Abstract
DNA methylation is an important epigenetic modification involved in transcriptional regulation, nuclear organization, development, aging, and disease. Although DNA methyltransferases have been characterized, the mechanisms for DNA demethylation remain poorly understood. Using a cell-based reporter assay, we performed a functional genomics screen to identify genes involved in DNA demethylation. Here we show that RNF4 (RING finger protein 4), a SUMO-dependent ubiquitin E3-ligase previously implicated in maintaining genome stability, plays a key role in active DNA demethylation. RNF4 reactivates methylation-silenced reporters and promotes global DNA demethylation. Rnf4 deficiency is embryonic lethal with higher levels of methylation in genomic DNA. Mechanistic studies show that RNF4 interacts with and requires the base excision repair enzymes TDG and APE1 for active demethylation. This activity appears to occur by enhancing the enzymatic activities that repair DNA G:T mismatches generated from methylcytosine deamination. Collectively, our study reveals a unique function for RNF4, which may serve as a direct link between epigenetic DNA demethylation and DNA repair in mammalian cells.
Keywords: epigenetics, DNA repair, base excision repair
DNA methylation plays important roles in transcriptional regulation, genomic imprinting, and mammalian development (1). Deregulation of this important epigenetic modification has been implicated in a number of diseases, including cancer and developmental defects (2). Methylation of DNA occurs at the 5C position of the CpG dinucleotide and is mediated by DNA methyltransferases (DNMTs) (3). The de novo methyltransferases DNMT3a and DNMT3b are mainly responsible for introducing cytosine methylation at previously unmethylated CpG sites, whereas the maintenance methyltransferase DNMT1 copies preexisting methylation patterns into the newly synthesized DNA strand during DNA replication (4).
Dynamic DNA methylation is critical during development (1, 5). Although maintenance and de novo methylation mediated by DNMTs are relatively well understood (4), the mechanisms of DNA demethylation are still elusive (6). Passive demethylation can occur through the inhibition of DNMT1 activity, causing a loss in the methylation pattern during DNA replication. Rapid genome-wide demethylation is observed during gametogenesis and postfertilization, suggesting an active demethylation mechanism independent of DNA replication. Recent studies have identified several factors that are involved in active DNA demethylation, including the activation-induced cytidine deaminase (AID), an enzyme catalyzing 5-methyl cytidine (m5C) deamination in single-stranded DNA to generate thymine and a G:T mismatch (7–9), and GADD45a, a nuclear protein involved in the maintenance of genomic stability and DNA repair (9–11). However, neither AID- nor Gadd45a-deficient mice (12, 13) exhibit catastrophic developmental defects (14, 15), suggesting other factors might be involved in the regulation of active DNA demethylation.
In plants, genetic and biochemical experiments suggest that active DNA demethylation is mediated through a base excision repair (BER) pathway initiated by m5C-specific DNA glycosylases (16, 17). It is possible that active demethylation in mammals uses similar mechanisms. However, the mammalian counterpart of the m5C-specific DNA glycosylase has yet to be identified. Spontaneous or enzymatic m5C deamination would generate a G:T mismatch in DNA duplex, and subsequent G:T mismatch repair has, therefore, been proposed as a possible mechanism for active demethylation in mammalian cells (8, 18).
Functional genomics approaches have been used to identify factors that are involved in diverse biological processes (19–21). Here we use a cell-based functional genomics screen to reveal a key role for RNF4 in active DNA demethylation. Rnf4 deficiency is embryonic lethal, resulting in a higher content of m5C in genomic DNA. Furthermore, RNF4 interacts with and requires BER enzymes for demethylation.
Results
Genome-Wide Gain-of-Function Screen Identifies RNF4 as a Regulator of DNA Demethylation.
To identify genes that promote DNA demethylation, we carried out a gain-of-function genome-wide screen using a cell-based assay in which the methylation-silenced p16INK4a promoter drives the expression of a luciferase reporter. The p16INK4a promoter is known to be aberrantly silenced by DNA methylation in many cancers (22). The reporter construct was methylated in vitro, purified, and cotransfected into HEK293 cells in 384-well format with an arrayed cDNA expression library composed of 9,624 mouse and 6,415 human full-length cDNAs from the Mammalian Gene Collection (MGC) (23). GADD45a was used as a positive control based on its previously reported demethylation activity and gave a 10-fold increase in luciferase activity 48 h posttransfection (10). From the primary screen, 19 genes were found to induce luciferase activity greater than 10-fold and were subsequently reconfirmed using an in vitro methylated pFMR1-luc reporter (24) (full hit list in Table S1). Among them, GADD45g has been reported to be involved in DNA methylation regulations (9, 10). Other genes with DNA repair activities have also been identified (25).
