was reported to be the key organ that relays photoperiodic information to a neuroendocrine output (17, 20, 21). To examine neural projections in this area, a neural tracer (DiI) was applied to the PVO. DiI-labeled fibers were observed in the external zone of the median eminence (Fig. 1F and Fig. S4). Interestingly, projections from the PVO to the median eminence and the pituitary gland have been reported in several other species (18).
Functional Characterization of Opsin 5 as a Photopigment.
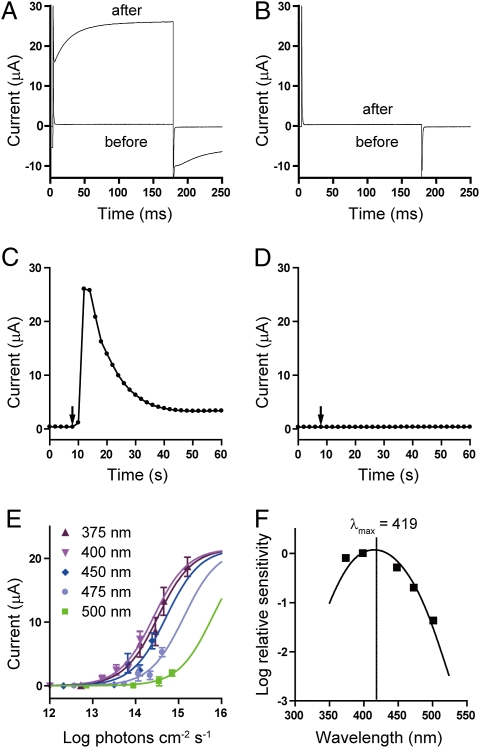
Vertebrate rhodopsin and cone opsins signal through transducin (Gt), whereas many invertebrate opsins signal through Gq protein. Sequence analysis of the opsin superfamily has shown that Opsin 5 is related to invertebrate opsins (22, 23). In addition, we detected the expression of the Gq protein (GNAQ) gene in the PVO (Fig. S5). We therefore predicted that Opsin 5 signals through Gq protein. Xenopus oocytes are known to possess an endogenous Ca2+-Cl− channel that is activated through Gq protein. To test the ability of Opsin 5 to form a functional sensory photopigment, we expressed quail Opsin 5 in Xenopus oocytes and analyzed the resulting current under voltage-clamp conditions (24). Illumination with bright white light (> 1,000 lx) induced light-dependent activation of membrane currents in oocytes injected with OPN5 5′-capped cRNA (Fig. 2 A and C). This photocurrent was not observed in noninjected oocytes (Fig. 2 B and D). These results clearly demonstrated that Opsin 5 is a functional photopigment. We next examined the spectral sensitivity of the Opsin 5-mediated photocurrent in oocytes. The effects of monochromatic light pulses were examined and the irradiance–response relationships for different wavelengths were measured (Fig. 2E). Relative quantum sensitivity to each wavelength was determined from the half-maximum response and an action spectrum was generated (25, 26). The action spectrum closely matched that predicted for a retinal1-based pigment with a λmax of ≈420 nm (419 nm and 418 nm in two independent experiments) (Fig. 2F).
Fig. 2.
Functional characterization of Opsin 5 as a photopigment. Current recordings in Opsin 5-expressing oocytes (A) and noninjected oocytes (B) before and after light exposure. (C and D) The time course of the response is shown by plotting the current amplitudes measured at the end of the depolarizing pulses over time. Arrows indicate the timing of light exposure. (E) Irradiance-response curves for monochromatic light pulses. Bars indicate mean ± SEM (n = 3–7). (F) Action spectrum for the Opsin 5-mediated photocurrent. Half-activation values derived from sigmoidal fits of irradiance-response curves were plotted versus the wavelength and fit with a curve for a retinal1-based photopigment.
Effects of Short-Wavelength Light on Testicular Growth in Eye-Patched, Pinealectomized Quail.