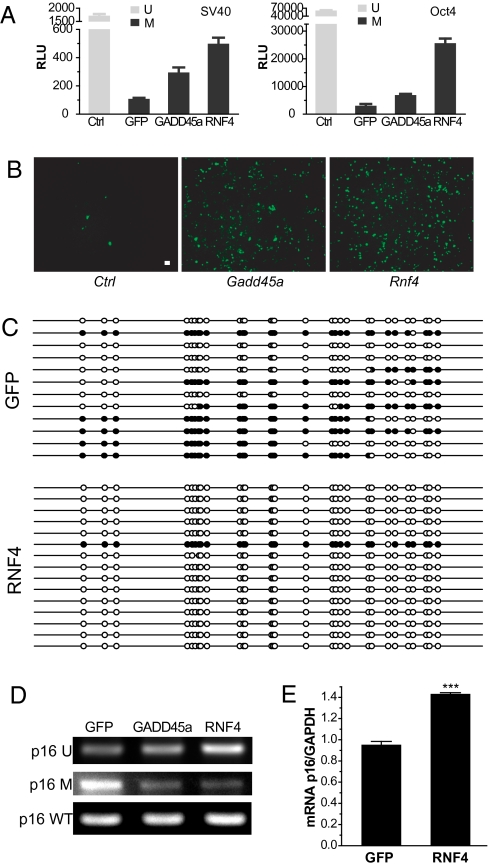
RNF4 (RING finger protein 4, or SNURF) was one of the most active hits in our screen with a >20-fold increase in reporter activity (Table S1). RNF4 has a conserved function in genome stability and DNA repair in eukaryotes (26, 27). In addition, we found that several methylation-silenced luciferase and GFP reporters were also reactivated by RNF4 overexpression in different cell lines (SV40-Luc, pOct4-Luc, and CMV-eGFP reporters; Fig. 1 A and B), indicating that the activity of RNF4 is both reporter and cell-type independent. Therefore, we focused our efforts on further characterization of the role of RNF4 in DNA demethylation. Analysis of the methylation status of endogenous CpG islands in the p16INK4a promoter by bisulfite sequencing revealed that RNF4 overexpression significantly reduces the methylation level of the p16INK4a promoter in the HCT116 colorectal cancer cell line, with complete demethylation in >80% of clones analyzed (Fig. 1C). We next examined the methylation status of the p16INK4a promoters using methylation-specific PCR (28). Overexpression of either GADD45a or RNF4 increases unmethylated-specific PCR product and decreases methylated-specific PCR product (Fig. 1D). Furthermore, removal of the repressive DNA methylation marks by RNF4 transfection led to partial reactivation of gene expression, as shown by 40% higher p16INK4a mRNA levels (P < 0.001) measured by qRT-PCR (Fig. 1E).
Fig. 1.
RNF4 promotes DNA demethylation. (A) Luciferase reporter assays transiently transfected with pCMV-sport6 (Ctrl) or the indicated genes. Luciferase expression was driven by the SV40 promoter in HEK293 cells, or the Oct4 promoter in P19 cells. Reporter plasmids were unmethylated (U) or in vitro methylated (M). Error bars indicate SEM (n = 6). (B) GFP reporter assay in HEK293 cells transiently transfected with the indicated genes. pEGFP-N1 vector was in vitro methylated. (Scale bar: 20 μm.) (C) Bisulfite sequence analysis of the p16INK4a promoter in HCT116 cells transiently transfected with the indicated genes. White and black circles represent unmethylated and methylated CpG, respectively. Images are representative result of three independent experiments. (D) Methylation-specific PCR was performed using bisulfate-treated genomic DNA as template with primers specific for unmethylated CpG template (p16 U) and methylated CpG template (p16 M) in HCT116 cells. Untreated genomic DNA was used as a loading control (p16 WT). (E) p16 mRNA was determined by qRT-PCR in HCT116 cells. GAPDH mRNA was used for normalization. P = 0.001; error bar indicate SEM (n = 3).
Rnf4 Deficiency Is Embryonic Lethal, and Loss of RNF4 Results in an Increase in Global DNA Methylation.
To address whether RNF4 plays an essential role in mammalian development, we took advantage of a recessive lethal gene-trap insertion mutation (29, 30) to generate Rnf4 knockout mice (Fig. S1A). Although heterozygous animals exhibited no obvious phenotype, homozygous null embryos were stunted and failed to develop to term (SI Materials and Methods, Figs. S1–S3, and Table S2). The embryos died between E14 and E15, and exhibited ventricular septal defects and cardiac insufficiency, which probably accounted for lethality. Genomic DNA from the Rnf4+/+, and Rnf4−/− mouse embryonic fibroblast (MEF) cells was extracted to analyze the global DNA methylation. Bisulfite sequencing analysis was performed for the representative maternally imprinted genes, Peg1 and Peg3, where ∼50% methylation was observed from wild-type genomic DNA; 75% and 78% of methylation was detected for Peg1 and Peg3, respectively, in Rnf4-deficient samples (Fig. 2A and Fig. S4A). Second, we performed Southern blot to probe the methylation status on minor satellite regions in genomic DNA (31). Global DNA hypermethylation was observed in DNA from Rnf4−/− MEFs, as shown by an increase in DNA fragment size from the methylation-sensitive HpaII digestion (Fig. 2B). Finally, the level of m5C in CCGG sites was measured with radioactive end-labeling and separation by TLC (14, 32). DNA from Rnf4−/− MEFs was found with higher (P < 0.05) m5C content (76%) than that of DNA from wildtype MEFs, in which about 60% of all CCGG sites were methylated (Fig. 2C). The content of m5C in the DNA of the heterozygous MEFs was similar to that of wild type. Collectively, these findings suggest that RNF4 is critical for embryonic development, and loss of RNF4 results in an increase in global DNA methylation.
Fig. 2.
Rnf4 deficiency increases global methylation level. (A) Bisulfite sequencing of a maternal imprinted locus, Peg3 (paternal expressed gene 3), on genomic DNA from Rnf4+/+ or Rnf4−/− MEFs. Representative results from three independent experiments are shown. (B) Southern blot of DNA methylation in minor satellite region. Genomic DNA was digested with the methylation-sensitive enzyme HpaII (H) or methylation-insensitive enzyme MspI (M), blotted, and probed by minor satellite pMR-150 probe. (C) m5C content on CCGG sequences in genomic DNA is determined using the end-labeling assay followed by TLC separation. The intensity of the spot corresponding to dm5CMP is quantified by scintillation counting. Representative results from three independent experiments are shown. P < 0.05; error bars indicate SEM (n = 3).
RNF4 Regulates Active Demethylation.
The reporter plasmids used in this study contain only bacterial replication origins, which are unable to replicate in mammalian cells. Therefore, we hypothesized that the reactivation of methylation-silenced reporters by RNF4 may involve an active demethylation process independent of DNA replication. To address this issue, we designed a probe for Southern blot analysis that hybridizes to a 240-bp fragment in between two HpaII sites on the pGL3 plasmid. As positive control, unmethylated pGL3 plasmid was readily digested by HpaII and released the 240-bp fragment (Fig. 3A, lane 2). Accordingly, methylated pGL3, as negative control, was resistant to HpaII digestion and did not release the 240-bp fragment (the two bands detected in lane 1 are presumably the supercoiled and nicked pGL3 plasmids; Fig. 3A). Next, we sought to determine if RNF4-induced demethylation is an active process or if it occurred passively during cell division. To this end, transiently transfected pGL3 plasmid was recovered from HEK293 cells and digested with HpaII. Whereas plasmids recovered from GFP-overexpressing cells did not change their methylation status (Fig. 3A, lanes 3 and 4), those recovered from RNF4-overexpressing cells were susceptible to HpaII digestion (Fig. 3A, lanes 5 and 6), indicating a loss of DNA methylation in these plasmids. Furthermore, we found that plasmids recovered from serum-starved nonproliferating cells were also demethylated upon RNF4 coexpression (Fig. S4B). Based on these experiments, demethylation by RNF4 is independent of DNA replication and cell proliferation, which suggests that RNF4 functions through an active demethylation process.
Fig. 3.