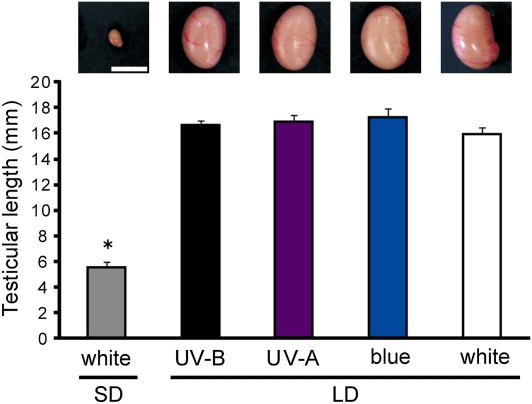
We examined testicular growth in eye-patched, pinealectomized quail exposed to 2 wk of photostimulation with UV-B, UV-A, or blue light (Fig. S6). The light intensity for each wavelength (UV-B, 0.2 mW/cm2; UV-A, 2 mW/cm2; blue, 1 mW/cm2) was less than that observed under direct sunlight in Japan, the natural habitat of the wild quail (UV-B, ∼0.2 mW/cm2; UV-A, ∼5 mW/cm2; blue, ∼13 mW/cm2). As shown in Fig. 3, long-day stimulus with each of the short wavelengths induced testicular growth, the magnitude of which was similar to that observed in birds exposed to white light (600 lx).
Fig. 3.
Effects of short-wavelength light on testicular growth in eye-patched, pinealectomized quail. Quail were subjected to 2 wk of photostimulation with UV-B, UV-A, blue, or white light, and testicular growth was examined. Values are mean + SEM (P < 0.01, ANOVA, F4,37 = 72.6, n = 7–10; *P < 0.01, Scheffé post hoc test). (Scale bar, 1 cm.) LD, long-day; SD, short-day.
Discussion
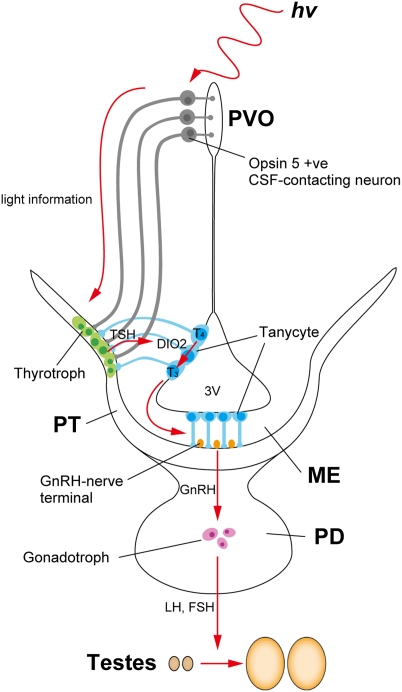
In this paper, we have described the identification of a previously uncharacterized photoreceptive molecule, Opsin 5, in CSF-contacting neurons of the PVO. We also found projection of Opsin 5 neuron to the external zone of the median eminence adjacent to the pars tuberalis of the pituitary gland. Thus, we propose that the Opsin 5-positive PVO neurons comprise one of the long-sought deep brain photoreceptors that mediate seasonal reproduction in birds (Fig. 4).
Fig. 4.
Model of photoperiodic signal transduction pathway in birds. Light detected by Opsin 5-positive PVO neurons that contact the CSF is transmitted to the pars tuberalis (PT) of the pituitary gland and induces thyroid-stimulating hormone (TSH) expression in the PT. PT TSH induces expression of type 2 deiodinase (DIO2) in tanycytes lining the ventrolateral walls of the third ventricle (3V) (17). DIO2 converts prohormone T4 to bioactive T3 (6). Long-day–induced T3 in the MBH causes morphologic changes in GnRH nerve terminals and glial processes and induces GnRH secretion.
Our present findings may have implications for evolutionary and developmental biology, because the retinal and pineal photosensory cells structurally resemble these neurons—e.g., they send dendritic processes into the pineal lumen and the photoreceptor space of the retina, and both are derived from diverticles of the third ventricle of the diencephalon (18). Although immunoreactivities for most of the opsins are generally observed in the photoreceptor outer segments or cilia-like structures of the modified photoreceptor cells, immunoreactivity of Opsin 5 was also observed in the axons of the external zone of the median eminence as well as in the cell bodies of the PVO. As Opsin 5 appears to activate the Ca2+-Cl− channel via the Gq protein, it is possible that not only Opsin 5 localized in the cell bodies but also Opsin 5 localized in the axons activate membrane currents. It is well established that the CSF-contacting neurons in the PVO contain serotonin or dopamine (18). Neurotransmitters used by Opsin 5 neurons remain to be identified in the future.