RNF4 interacts and requires BER pathway enzymes for active demethylation. (A) RNF4 overexpression induces demethylation of pGL3 plasmid. Methylated or unmethylated pGL3 plasmids were used as negative or positive controls, respectively (lanes 1 and 2). Plasmids recovered from cell culture were digested with HpaII, and the products were analyzed by Southern blot. Arrow, methylated plasmid; Arrowhead, unmethylated fragment. (B) RNF4 interacts with TDG and APE1 in cells. Endogenous TDG and APE1 were coimmunoprecipitated from Flag-tagged RNF4-transfected HEK293 cells. (C) Reverse Co-IP detected RNF4 association with myc-tagged TDG and APE1. (D) TDG and APE1 can synergize with RNF4 for active demethylation. Methylated pGL3 plasmid was recovered from cells cotransfected with suboptimal amounts of RNF4 (0.5 μg) and the designated genes (0.5 μg), digested with HpaII, and analyzed by Southern blot. Arrow, methylated plasmid; arrowhead, unmethylated fragment. (E and F) TDG and APE1 are required for RNF4-induced active demethylation. shRNAs targeting TDG and APE1 were used to knock down the expression of the corresponding protein (Western blot, Lower). Active demethylation by RNF4 is analyzed by Southern blot using recovered plasmids from cells cotransfected with corresponding shRNAs (Upper). Arrow, methylated plasmid; arrowhead, unmethylated fragment.
RNF4 is a SUMO-targeted ubiquitin E3 ligase (STUbL) with four N-terminal SUMO-interacting motifs (SIM) that trigger interaction with SUMOylated proteins, and a C-terminal RING finger motif critical for ubiquitination activity (33–35). Directed mutagenesis was used to generate the SIM and CS3 loss-of-function mutants that correspond to the SUMO-interacting motifs and RING motif, respectively (Fig. S5A). Both SIM and CS3 mutants were unable to reactivate the methylation-silenced luciferase reporter (Fig. S5B). In a Southern blot experiment using HpaII-digested recovered plasmids, the small fragments representing demethylated plasmid were not observed with either the SIM or CS3 mutants (Figs. S4B and S5C). These findings suggest that both motifs are essential for the demethylation function of RNF4.
RNF4 Regulates DNA Demethylation Through BER Pathway and Enhances DNA G:T Mismatch Repair.
Although genetic and biochemical evidence has suggested that DNA demethylation in plants is mediated through a BER pathway initiated by a m5C-specific DNA glycosylase, the corresponding mammalian glycosylase has not been identified (16, 17). Instead, m5C deamination generates a G:T mismatch in DNA duplex in mammalian DNA (36, 37). This mismatch is recognized and removed by DNA glycosylases, such as thymine DNA glycosylase (TDG), to create an apurinic/apyrimidinic (AP) site, which is then processed by an AP endonuclease (APE1) through the BER pathway. Interestingly, we found that RNF4 interacts with both TDG and APE1 in coimmunoprecipitation experiments (Fig. 3 B and C). However, mutations in all four SIMs and the RING domain did not interfere with TDG and APE1 association (Fig. 3 B and C). Interestingly, we observed that the N-terminal 100-aa fragment, which contains all of the SIMs, is sufficient for interaction with both TDG and APE1 (Fig. S4C), suggesting sequences other than the SUMO-interacting motifs in the N-terminal region are mediating the interaction (38).
To investigate whether TDG and APE1 play a role in active demethylation, we examined the synergistic effects of these genes when combined with a low level of RNF4, which by itself is not sufficient for demethylation (Fig. S6A). Methylated plasmid was recovered from cells cotransfected with suboptimal amounts of RNF4 and either TDG or APE1. The release of a small fragment from HpaII-digested TDG and APE1 samples indicated TDG/APE1 enhanced RNF4-induced demethylation (Fig. 3D). The same synergy was observed in the reactivation of methylation-silenced luciferase reporter assay (pFMR-Luc; Fig. S6B). The AID/Apobec (activation-induced deaminase/apolipoprotein B RNA-editing complex) family of RNA cytidine deaminases has been reported to have m5C deaminase activities (7). G:T mismatches could also be generated by such enzymatic catalysis. However, no synergistic effects were observed between RNF4 and AID or APOBEC1 in both reporter and Southern blot experiments.
To confirm that TDG and APE1 are required for demethylation, we used shRNAs to specifically silence the endogenous expression of TDG and APE1 in HEK293 cells. Methylated plasmids were recovered from cells transfected with both RNF4 and shRNAs targeting TDG (90% knockdown efficiency; Fig. 3E). The recovered methylated plasmid was resistant to HpaII digestion (Fig. 3E), indicating the methylation marks are intact and that RNF4 failed to induce demethylation upon TDG knockdown. We observed similar results in samples recovered from cells transfected with RNF4 and APE1 shRNAs (50% knockdown efficiency; Fig. 3F). Both shRNAs also inhibited RNF4-induced reactivation of methylation-silenced luciferase reporter (pFMR-Luc; Fig. S6 C and D). Together, these findings suggest that RNF4 interacts with and requires both BER pathway enzymes TDG and APE1 for active demethylation.
We next examined the enzymatic activities of TDG and APE1 in processing G:T mismatch lesions. A fluorescein-labeled, synthetic duplex (60 bp) carrying a G:T mismatch 24 bp away from the labeled 5′ end was used as substrate and incubated with immunoprecipitated Flag-tagged TDG and APE1 (39). As expected, TDG recognized the lesion site G:T mismatch and removed the thymine base. This generated an abasic site that was subsequently processed by APE1, and the cleaved 24-bp product was separated from the substrate by denaturing gels. RNF4 overexpression significantly increased the enzymatic activities of both TDG and APE1, compared with those of the GFP control (Fig. 4A and Fig. S7). To analyze cellular G:T mismatch repair activity, the luciferase start codon ATG was engineered to pair with TAT on the template strand in the pGL3 plasmid (named pGL3G:T hereafter). Failure to repair the G:T mismatch and convert TAT back to CAT would transcribe an ATA codon in mRNA, which would not be recognized by the ribosome for translation initiation. The luciferase reading is, therefore, an indirect indication of the G:T mismatch repair efficiency in cells. pGL3G:T cotransfected with RNF4 resulted in significantly higher (>8-fold, P < 0.01) relative response ratio (RRR) than cotransfection with GFP, indicating that RNF4 overexpression increases G:T mismatch repair efficiency (Fig. 4B). Using a probe (ARP) that reacts specifically with the aldehyde group in the open-ring form of the AP sites, the damage on genomic DNA was quantified by a colorimetric detection. As shown in Fig. 4C, DNA damage in RNF4-overexpressing cells was significantly less than that in control cells (P < 0.01), whereas DNA damage in Rnf4−/− MEFs was 2-fold higher than that in the wild-type/heterozygous controls (P < 0.01; Fig. 4D). These observations suggest that RNF4 induces DNA demethylation by enhancing DNA repair in mammalian cells.
Fig. 4.
RNF4 promotes DNA G:T mismatch repair. (A) The time-dependent generation of DNA nicking was assayed by incubation of immunoprecipitated TDG and APE1 with double-stranded 60-mer substrate (50 nM) containing a single G:T mismatch (39). Average numbers of three independent assays were graphed. (B) Relative response ratio for G:T mismatch firefly luciferase reading vs. Renilla luciferase reading (Materials and Methods). P = 0.01; error bars indicate SEM (n = 6). The number of AP sites per 105-bp genomic DNA was assayed using aldehyde reactive probe and colorimetric detection (Materials and Methods). P < 0.0001; error bars indicate SEM (n = 6). HEK293 genomic DNA (C) and RNF4 MEFs genomic DNA (D) were assayed.
Discussion
Gain-of-function screens have been used to successfully identify demethylating factors (10). Our studies extend these efforts to the identification of genes involved in mammalian DNA demethylation on a genome-wide scale. We show here that RNF4 plays a key role in active DNA demethylation through the BER pathway in mammalian cells. Indeed, previous studies have indicated a role for the BER pathway in active DNA demethylation in other organisms (9, 17). RNF4 interacts with and requires both BER enzymes TDG and APE1 for demethylation, likely by enhancing their enzymatic activities to repair G:T mismatch in DNA duplexes. Consistent with this notion, we observed that DNA damage in RNF4-overexpressing cells is significantly less than that in GFP-overexpressing control cells, whereas higher content of DNA damage sites is observed in Rnf4−/− MEFs.
Due to the lack of base coding information, and susceptibility to single-strand breaks in DNA, the AP sites generated during BER repair are both mutagenic and cytotoxic (40). This could explain the fact that many DNA glycosylases remain tightly bound to their AP-DNA product, which greatly reduces their enzymatic turnover (41). Recent studies have shown that human APE1 is able to stimulate TDG activity >42-fold on G:T mismatch substrates by disrupting the TDG-product complex in vitro (42). However, a stable molecular interaction between TDG and APE1 has not been detected in previous studies (43, 44). Our observation that RNF4 interacts with both TDG and APE1 suggests that RNF4 may function as a molecular scaffold to bring together the BER enzymes for efficient DNA repair in cells.