Photons with longer wavelengths are generally thought to penetrate into the brain more effectively than photons with shorter wavelengths (10, 27, 28), which seems to contradict our results. Foster et al., Foster and Follett, and Halford et al. reported an action spectrum for the photoperiodic response in quail with a λmax of ≈483–492 nm (9, 10, 16). Careful analysis of their results, however, revealed that light with a wavelength of 410 nm also caused a photoperiodic response. Furthermore, Oishi used a large spectrograph (National Institute for Basic Biology) to show that light with a wavelength of 350 nm also induced testicular growth (29). This result seems reasonable because UV light reaches the quail hypothalamus (10, 28), and feathers, skin, bone, and brain tissue are known to autofluoresce in the range from UV to blue light in response to UV irradiation (30–32). Although another unidentified UV photoreceptor may be present in the quail brain, Opsin 5 in the PVO appears to cover a relatively wide range of short-wavelength light from UV to blue light. The effects of short-wavelength light on avian photoperiodism have not been extensively examined because of the low transmission efficiency. However, it is reasonable to hypothesize that birds also use short-wavelength light in their natural habitats, because UV and blue light are known to regulate photoperiodism in mammals and insects (33, 34).
Although we failed to detect opsin gene expression in the septal region, it is important to bear in mind that local illumination of the septal region of the telencephalon also induces testicular growth (1, 8). Because nucleotide sequences are higly conserved between chicken and quail, chicken and quail probes are widely used for both species in methods such as Northern hybridization, Southern hybridization, in situ hybridization, chromosomal mapping using FISH, and microarrays (17, 35–40). Therefore, we used chicken DNA sequences for in situ hybridization with 33P-labeled riboprobes. However, we cannot exclude the possibility that some of the chicken probes did not work in quail. In addition, it is reported that expression of some opsin genes is rhythmically regulated (15, 41). Because we collected brains in the middle of the day, the expression of some genes may have been too low for detection at that time point. It is also possible that the density of opsin-expressing cells and/or the levels of opsin mRNA are below the limits of detection with our current methods. Multiple photoreceptors are likely involved in the photoperiodic response, as has been reported for circadian photoreception, which involves both visual photoreceptors (rod and cones) and nonvisual photoreceptors (melanopsin) (42, 43). Although rhodopsin-like immunoreactivity has been observed in the pigeon septal region (11, 12), multiple independent studies failed to detect similar signals in the quail brain (13, 14). Expression of melanopsin has been demonstrated in the lateral septal region as well as the retina and pineal gland in chicks (15). Recently, vertebrate ancient (VA) opsin-like immunoreactivity was detected in the avian anterior hypothalamus (16). Although spectral sensitivities of avian melanopsin and VA opsin remain to be determined, both mammalian melanopsin and teleost VA opsin have a λmax of ∼480 nm (44, 45). This λmax is consistent with the action spectrum for photoinduced LH release, suggesting possible involvement of melanopsin and VA opsin in the photoperiodic response. However, neural projections from melanopsin- and VA opsin- expressing neurons to the pars tuberalis, and their physiological significance remains to be clarified. It is particularly noteworthy that local illumination of MBH, including PVO, induces testicular growth, and PVO lesions block the photoperiodic response of the gonads; this highlights the physiological role of the PVO in the regulation of seasonal reproduction (8, 46). The gene-targeting technique is still unavailable for research in avian species. Analyses of the effect of Opsin 5 and/or melanopsin and/or VA opsin knock-outs on the photoperiodic response are required for conclusive identification of avian deep brain photoreceptors in future.