Proteins with RING finger motifs are involved in diverse cellular processes. For example, RNF8 was found to recognize DNA double-strand breaks and promote assembly of repair proteins (45, 46). Yeast homologs (Rfp1 and Rfp2) of RNF4 are essential for DNA repair (26), and mammalian RNF4 is required for arsenic-induced promyelocytic leukemia (PML) protein degradation in acute promyelocytic leukemia (33, 34). Although RNF4 is characterized as the only STUbL member in mammalian cells, recent yeast genetics studies suggest that SUMOylation is not required for RNF4-mediated substrate ubiquitination (38). Nonetheless, both SIMs and RING finger motif are essential for RNF4-induced active demethylation, indicating that a SUMOylated unknown substrate of RNF4 may be involved. Interestingly, SUMOylation of PML is required for the maturation of PML nuclear body (PML-NB), which recruits a number of nuclear proteins involved in gene transcription (47), apoptosis (48), DNA damage and repair (49), and virus infection (50). TDG has been reported to colocalize with PML in the cell nucleus in a SUMO-dependent manner (51), and PML exhibits repressive functions for genes in the BER repair pathway (52), suggesting that PML may negatively regulate the DNA repair pathway and that SUMO-dependent polyubiquitination of PML by RNF4 may account for the enhanced BER repair for active demethylation.
Our study shows that RNF4, a gene essential for mammalian embryonic development, plays a critical role in active DNA demethylation through mechanisms that involve the BER pathway. These findings suggest that active demethylation requires the DNA repair machinery, a mechanism likely conserved in both plant and mammalian cells.
Materials and Methods
Luciferase Reporter Assay.
HEK293 and P19 cells were transiently transfected in 384-well plates with a total of 50 ng DNA per well, containing 25 ng luciferase reporter and 25 ng effector plasmid. Luciferase reporter plasmids were produced in the dam−/dcm− bacteria strain SCS110 (Stratagene) and in vitro methylated using the CpG DNA methylase M.SssI (New England BioLabs). All luciferase readings were acquired following addition of Bright-Glo reagent (Promega) on the CLIPR instrument (Molecular Devices). Each experiment was repeated at least three times.
Southern Blot.
Southern blot analysis was carried out using 4–20% TBE PAGE (Invitrogen) gel electrophoresis and transferred to Nylon+ membrane (0.45-μm pore size; Invitrogen) according to manufacturer's instructions. 32P-labeled probe was hybridized at 65 °C overnight, washed, and developed by phosphorimager using the Noth2South system (Pierce) according to manufacturer's instructions. The minor satellite methylation was carried out using the pMR-150 probe according to Zhu et al. (31).
To analyze methylation status of the recovered plasmid, pGL3-promoter plasmid was in vitro methylated by M.SssI (New England BioLabs) and cotransfected into HEK293 cells with effector genes. The plasmids were recovered after 48 h by Miniprep Kit (ZymoResearch). Recovered plasmids were subject to HpaII or MspI (New England BioLabs) restriction digestion, purification, and Southern blot using probe 5′-CCATTCTATCCGCTGGAAGATGGAACCGCTGG. Each Southern experiment was repeated three times.
G:T Mismatch Repair Activity.
Generation of the G:T mismatch repair reporter in cells was adapted from Lei et al. (53). Point mutagenesis was used to insert an EcoRI site on pGL3-promoter plasmid where a Glu was introduced between Pro13 and Phe14. Luciferase activity for this construct is not affected by this insertion. This plasmid DNA was then digested with HindIII and EcoRI, treated with phosphatase (New England BioLabs) to remove the 5′ phosphates before ligation. A mismatch was introduced at the ATG codon matching with TAT where the G:T are underlined. Annealed oligos corresponding to the HindIII/EcoRI-released fragment (74 bp) were treated with PNK (New England BioLabs) to add the 5′ phosphyl group.