The eye is believed to be the only photoreceptive organ in mammals. The mammalian ortholog of OPN5 is reportedly expressed in the neural tissues (retina, brain, and spinal cord) and the testis (22). The photoresponsiveness and physiological roles of mammalian Opsin 5 should be addressed in future studies.
Materials and Methods
Animals.
Japanese quail (Coturnix japonica) and Xenopus laevis were used in this study, which was approved by the Animal Experiment Committee of Nagoya University and the National Institute for Physiological Sciences.
In Situ Hybridization.
Nonperfused frozen sections were examined with 33P-labeled oligonucleotide probes as previously described (40). The probe sequences are shown in Table S1. Paraffin sections were examined with DIG-labeled riboprobes as previously described (47). The entire ORF of OPN5 was used to generate the DIG-labeled riboprobe. BM purple AP substrate (Roche) was used to visualize hybridization, which was followed by counterstaining with nuclear fast red (Merck).
Antibodies.
We generated rabbit polyclonal antibodies against a C-terminal peptide from quail Opsin 5 (CISSHRDSAALSETQLEV). Purified peptides (ΔOpsin 5-KLH) were dissolved in 8 M urea containing 1,3-diaza-2,4-cyclopentadiene (imidazole) and used as an immunogen. After injecting the immunogen into each rabbit six times, blood was collected and IgG molecules were purified using peptide-Sepharose affinity chromatography. Purified antibodies were dialyzed in PBS and concentrated. IgG in PBS containing 50% glycerol was stored at –30 °C. Antibodies were diluted at 1:500–5,000 for Western blotting and 1:100–4,000 for immunohistochemistry.
Western Blotting.
Western blotting was performed as previously reported (48). Brain slices (3 mm) were generated by a mouse brain matrix (ASI Instruments), and the MBH, including the PVO (2.5-mm diameter), was punched out (6). Membranes were incubated with anti-quail Opsin 5 antibodies (1:5,000; final concentration of 0.278 μg/mL). To ascertain the specificity of the immunostaining, immunoadsorption analysis was performed with a solution in which the molar ratio of anti-quail Opsin 5 antibodies and the indicated antigen peptides was 1:20.
Immunohistochemistry.
Immunohistochemistry was performed using anti-quail Opsin 5 antibodies (1:1,000) and a Vectastain Elite ABC rabbit IgG kit (Vector Laboratories) with a standard protocol (48).
Carbocyanine Dye (DiI) Experiments.
Following deep anesthesia with pentobarbital, quail were perfused with 0.75% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brain was postfixed in fresh fixative for 2 d. After postfixation, the brain was cut in half along the midsagittal plane with a razor blade. A small carbocyanine dye (1,1'-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate: DiI) crystal (Molecular Probes) was placed onto the paraventricular organ. The insertion site was covered with 5% gelatin and stored in the same paraformaldehyde fixative at 37 °C in darkness for 14 d. Brains were then sectioned coronally using a microslicer (D.S.K., DTK-3000) at 50 μm. Sections were observed under a BZ-9000 microscope (Keyence).
Preparation of Xenopus Oocytes.
Xenopus oocytes were collected from frogs anesthetized in water containing 0.15% tricaine (24). 5′-capped cRNA was prepared from quail OPN5 inserted in the pGEMHE vector using an in vitro transcription kit (mMESSAGE mMACHINE kit, Ambion), and the yield was estimated by loading cRNA onto an agarose gel. The isolated oocytes were treated with 2 mg/mL collagenase type 1 (Sigma-Aldrich) for 6 h to remove follicular cells. Oocytes were injected with 55 nL cRNA solution and incubated in standard frog saline [88 mM NaCl, 1 mM KCl, 0.3 mM Ca(NO3)2, 0.41 mM CaCl2, 0.8 mM MgSO4, 2.4 mM NaHCO3, and 15 mM Hepes at pH 7.6] at 17 °C overnight in a dark box.
Electrophysiology.