HindIII oligo: 5′-AGCTTGGCATTCCGGTACTGTTGGTAAAGCCACCATGGAAGACGCCAAAAACATAAAGAAAGGCCCGGCGCCAG
EcoRI oligo: 5′-AATTCTGGCGCCGGGCCTTTCTTTATGTTTTTGGCGTCTTCTATGGTGGCTTTACCAACAGTACCGGAATGCCA
Ligation was carried out with T4 DNA ligase (New England BioLabs) at 16 °C overnight. The ligation products were then treated with plasmid-safe ATP-dependent DNase (Epicenter) to remove the linear vector substrates before purification. Purified plasmid (designated as pGL3G:T) was cotransfected with renilla luciferase and effector genes for 48 h before both firefly and renilla luciferase activities were read using Dual-Glo reagents (Promega). RRR was calculated according to manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Drs. Y. N. Chen (Novartis Institutes for BioMedical Research, Cambridge, MA), D. Reines (Emory University, Atlanta), and R. Hay (University of Dundee, Dundee, Scotland) for constructs and Drs. E. Li (Novartis Institutes for BioMedical Research, Shanghai, China), D. Schübeler (Friedrich Miescher Institute, Basel), S. Gasser (Friedrich Miescher Institute, Basel), T. Hunter (Salk Institute, La Jolla, CA), C. Cho (Genomics Institute of the Novartis Foundation, San Diego), and C. A. Lyssiotis (The Scripps Research Institute) for helpful discussions. X.V.H. is a Gilead Fellow of the Life Sciences Research Foundation. R.K.B. was supported by postdoctoral fellowships from the American Heart Association. This work is supported by the Skaggs Institute of Chemical Biology (P.G.S.), National Institutes of Health (G.E.L.), and Novartis Research Foundation (P.G.S. and X.W.). This article is Manuscript 19948 of the Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009025107/-/DCSupplemental.
References
- 1.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 3.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 4.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 5.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 6.Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–1148. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Morgan HD, Dean W, Coker HA, Reik W, Petersen-Mahrt SK. Activation-induced cytidine deaminase deaminates 5-methylcytosine in DNA and is expressed in pluripotent tissues: Implications for epigenetic reprogramming. J Biol Chem. 2004;279:52353–52360. doi: 10.1074/jbc.M407695200. [DOI] [PubMed] [Google Scholar]
- 8.Popp C, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai K, et al. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–1212. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barreto G, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–675. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz KM, et al. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol Cell. 2009;33:344–353. doi: 10.1016/j.molcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Salvador JM, et al. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity. 2002;16:499–508. doi: 10.1016/s1074-7613(02)00302-3. [DOI] [PubMed] [Google Scholar]
- 13.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 14.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 15.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 16.Gehring M, Reik W, Henikoff S. DNA demethylation by DNA repair. Trends Genet. 2009;25:82–90. doi: 10.1016/j.tig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhu JK. Active DNA demethylation mediated by DNA glycosylases. Annu Rev Genet. 2009;43:143–166. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan BK, Miller JH. Mutagenic deamination of cytosine residues in DNA. Nature. 1980;287:560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, et al. Identification of the Wnt signaling activator leucine-rich repeat in Flightless interaction protein 2 by a genome-wide functional analysis. Proc Natl Acad Sci USA. 2005;102:1927–1932. doi: 10.1073/pnas.0409472102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luesch H, et al. A functional genomics approach to the mode of action of apratoxin A. Nat Chem Biol. 2006;2:158–167. doi: 10.1038/nchembio769. [DOI] [PubMed] [Google Scholar]
- 21.Willingham AT, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–1573. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 22.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 23.Chanda SK, et al. Genome-scale functional profiling of the mammalian AP-1 signaling pathway. Proc Natl Acad Sci USA. 2003;100:12153–12158. doi: 10.1073/pnas.1934839100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KT, Coffee B, Reines D. Occupancy and synergistic activation of the FMR1 promoter by Nrf-1 and Sp1 in vivo. Hum Mol Genet. 2004;13:1611–1621. doi: 10.1093/hmg/ddh172. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 26.Kosoy A, Calonge TM, Outwin EA, O'Connell MJ. Fission yeast Rnf4 homologs are required for DNA repair. J Biol Chem. 2007;282:20388–20394. doi: 10.1074/jbc.M702652200. [DOI] [PubMed] [Google Scholar]
- 27.Sun H, Leverson JD, Hunter T. Conserved function of RNF4 family proteins in eukaryotes: Targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 2007;26:4102–4112. doi: 10.1038/sj.emboj.7601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedrich G, Soriano P. Promoter traps in embryonic stem cells: A genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- 30.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, et al. Lsh is involved in de novo methylation of DNA. EMBO J. 2006;25:335–345. doi: 10.1038/sj.emboj.7600925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bestor TH, Hellewell SB, Ingram VM. Differentiation of two mouse cell lines is associated with hypomethylation of their genomes. Mol Cell Biol. 1984;4:1800–1806. doi: 10.1128/mcb.4.9.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lallemand-Breitenbach V, et al. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10:547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 34.Tatham MH, et al. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 35.Prudden J, et al. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 2007;26:4089–4101. doi: 10.1038/sj.emboj.7601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walsh CP, Xu GL. Cytosine methylation and DNA repair. Curr Top Microbiol Immunol. 2006;301:283–315. doi: 10.1007/3-540-31390-7_11. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer GP. Mutagenesis at methylated CpG sequences. Curr Top Microbiol Immunol. 2006;301:259–281. doi: 10.1007/3-540-31390-7_10. [DOI] [PubMed] [Google Scholar]
- 38.Xie Y, Rubenstein EM, Matt T, Hochstrasser M. SUMO-independent in vivo activity of a SUMO-targeted ubiquitin ligase toward a short-lived transcription factor. Genes Dev. 2010;24:893–903. doi: 10.1101/gad.1906510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardeland U, Bentele M, Jiricny J, Schär P. Separating substrate recognition from base hydrolysis in human thymine DNA glycosylase by mutational analysis. J Biol Chem. 2000;275:33449–33456. doi: 10.1074/jbc.M005095200. [DOI] [PubMed] [Google Scholar]
- 40.Boiteux S, Guillet M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair (Amst) 2004;3:1–12. doi: 10.1016/j.dnarep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Dizdaroglu M. Substrate specificities and excision kinetics of DNA glycosylases involved in base-excision repair of oxidative DNA damage. Mutat Res. 2003;531:109–126. doi: 10.1016/j.mrfmmm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald ME, Drohat AC. Coordinating the initial steps of base excision repair. Apurinic/apyrimidinic endonuclease 1 actively stimulates thymine DNA glycosylase by disrupting the product complex. J Biol Chem. 2008;283:32680–32690. doi: 10.1074/jbc.M805504200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waters TR, Gallinari P, Jiricny J, Swann PF. Human thymine DNA glycosylase binds to apurinic sites in DNA but is displaced by human apurinic endonuclease 1. J Biol Chem. 1999;274:67–74. doi: 10.1074/jbc.274.1.67. [DOI] [PubMed] [Google Scholar]
- 44.Privezentzev CV, Saparbaev M, Laval J. The HAP1 protein stimulates the turnover of human mismatch-specific thymine-DNA-glycosylase to process 3,N(4)-ethenocytosine residues. Mutat Res. 2001;480-481:277–284. doi: 10.1016/s0027-5107(01)00186-5. [DOI] [PubMed] [Google Scholar]
- 45.Mailand N, et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887–900. doi: 10.1016/j.cell.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 46.Huen MS, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shima Y, et al. PML activates transcription by protecting HIPK2 and p300 from SCFFbx3-mediated degradation. Mol Cell Biol. 2008;28:7126–7138. doi: 10.1128/MCB.00897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernardi R, Papa A, Pandolfi PP. Regulation of apoptosis by PML and the PML-NBs. Oncogene. 2008;27:6299–6312. doi: 10.1038/onc.2008.305. [DOI] [PubMed] [Google Scholar]
- 49.Conlan LA, McNees CJ, Heierhorst J. Proteasome-dependent dispersal of PML nuclear bodies in response to alkylating DNA damage. Oncogene. 2004;23:307–310. doi: 10.1038/sj.onc.1207119. [DOI] [PubMed] [Google Scholar]
- 50.Chelbi-Alix MK, Quignon F, Pelicano L, Koken MH, de Thé H. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J Virol. 1998;72:1043–1051. doi: 10.1128/jvi.72.2.1043-1051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi H, Hatakeyama S, Saitoh H, Nakayama KI. Noncovalent SUMO-1 binding activity of thymine DNA glycosylase (TDG) is required for its SUMO-1 modification and colocalization with the promyelocytic leukemia protein. J Biol Chem. 2005;280:5611–5621. doi: 10.1074/jbc.M408130200. [DOI] [PubMed] [Google Scholar]
- 52.Alcalay M, et al. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J Clin Invest. 2003;112:1751–1761. doi: 10.1172/JCI17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lei X, Zhu Y, Tomkinson A, Sun L. Measurement of DNA mismatch repair activity in live cells. Nucleic Acids Res. 2004;32:e100. doi: 10.1093/nar/gnh098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.