Oocytes were positioned and impaled for recordings under dim light,and recordings were carried out under dark conditions. Oocytes were incubated in 40 μM 11-cis retinal (a gift from R.K. Crouch, Medical University of South Carolina, Charleston, SC) in standard frog saline supplemented with 0.1% penicillin–streptomycin (Sigma-Aldrich) for 1 h in a dark box at 17 °C before recording. Recordings were made in Ca2+-free saline (96 mM NaCl, 2 mM KCl, 3 mM MgCl2, and 5 mM Hepes at pH 7.4) to reduce the basal Ca2+-Cl− current (24). The Ca2+-Cl− current was monitored by applying a 175-ms depolarizing pulse to 60 mV from a holding potential of –80 mV every 2 s and light irradiation began 8 s after recording was initiated.
Spectral Analysis.
Monochromatic light was produced using an interference filter (half-bandwidth, 7.0–14.2 nm; Vacuum Optics Co.) and the light intensity was adjusted using neutral density filters (25). A halogen lamp (NPI PCS-UNX; 400, 450, 475, and 500 nm) and UV LED (375 nm; Nitride Semiconductors Co.) were used as light sources. Light intensities (μW/cm2) were measured using a radiometer (S371, UDT Instruments; UVR-300, Topcon). Measurements at 375 nm were fitted to the Hill function using the least-squares method, wherein the mean amplitude (22 μA) of the saturated response evoked by full-strength white light was used as the peak value for the fitting. Based on the parallel nature of these curves, the Hill coefficient for the data collected at 375 nm was used for curve fitting data obtained for other wavelengths (25). Relative quantum sensitivity to each wavelength was determined from the half-maximum response and fit with a retinal1-based photopigment template function (26).
Effect of Short-Wavelength Light on Testicular Growth in Eye-Patched, Pinealectomized Quail.
Four-week-old male quail were kept under short-day conditions (6 h/18 h light/dark cycle) for 4 wk in light-tight boxes. At 7 wk of age, the pineal gland was removed. At 8 wk of age, both eyes from each pinealectomized quail were covered with eye patches to block light. Eye patches were made by cutting the adhesive part of a Band-Aid (10 mm in diameter), and pasting black tape on the nonadhesive side. After feathers from the area surrounding the eye were removed, rubber cement was applied both around the eye and around the edge of the patch, and the patch was placed over the eye. These quail had no difficulty feeding. To assess the effects of 2 wk of photostimulation on testicular growth, long-day quail were transferred from short-day to long-day conditions (20 h/4 h light/dark cycle) for 2 wk, whereas short-day quail were kept under short-day conditions. Photic stimuli were produced using low-pressure mercury vapor fluorescent lamps (Philips UV-B, TL/12; UV-A, TL/08; blue, TL/52) (Fig. S6). The light intensity for each wavelength (UV-B, 0.2 mW/cm2; UV-A, 2 mW/cm2; blue, 1 mW/cm2) was less than that observed under direct sunlight in Japan, the natural habitat of the wild quail (UV-B, ∼0.2 mW/cm2; UV-A, ∼5 mW/cm2; blue, ∼13 mW/cm2).
Supplementary Material
Acknowledgments
We thank Nobuhiro Nakao, Naoki Takai, Tsuyoshi Shimmura, Yuta Hoshino, Itaru Murayama, Eriko Takarada, Tomomi Yamamoto, Mamiko Sukeno, Hideki Abe, Hideki Ukai, and Hiroki R. Ueda for technical support and helpful discussions. We are grateful to R. K. Crouch for the gift of 11-cis retinal. We thank Nagoya University Radioisotope Center for use of facilities. This work was supported in part by Grant-in-Aid for Young Scientists (S) 19678002, Grant-in-Aid for Scientific Research on Innovative Areas, ‘Regulatory Mechanism of Gamete Stem Cells’ 21116504, Japan Society for the Promotion of Science Research Fellow, the Global COE program “Advanced Systems-Biology” from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Mitsubishi Foundation, and Toyoaki Scholarship Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB547151).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006393107/-/DCSupplemental.
References
- 1.Oliver J, Bayle JD. Brain photoreceptors for the photoinduced testicular response in birds. Experientia. 1982;38:1020–1029. doi: 10.1007/BF01955346. [DOI] [PubMed] [Google Scholar]
- 2.von Frisch K. Beitrage zur physiologie der pigmentzellen in der fischhaut. Pflugers Arch Gesamte Physiol Menschen Tiere. 1911;138:319–387. [Google Scholar]
- 3.Benoit J. Le role des yeux dans l'action stimulante de la lumiere sure le developpement testiulaire chez le canard. CR Soc Biol (Paris) 1935;118:669–671. [Google Scholar]
- 4.Menaker M, Roberts R, Elliott J, Underwood H. Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci USA. 1970;67:320–325. doi: 10.1073/pnas.67.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Follett BK, King VM, Meddle SL. In: Biological Rhythms and Photoperiodism in Plants. Lumsden PJ, Miller AJ, editors. Oxford: BIOS; 1998. pp. 231–242. [Google Scholar]
- 6.Yoshimura T, et al. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- 7.Siopes TD, Wilson WO. Extraocular modification of photoreception in intact and pinealectomized coturnix. Poult Sci. 1974;53:2035–2041. doi: 10.3382/ps.0532035. [DOI] [PubMed] [Google Scholar]
- 8.Homma K, Ohta M, Sakakibara Y. In: Biological Rhythms and Their Central Mechanism. Suda M, Hayaishi O, Nakagawa H, editors. Amsterdam: Elsevier; 1979. pp. 85–94. [Google Scholar]
- 9.Foster RG, Follett BK, Lythgoe JN. Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature. 1985;313:50–52. doi: 10.1038/313050a0. [DOI] [PubMed] [Google Scholar]
- 10.Foster RG, Follett BK. The involvement of a rhodopsin-like photopigment in the photoperiodic response of the Japanese quail. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157:519–528. [Google Scholar]
- 11.Silver R, et al. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- 12.Wada Y, Okano T, Adachi A, Ebihara S, Fukada Y. Identification of rhodopsin in the pigeon deep brain. FEBS Lett. 1998;424:53–56. doi: 10.1016/s0014-5793(98)00138-0. [DOI] [PubMed] [Google Scholar]
- 13.Foster RG, Korf HW, Schalken JJ. Immunocytochemical markers revealing retinal and pineal but not hypothalamic photoreceptor systems in the Japanese quail. Cell Tissue Res. 1987;248:161–167. doi: 10.1007/BF01239977. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa T, Oishi T. Extraretinal photoreception and circadian systems in nonmammalian vertebrates. Comp Biochem Physiol. 1998;119B:65–72. [Google Scholar]
- 15.Chaurasia SS, et al. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): Differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 16.Halford S, et al. VA opsin-based photoreceptors in the hypothalamus of birds. Curr Biol. 2009;19:1–7. doi: 10.1016/j.cub.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 17.Nakao N, et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452:317–322. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 18.Vigh B, Vigh-Teichmann I. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc Res Tech. 1998;41:57–83. doi: 10.1002/(SICI)1097-0029(19980401)41:1<57::AID-JEMT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 19.Vigh-Teichmann I, Röhlich P, Vigh B, Aros B. Comparison of the pineal complex, retina and cerebrospinal fluid contacting neurons by immunocytochemical antirhodopsin reaction. Z Mikrosk Anat Forsch. 1980;94:623–640. [PubMed] [Google Scholar]
- 20.Ono H, et al. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA. 2008;105:18238–18242. doi: 10.1073/pnas.0808952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanon EA, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 22.Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): A novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 23.Koyanagi M, et al. Jellyfish vision starts with cAMP signaling mediated by opsin-G(s) cascade. Proc Natl Acad Sci USA. 2008;105:15576–15580. doi: 10.1073/pnas.0806215105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubo Y, Miyashita T, Murata Y. Structural basis for a Ca2+-sensing function of the metabotropic glutamate receptors. Science. 1998;279:1722–1725. doi: 10.1126/science.279.5357.1722. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimura T, Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+)mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- 26.Lamb TD. Photoreceptor spectral sensitivities: Common shape in the long-wavelength region. Vision Res. 1995;35:3083–3091. doi: 10.1016/0042-6989(95)00114-f. [DOI] [PubMed] [Google Scholar]
- 27.Hartwig HG, van Veen T. Spectral characteristics of visible radiation penetrating into the brain and stimulating extraretinal photoreceptors. J Comp Physiol. 1979;130:277–282. [Google Scholar]
- 28.Oishi T, Ohashi K. Effects of wavelength of light on the photoperiodic gonadal response of blinded-pinealectomized Japanese quail. Zoolog Sci. 1993;10:757–762. [Google Scholar]
- 29.Oishi T. Spectral sensitivity of the encephalic photoreceptor in the photoperiodic-gonadal response of Japanese quail. Doubutsugaku Zasshi. 1981;90:511. [Google Scholar]
- 30.Bachman CH, Ellis EH. Fluorescence of bone. Nature. 1965;206:1328–1331. doi: 10.1038/2061328a0. [DOI] [PubMed] [Google Scholar]
- 31.Kodrnja D. Fluorescence properties of biological tissues. Periodicum Biologorum. 1982;84:33–37. [Google Scholar]
- 32.Rost FWD. Fluorescence Microscopy. Vol. 2. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- 33.Brainard GC, Vaughan MK, Reiter RJ. Effect of light irradiance and wavelength on the Syrian hamster reproductive system. Endocrinology. 1986;119:648–654. doi: 10.1210/endo-119-2-648. [DOI] [PubMed] [Google Scholar]
- 34.Lees AD. Action spectra for the photoperiodic control of polymorphism in the aphid Megoura viciae. J Insect Physiol. 1981;27:761–771. [Google Scholar]
- 35.Noce T, et al. Molecular cloning and nucleotide sequence analysis of the putative cDNA for the precursor molecule of the chicken LH-β subunit. J Mol Endocrinol. 1989;3:129–137. doi: 10.1677/jme.0.0030129. [DOI] [PubMed] [Google Scholar]
- 36.Hanks MC, Alonzi JA, Sharp PJ, Sang HM. Molecular cloning and sequence analysis of putative chicken prolactin cDNA. J Mol Endocrinol. 1989;2:21–30. doi: 10.1677/jme.0.0020021. [DOI] [PubMed] [Google Scholar]
- 37.Boswell T, Millam JR, Li Q, Dunn IC. Cellular localization of neuropeptide Y mRNA and peptide in the brain of the Japanese quail and domestic chicken. Cell Tissue Res. 1998;293:31–38. doi: 10.1007/s004410051095. [DOI] [PubMed] [Google Scholar]
- 38.Nakao N, et al. Possible involvement of organic anion transporting polypeptide 1c1 in the photoperiodic response of gonads in birds. Endocrinology. 2006;147:1067–1073. doi: 10.1210/en.2005-1090. [DOI] [PubMed] [Google Scholar]
- 39.Shibusawa M, et al. A comparative cytogenetic study of chromosome homology between chicken and Japanese quail. Cytogenet Cell Genet. 2001;95:103–109. doi: 10.1159/000057026. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura T, et al. Molecular analysis of avian circadian clock genes. Brain Res Mol Brain Res. 2000;78:207–215. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 41.Pierce ME, et al. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- 42.Panda S, et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 43.Hattar S, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu X, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 45.Jenkins A, et al. VA opsin, melanopsin, and an inherent light response within retinal interneurons. Curr Biol. 2003;13:1269–1278. doi: 10.1016/s0960-9822(03)00509-8. [DOI] [PubMed] [Google Scholar]
- 46.Sharp PJ, Follett BK. The effect of hypothalamic lesions on gonadotrophin release in Japanese quail (Coturnix coturnix japonica) Neuroendocrinology. 1969;5:205–218. doi: 10.1159/000121861. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida S, et al. Sgn1, a basic helix-loop-helix transcription factor delineates the salivary gland duct cell lineage in mice. Dev Biol. 2001;240:517–530. doi: 10.1006/dbio.2001.0473. [DOI] [PubMed] [Google Scholar]
- 48.Ikegami K, Katou Y, Higashi K, Yoshimura T. Localization of circadian clock protein BMAL1 in the photoperiodic signal transduction machinery in Japanese quail. J Comp Neurol. 2009;517:397–404. doi: 10.1002/cne.22165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.